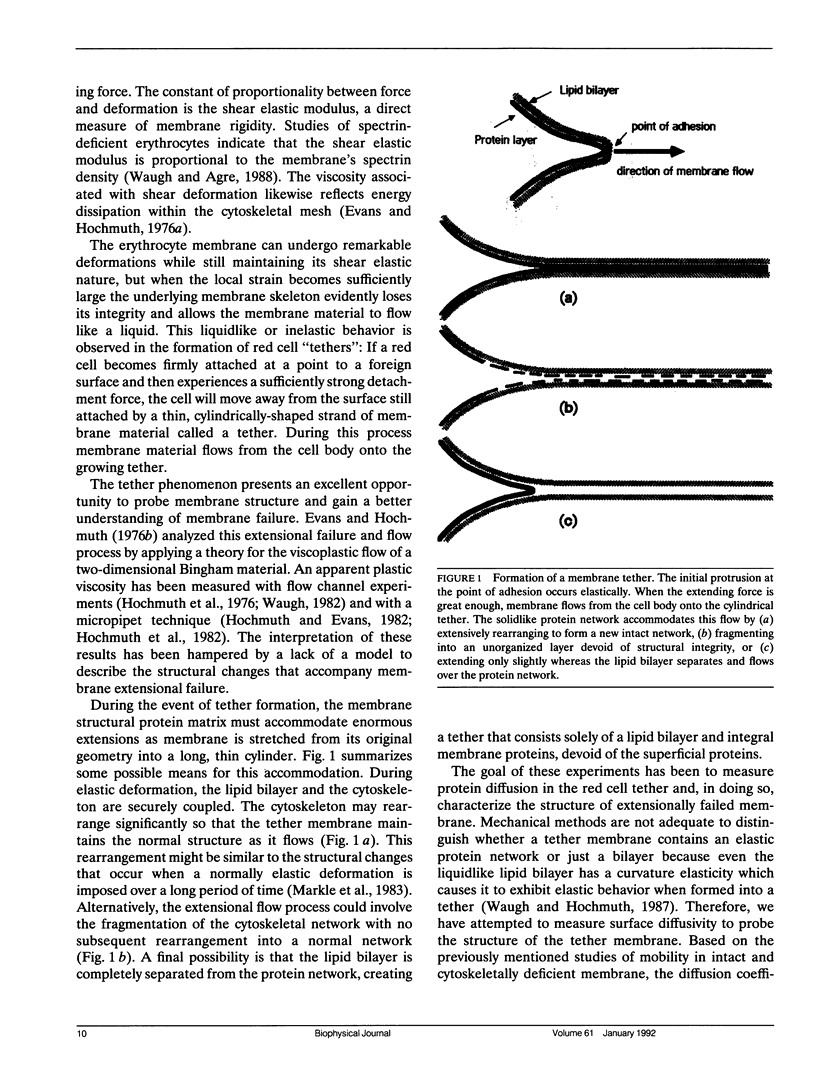
Abstract
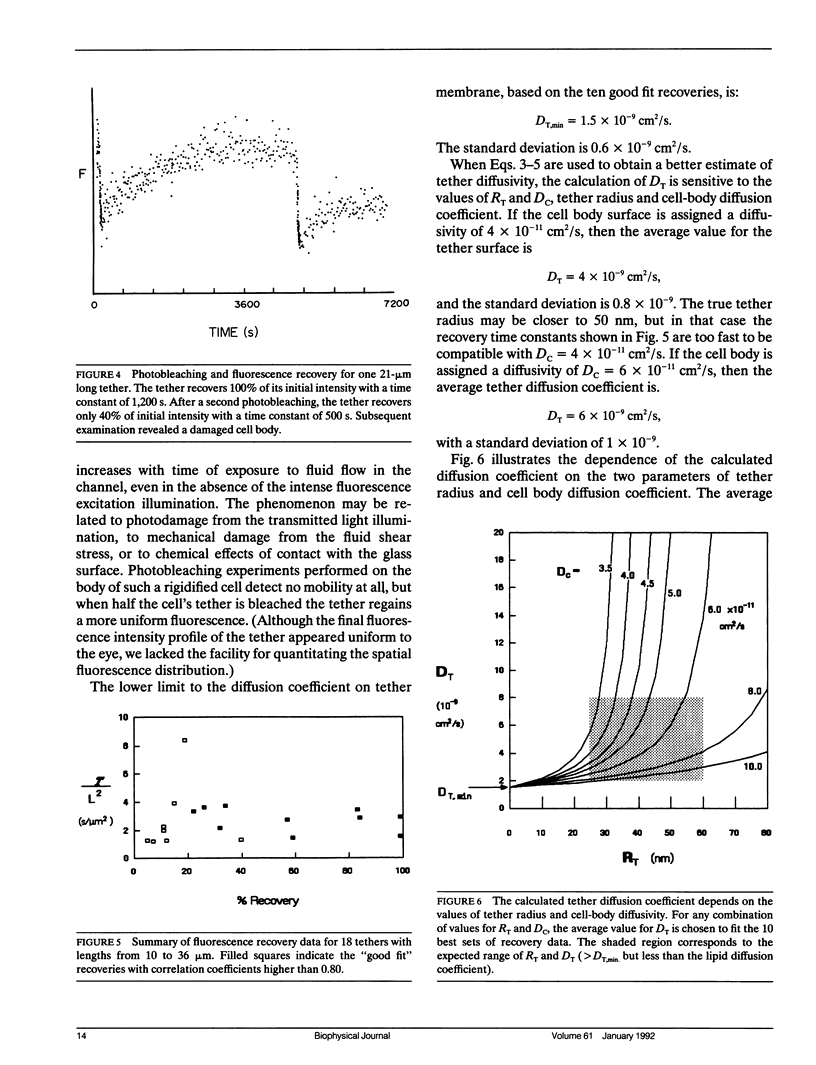
The red blood cell membrane is a complex material that exhibits both solid- and liquidlike behavior. It is distinguished from a simple lipid bilayer capsule by its mechanical properties, particularly its shear viscoelastic behavior and by the long-range mobility of integral proteins on the membrane surface. Subject to sufficiently large extension, the membrane loses its shear rigidity and flows as a two-dimensional fluid. These experiments examine the change in integral protein mobility that accompanies the mechanical phenomenon of extensional failure and liquidlike flow. A flow channel apparatus is used to create red cell tethers, hollow cylinders of greatly deformed membrane, up to 36-microns long. The diffusion of proteins within the surface of the membrane is measured by the technique of fluorescence redistribution after photobleaching (FRAP). Integral membrane proteins are labeled directly with a fluorescein dye (DTAF). Mobility in normal membrane is measured by photobleaching half of the cell and measuring the rate of fluorescence recovery. Protein mobility in tether membrane is calculated from the fluorescence recovery rate after the entire tether has been bleached. Fluorescence recovery rates for normal membrane indicate that more than half the labeled proteins are mobile with a diffusion coefficient of approximately 4 x 10(-11) cm2/s, in agreement with results from other studies. The diffusion coefficient for proteins in tether membrane is greater than 1.5 x 10(-9) cm2/s. This dramatic increase in diffusion coefficient indicates that extensional failure involves the uncoupling of the lipid bilayer from the membrane skeleton.
