Abstract
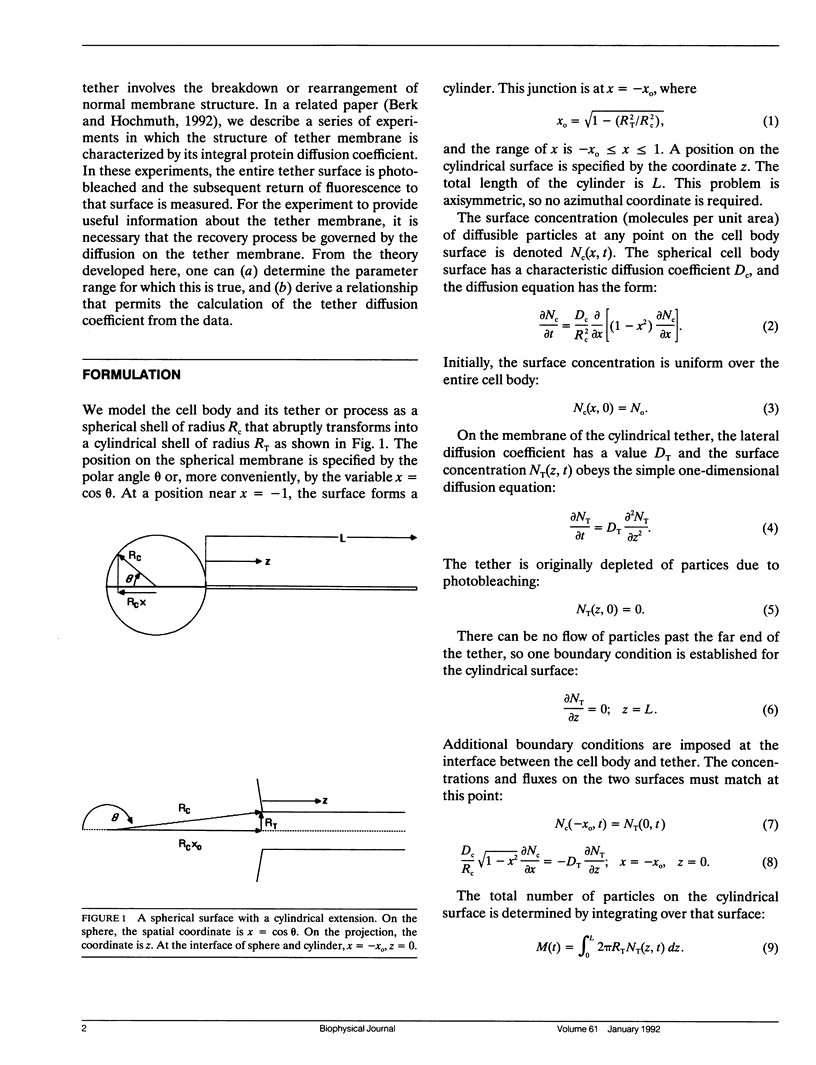
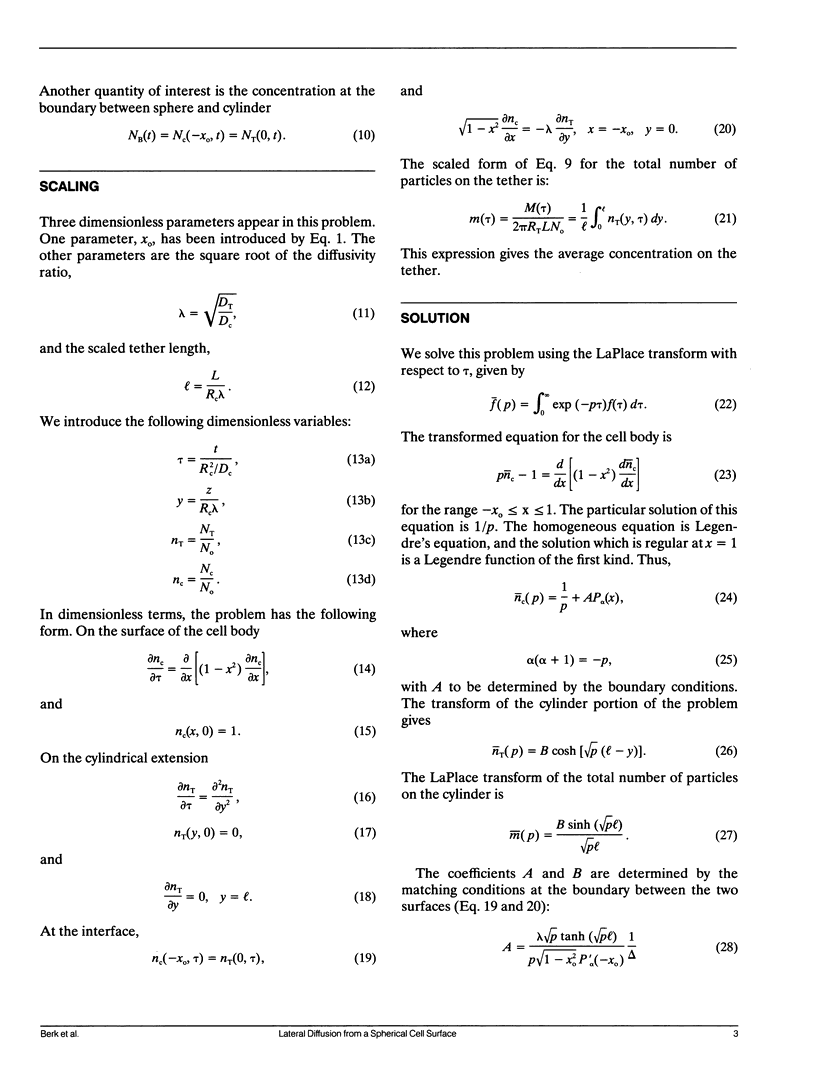
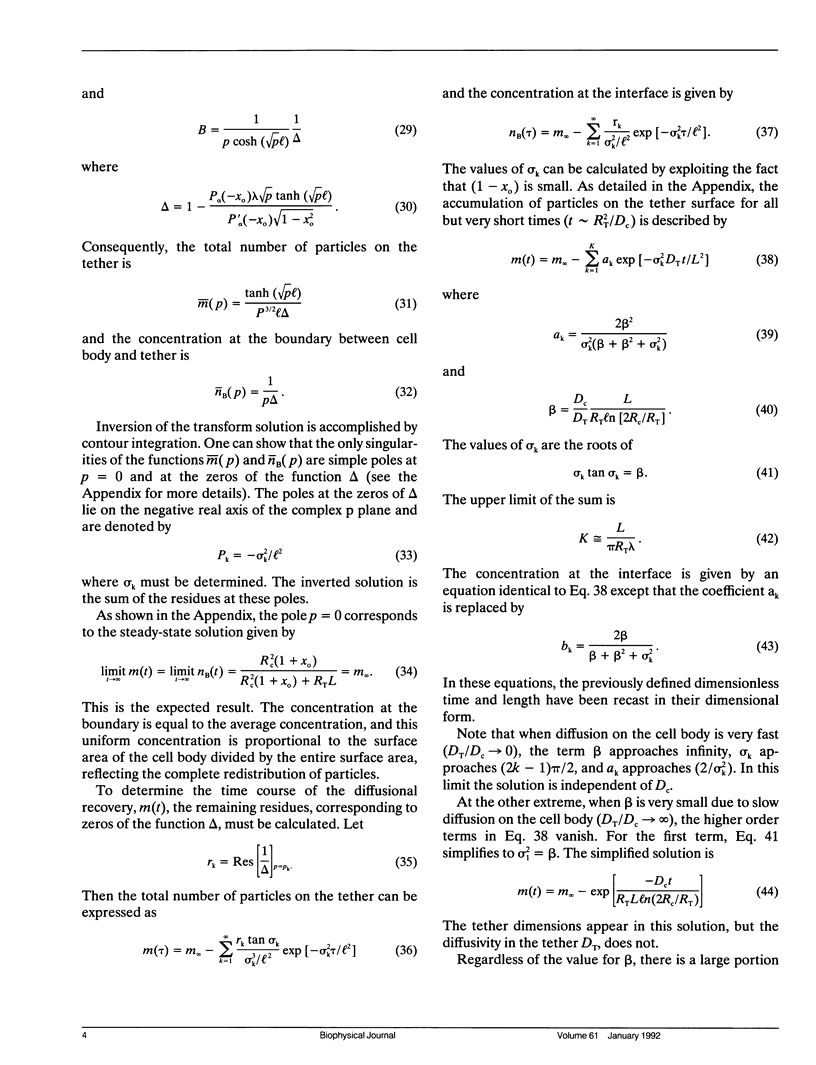
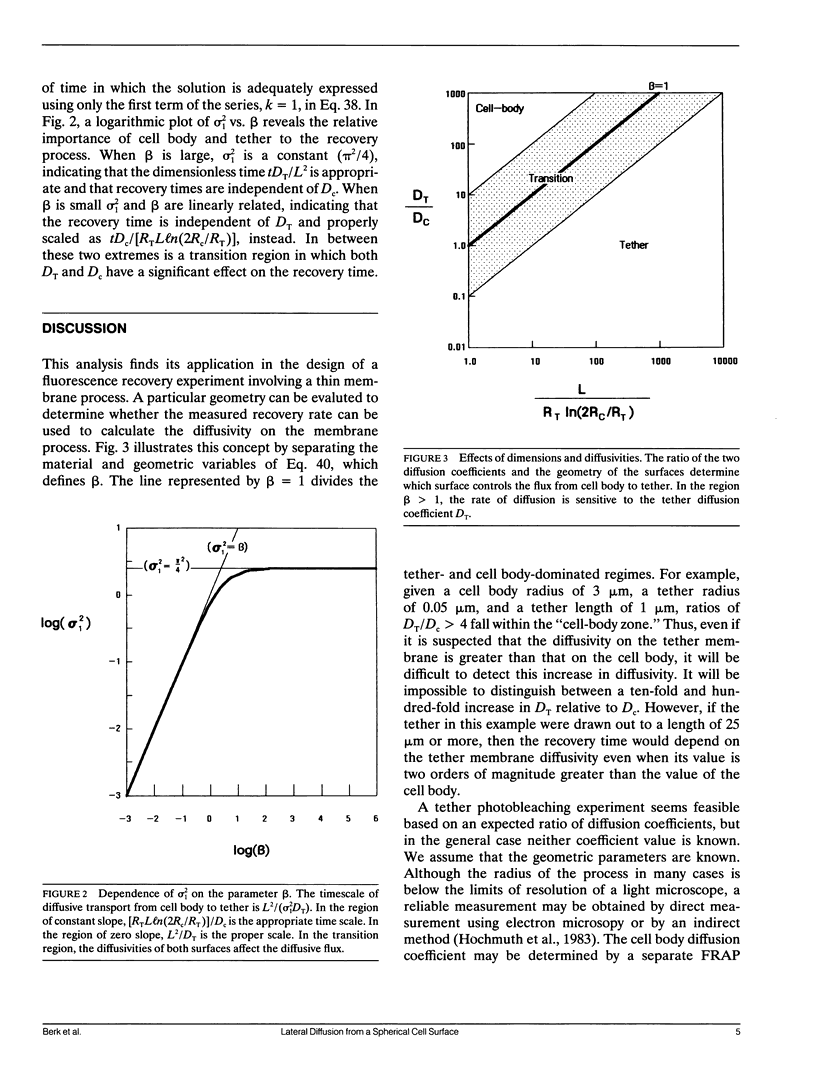
Cell surfaces are often heterogeneous with respect to the lateral distribution and mobility of membrane components. Because lateral mobility is related to membrane structure, measurement of a particular component's local diffusion coefficient within a distinct surface region provides useful information about the formation and maintenance of that region. Many structurally interesting cell surface features can be described as narrow tubular projections from the body of the cell. In a companion paper, we consider the thin "tethers" that can be mechanically drawn from the red blood cell membrane, and we measure the transport of fluorescent integral proteins from the surface of the cell body onto the tether. In this paper we present an analysis to describe the surface diffusion of membrane particles from a spherical shell onto a thin cylindrical process. Provision is made for different rates of diffusion within the two morphologically distinct regions. The relative role of each region in controlling the diffusive flux between regions is determined primarily by a single dimensionless parameter. This parameter