Abstract
Orientation of the green alga Chlamydomonas in light (phototaxis and stop responses) is controlled by a visual system with a rhodopsin as the functional photoreceptor. Here, we present evidence that in Chlamydomonas wild-type cells all-trans retinal is the predominant isomer and that it is present in amounts similar to that of the rhodopsin itself.
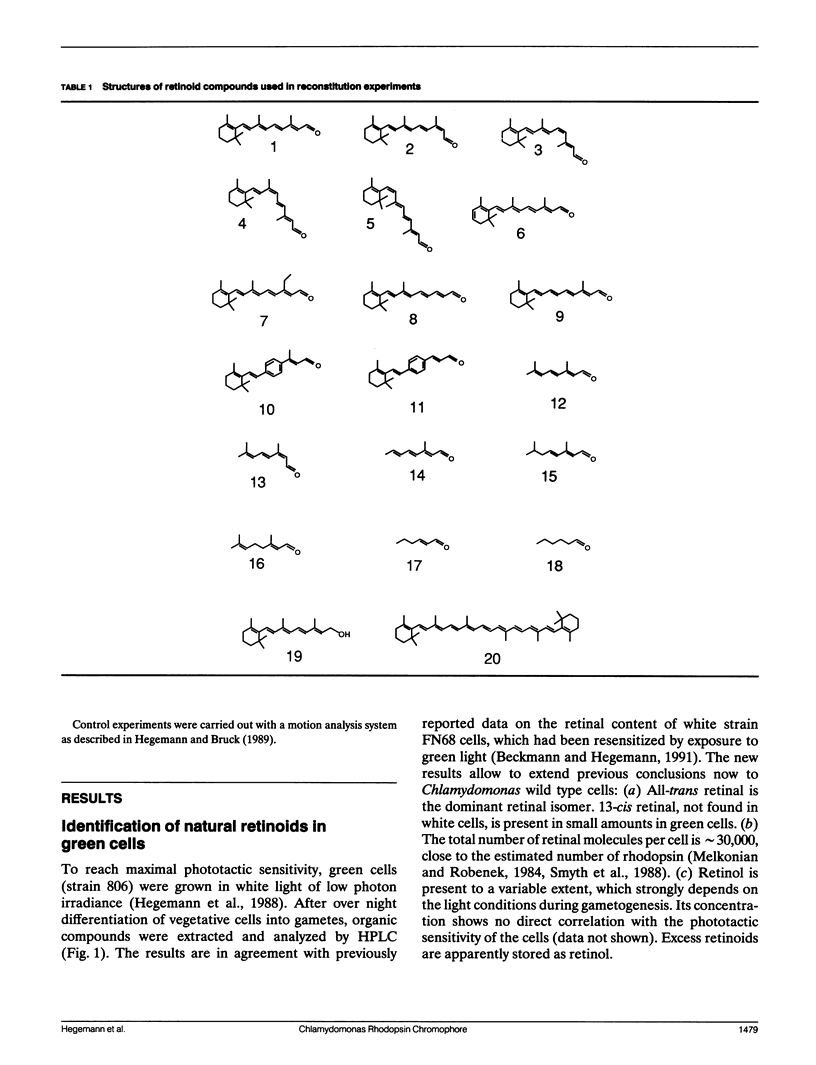
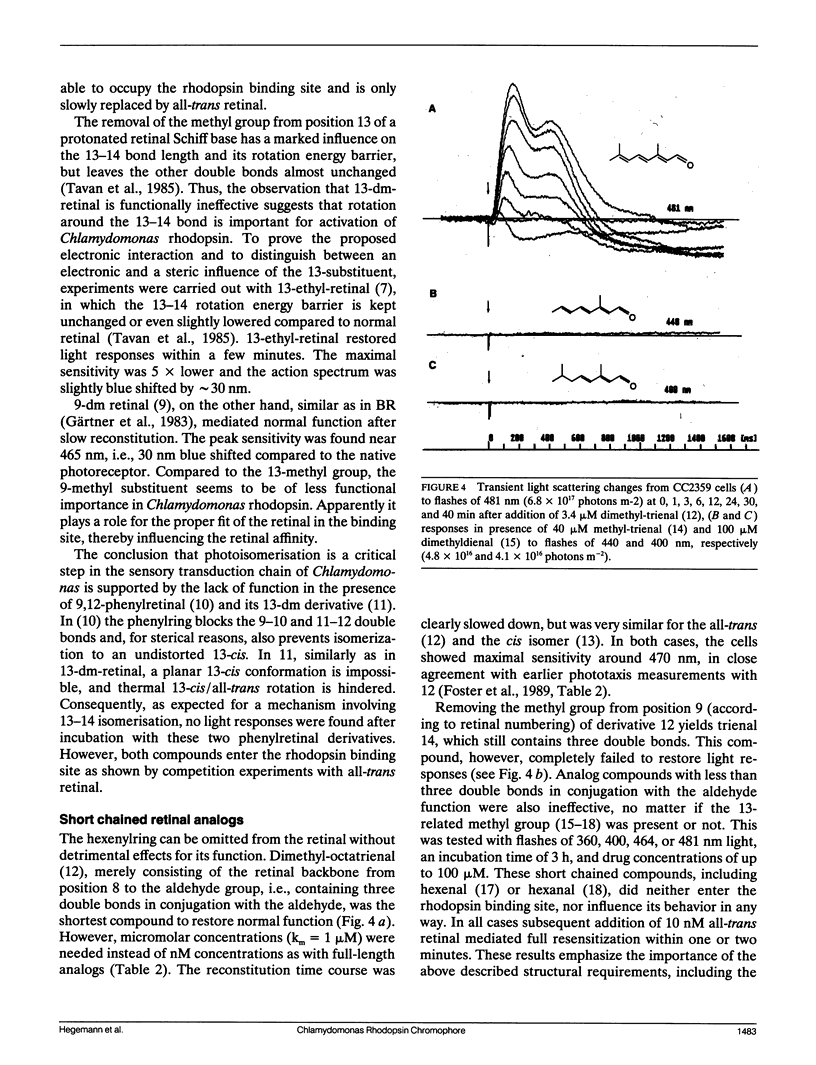
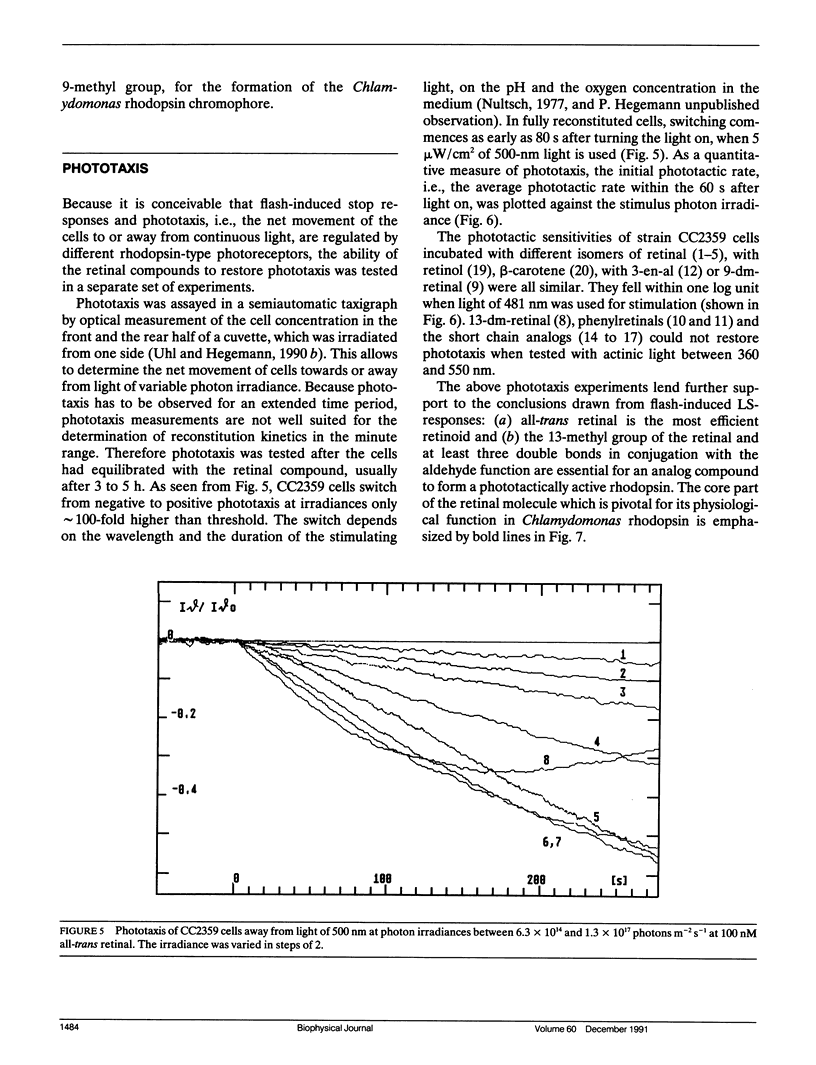
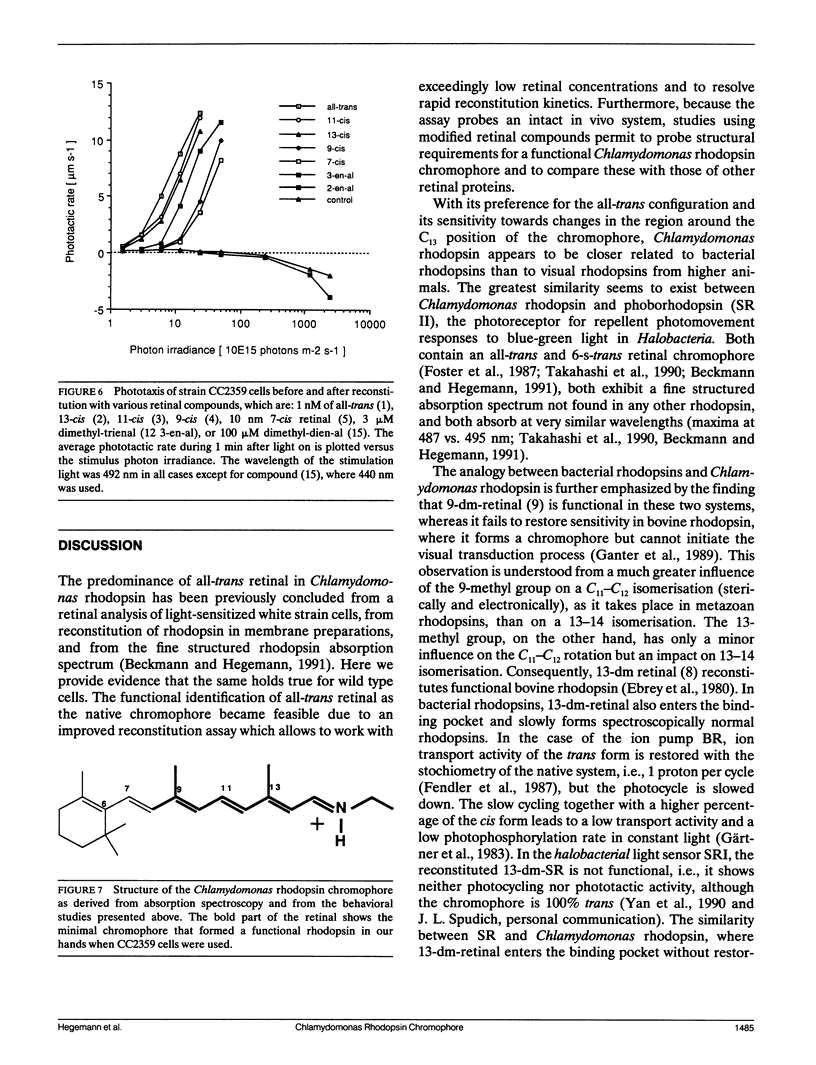
The ability of different retinal isomers and analog compounds to restore photosensitivity in blind Chlamydomonas cells (strain CC2359) was tested by means of flash-induced light scattering transients or by measuring phototaxis in a taxigraph. All-trans retinal reconstitutes behavioral light responses within one minute, whereas cis-isomers require at least 50 × longer incubation times, suggesting that the retinal binding site is specific for all-trans retinal. Experiments with 13-demethyl(dm)-retinal and short-chained analogs reveal that only chromophores with a β-methyl group and at least three double bonds in conjugation with the aldehyde mediate function. Because neither 13-dm-retinal, nor 9,12-phenylretinal restores a functional rhodopsin, a trans/13-cis isomerisation seems to take place in the course of the activation mechanism. We conclude that with respect to its chromophore, Chlamydomonas rhodopsin bears a closer resemblence to bacterial rhodopsins than to visual rhodopsins of higher animals.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann M., Hegemann P. In vitro identification of rhodopsin in the green alga Chlamydomonas. Biochemistry. 1991 Apr 16;30(15):3692–3697. doi: 10.1021/bi00229a014. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T., Tsuda M., Sassenrath G., West J. L., Waddell W. H. Light activation of bovine rod phosphodiesterase by non-physiological visual pigments. FEBS Lett. 1980 Jul 28;116(2):217–219. doi: 10.1016/0014-5793(80)80647-8. [DOI] [PubMed] [Google Scholar]
