Abstract
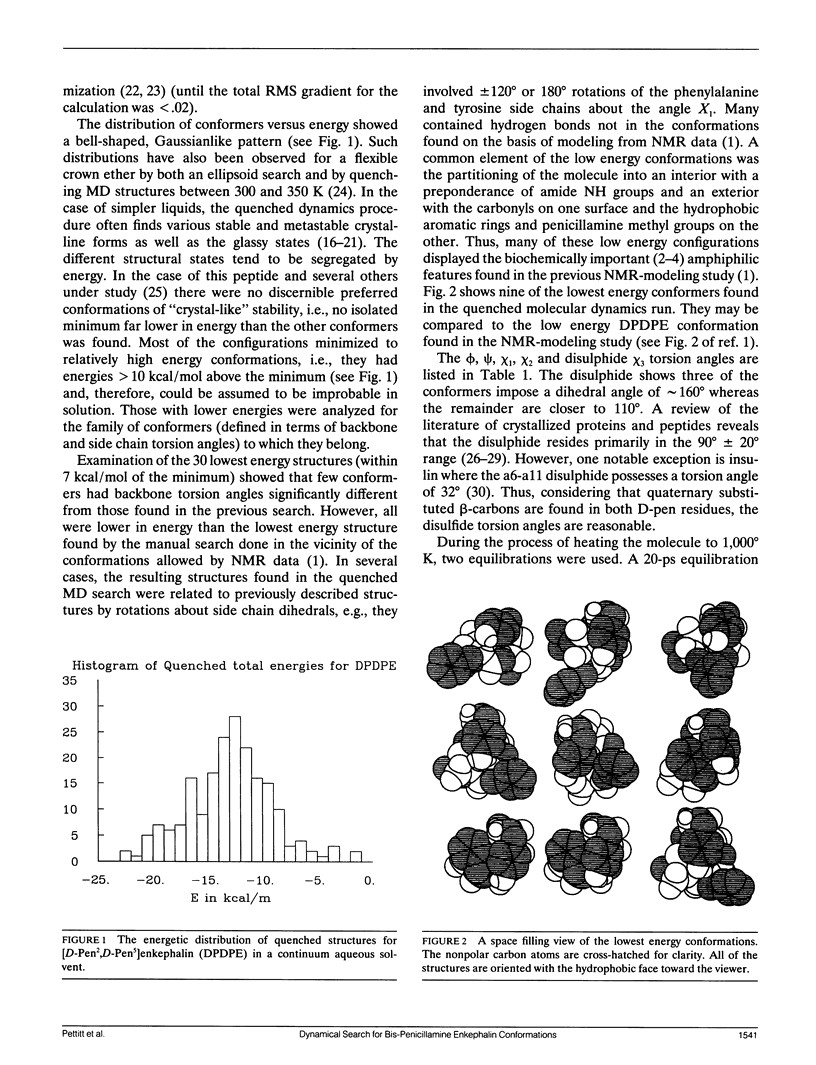
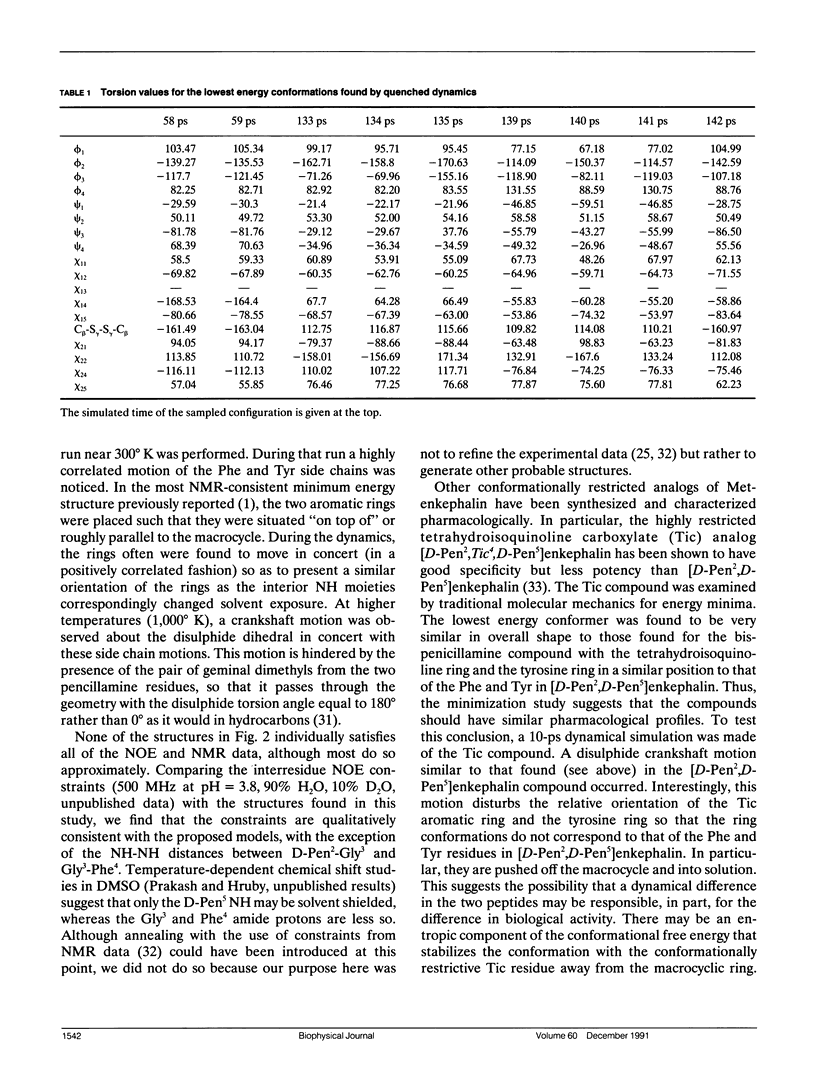
Quenched molecular dynamics is used as a conformational search technique for the constrained cyclic analog [D-Pen2,D-Pen5]enkephalin (DPDPE) in a continuum solvent. The results show a Gaussianlike distribution of conformations as a function of energy, unlike the distributions found for simple liquids which have sharp bands for different crystal forms and broad glasslike states are found. The lowest energy conformers have structural features in common with those obtained from constrained searches based on energy minimization. (Hruby, V. J., L-.F. Kao, B. M. Pettitt, and M. Karplus. 1988. J. Am. Chem. Soc. 110:3351-3359). Many of the low energy configurations are amphiphilic with the carbonyl groups on one surface and the hydrophobic groups on the other. This supports the conclusions from the previous modeling study, which yielded amphiphilic structures as the most probable conformations of DPDPE when NOE data were included.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Dodson G. G., Dodson E., Hodgkin D. C., Vijayan M. X-ray analysis and the structure of insulin. Recent Prog Horm Res. 1971;27:1–40. doi: 10.1016/b978-0-12-571127-2.50025-0. [DOI] [PubMed] [Google Scholar]
- Bruccoleri R. E., Karplus M. Conformational sampling using high-temperature molecular dynamics. Biopolymers. 1990 Dec;29(14):1847–1862. doi: 10.1002/bip.360291415. [DOI] [PubMed] [Google Scholar]
- Bruccoleri R. E., Karplus M. Prediction of the folding of short polypeptide segments by uniform conformational sampling. Biopolymers. 1987 Jan;26(1):137–168. doi: 10.1002/bip.360260114. [DOI] [PubMed] [Google Scholar]
- Dammkoehler R. A., Karasek S. F., Shands E. F., Marshall G. R. Constrained search of conformational hyperspace. J Comput Aided Mol Des. 1989 Mar;3(1):3–21. doi: 10.1007/BF01590992. [DOI] [PubMed] [Google Scholar]
- Elber R., Karplus M. Multiple conformational states of proteins: a molecular dynamics analysis of myoglobin. Science. 1987 Jan 16;235(4786):318–321. doi: 10.1126/science.3798113. [DOI] [PubMed] [Google Scholar]