incorporates the ratio of the two diffusion coefficients as well as the dimensions of each region. The analysis can be applied to a fluorescence photobleaching experiment in which the extended process is bleached. If the dimensions of the spherical cell body and the cylindrical extension are known, then the diffusion coefficients of both regions can be determined from the experimental fluorescence recovery curve.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelides K. J. Fluorescently labelled Na+ channels are localized and immobilized to synapses of innervated muscle fibres. Nature. 1986 May 1;321(6065):63–66. doi: 10.1038/321063a0. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk D. A., Hochmuth R. M. Lateral mobility of integral proteins in red blood cell tethers. Biophys J. 1992 Jan;61(1):9–18. doi: 10.1016/S0006-3495(92)81811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. Membrane viscoplastic flow. Biophys J. 1976 Jan;16(1):13–26. doi: 10.1016/S0006-3495(76)85659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M., Evans C. A., Wiles H. C., McCown J. T. Mechanical measurement of red cell membrane thickness. Science. 1983 Apr 1;220(4592):101–102. doi: 10.1126/science.6828875. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Mohandas N., Blackshear P. L., Jr Measurement of the elastic modulus for red cell membrane using a fluid mechanical technique. Biophys J. 1973 Aug;13(8):747–762. doi: 10.1016/S0006-3495(73)86021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. W. Mobility and diffusion in the plane of cell membrane. J Theor Biol. 1973 Jul;40(1):11–17. doi: 10.1016/0022-5193(73)90161-6. [DOI] [PubMed] [Google Scholar]
- Jacobson K., O'Dell D., August J. T. Lateral diffusion of an 80,000-dalton glycoprotein in the plasma membrane of murine fibroblasts: relationships to cell structure and function. J Cell Biol. 1984 Nov;99(5):1624–1633. doi: 10.1083/jcb.99.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E. Normal-mode analysis of lateral diffusion on a bounded membrane surface. Biophys J. 1985 Mar;47(3):337–347. doi: 10.1016/S0006-3495(85)83924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Lateral diffusion in biological membranes. A normal-mode analysis of diffusion on a spherical surface. Biophys J. 1980 Apr;30(1):187–192. doi: 10.1016/S0006-3495(80)85087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Matrix control of protein diffusion in biological membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3576–3580. doi: 10.1073/pnas.78.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Koppel D. E., Sheetz M. P. Modulation of membrane protein lateral mobility by polyphosphates and polyamines. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1457–1461. doi: 10.1073/pnas.77.3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Wu E. S., Webb W. W. Enhanced molecular diffusibility in muscle membrane blebs: release of lateral constraints. J Cell Biol. 1982 Jan;92(1):207–212. doi: 10.1083/jcb.92.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E., Hagopian S. S., Lewis R. G., Voglmayr J. K., Fairbanks G. Lateral regionalization and diffusion of a maturation-dependent antigen in the ram sperm plasma membrane. J Cell Biol. 1986 May;102(5):1826–1831. doi: 10.1083/jcb.102.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E. S., Tank D. W., Webb W. W. Unconstrained lateral diffusion of concanavalin A receptors on bulbous lymphocytes. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4962–4966. doi: 10.1073/pnas.79.16.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat S. W., van der Saag P. T., Elson E. L., Schlessinger J. Lateral diffusion of membrane lipids and proteins is increased specifically in neurites of differentiating neuroblastoma cells. Biochim Biophys Acta. 1979 Dec 4;558(2):247–250. doi: 10.1016/0005-2736(79)90064-6. [DOI] [PubMed] [Google Scholar]