- Foster K. W., Saranak J., Derguini F., Zarrilli G. R., Johnson R., Okabe M., Nakanishi K. Activation of Chlamydomonas rhodopsin in vivo does not require isomerization of retinal. Biochemistry. 1989 Jan 24;28(2):819–824. doi: 10.1021/bi00428a061. [DOI] [PubMed] [Google Scholar]
- Foster K. W., Saranak J., Patel N., Zarilli G., Okabe M., Kline T., Nakanishi K. A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature. 1984 Oct 25;311(5988):756–759. doi: 10.1038/311756a0. [DOI] [PubMed] [Google Scholar]
- Foster K. W., Saranak J., Zarrilli G. Autoregulation of rhodopsin synthesis in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6379–6383. doi: 10.1073/pnas.85.17.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K. W., Smyth R. D. Light Antennas in phototactic algae. Microbiol Rev. 1980 Dec;44(4):572–630. doi: 10.1128/mr.44.4.572-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter U. M., Schmid E. D., Perez-Sala D., Rando R. R., Siebert F. Removal of the 9-methyl group of retinal inhibits signal transduction in the visual process. A Fourier transform infrared and biochemical investigation. Biochemistry. 1989 Jul 11;28(14):5954–5962. doi: 10.1021/bi00440a036. [DOI] [PubMed] [Google Scholar]
- Groenendijk G. W., Jansen P. A., Bonting S. L., Daemen F. J. Analysis of geometrically isomeric vitamin A compounds. Methods Enzymol. 1980;67:203–220. doi: 10.1016/s0076-6879(80)67029-3. [DOI] [PubMed] [Google Scholar]
- Hara T., Hara R. Cephalopod retinochrome. Methods Enzymol. 1982;81:190–197. doi: 10.1016/s0076-6879(82)81031-8. [DOI] [PubMed] [Google Scholar]