- Hruby V. J., al-Obeidi F., Kazmierski W. Emerging approaches in the molecular design of receptor-selective peptide ligands: conformational, topographical and dynamic considerations. Biochem J. 1990 Jun 1;268(2):249–262. doi: 10.1042/bj2680249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Amphiphilic secondary structure: design of peptide hormones. Science. 1984 Jan 20;223(4633):249–255. doi: 10.1126/science.6322295. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T. The design of peptides and proteins. Ann N Y Acad Sci. 1986;471:233–238. doi: 10.1111/j.1749-6632.1986.tb48039.x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick S., Gelatt C. D., Jr, Vecchi M. P. Optimization by simulated annealing. Science. 1983 May 13;220(4598):671–680. doi: 10.1126/science.220.4598.671. [DOI] [PubMed] [Google Scholar]
- Nilges M., Clore G. M., Gronenborn A. M. Determination of three-dimensional structures of proteins from interproton distance data by dynamical simulated annealing from a random array of atoms. Circumventing problems associated with folding. FEBS Lett. 1988 Oct 24;239(1):129–136. doi: 10.1016/0014-5793(88)80559-3. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Ripoll D. R., Scheraga H. A. On the multiple-minima problem in the conformational analysis of polypeptides. II. An electrostatically driven Monte Carlo method--tests on poly(L-alanine). Biopolymers. 1988 Aug;27(8):1283–1303. doi: 10.1002/bip.360270808. [DOI] [PubMed] [Google Scholar]
- Wood S. P., Tickle I. J., Treharne A. M., Pitts J. E., Mascarenhas Y., Li J. Y., Husain J., Cooper S., Blundell T. L., Hruby V. J. Crystal structure analysis of deamino-oxytocin: conformational flexibility and receptor binding. Science. 1986 May 2;232(4750):633–636. doi: 10.1126/science.3008332. [DOI] [PubMed] [Google Scholar]
- Wyckoff H. W., Tsernoglou D., Hanson A. W., Knox J. R., Lee B., Richards F. M. The three-dimensional structure of ribonuclease-S. Interpretation of an electron density map at a nominal resolution of 2 A. J Biol Chem. 1970 Jan 25;245(2):305–328. [PubMed] [Google Scholar]