- Hegemann P., Hegemann U., Foster K. W. Reversible bleaching of Chlamydomonas reinhardtii rhodopsin in vivo. Photochem Photobiol. 1988 Jul;48(1):123–128. doi: 10.1111/j.1751-1097.1988.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Kamiya R., Witman G. B. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol. 1984 Jan;98(1):97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. The molecular mechanism of excitation in visual transduction and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):549–553. doi: 10.1073/pnas.75.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nultsch W. Effect of external factors on phototaxis of Chlamydomonas reinhardtii. II. Charbon dioxide, oxygen and pH. Arch Microbiol. 1977 Mar 1;112(2):179–185. doi: 10.1007/BF00429333. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Salem L., Bruckmann P. Conversion of a photon to an electrical signal by sudden polarisation in the N-retinylidene visual chromophore. Nature. 1975 Dec 11;258(5535):526–528. doi: 10.1038/258526a0. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Eckert R. Calcium couples flagellar reversal to photostimulation in Chlamydomonas reinhardtii. Nature. 1976 Aug 19;262(5570):713–715. doi: 10.1038/262713a0. [DOI] [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Specificity of the retinal binding site of bacteriorhodopsin: chemical and stereochemical requirements for the binding of retinol and retinal. Biochemistry. 1978 Dec 12;17(25):5353–5359. doi: 10.1021/bi00618a005. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., McCain D. A., Nakanishi K., Okabe M., Shimizu N., Rodman H., Honig B., Bogomolni R. A. Chromophore/protein interaction in bacterial sensory rhodopsin and bacteriorhodopsin. Biophys J. 1986 Feb;49(2):479–483. doi: 10.1016/S0006-3495(86)83657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Fujita Y., Noda Y., Miyata S. A simple procedure for the extraction of the native chromophore of visual pigments: the formaldehyde method. Vision Res. 1986;26(3):425–429. doi: 10.1016/0042-6989(86)90185-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Makino-Tasaka M. Analysis of retinal and 3-dehydroretinal in the retina by high-pressure liquid chromatography. Anal Biochem. 1983 Feb 15;129(1):111–119. doi: 10.1016/0003-2697(83)90059-3. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Yan B., Mazur P., Derguini F., Nakanishi K., Spudich J. L. Color regulation in the archaebacterial phototaxis receptor phoborhodopsin (sensory rhodopsin II). Biochemistry. 1990 Sep 11;29(36):8467–8474. doi: 10.1021/bi00488a038. [DOI] [PubMed] [Google Scholar]
- Tavan P., Schulten K., Gärtner W., Oesterhelt D. Substituents at the c(13) position of retinal and their influence on the function of bacteriorhodopsin. Biophys J. 1985 Mar;47(3):349–355. doi: 10.1016/s0006-3495(85)83925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl R., Hegemann P. Probing visual transduction in a plant cell: Optical recording of rhodopsin-induced structural changes from Chlamydomonas reinhardtii. Biophys J. 1990 Nov;58(5):1295–1302. doi: 10.1016/S0006-3495(90)82469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A. Charge stabilization mechanism in the visual and purple membrane pigments. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2558–2562. doi: 10.1073/pnas.75.6.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Takahashi T., Johnson R., Derguini F., Nakanishi K., Spudich J. L. All-trans/13-cis isomerization of retinal is required for phototaxis signaling by sensory rhodopsins in Halobacterium halobium. Biophys J. 1990 Apr;57(4):807–814. doi: 10.1016/S0006-3495(90)82600-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