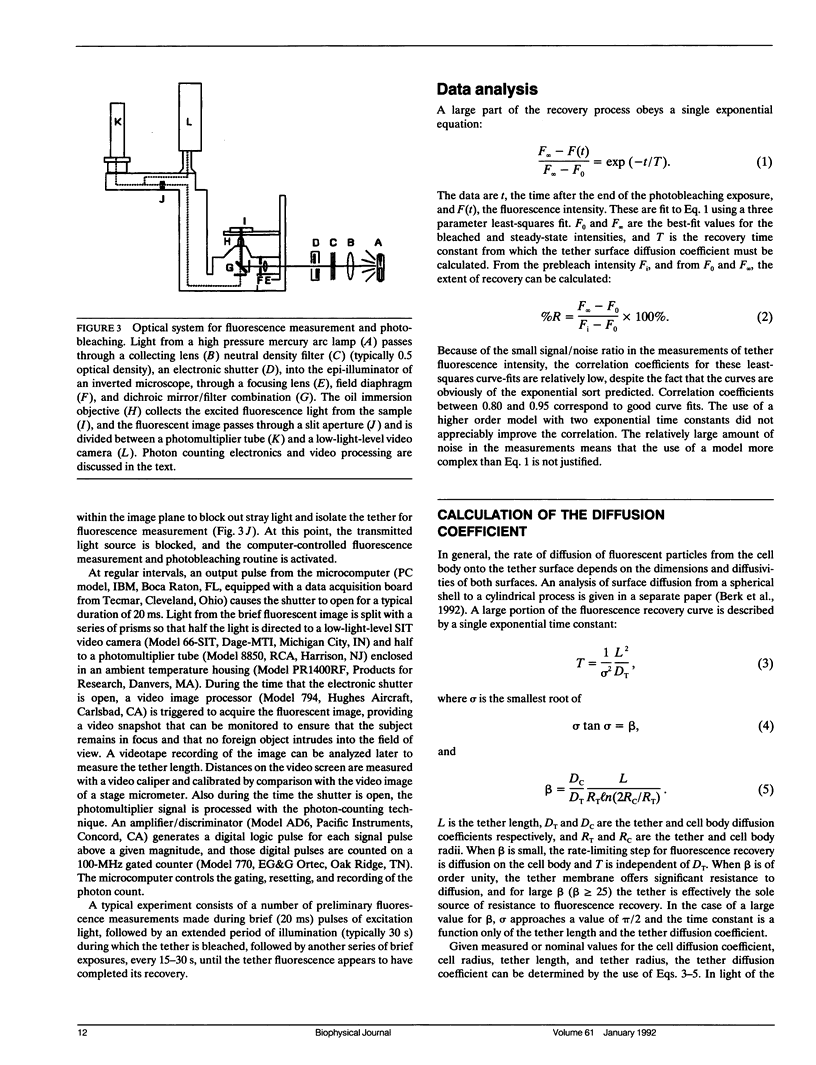
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barak L. S., Webb W. W. Diffusion of low density lipoprotein-receptor complex on human fibroblasts. J Cell Biol. 1982 Dec;95(3):846–852. doi: 10.1083/jcb.95.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Berk D. A., Clark A., Jr, Hochmuth R. M. Analysis of lateral diffusion from a spherical cell surface to a tubular projection. Biophys J. 1992 Jan;61(1):1–8. doi: 10.1016/S0006-3495(92)81810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom J. A., Webb W. W. Lipid diffusibility in the intact erythrocyte membrane. Biophys J. 1983 Jun;42(3):295–305. doi: 10.1016/S0006-3495(83)84397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Zuniga M. Lateral diffusion of wild-type and mutant Ld antigens in L cells. J Cell Biol. 1984 Dec;99(6):2333–2335. doi: 10.1083/jcb.99.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. Membrane viscoelasticity. Biophys J. 1976 Jan;16(1):1–11. doi: 10.1016/S0006-3495(76)85658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. Membrane viscoplastic flow. Biophys J. 1976 Jan;16(1):13–26. doi: 10.1016/S0006-3495(76)85659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler V., Branton D. Lateral mobility of human erythrocyte integral membrane proteins. Nature. 1977 Jul 7;268(5615):23–26. doi: 10.1038/268023a0. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M., Evans C. A., Wiles H. C., McCown J. T. Mechanical measurement of red cell membrane thickness. Science. 1983 Apr 1;220(4592):101–102. doi: 10.1126/science.6828875. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Evans E. A., Colvard D. F. Viscosity of human red cell membrane in plastic flow. Microvasc Res. 1976 Mar;11(2):155–159. doi: 10.1016/0026-2862(76)90047-9. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Evans E. A. Extensional flow of erythrocyte membrane from cell body to elastic tether. I. Analysis. Biophys J. 1982 Jul;39(1):71–81. doi: 10.1016/S0006-3495(82)84492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M., Mohandas N., Blackshear P. L., Jr Measurement of the elastic modulus for red cell membrane using a fluid mechanical technique. Biophys J. 1973 Aug;13(8):747–762. doi: 10.1016/S0006-3495(73)86021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M., Wiles H. C., Evans E. A., McCown J. T. Extensional flow of erythrocyte membrane from cell body to elastic tether. II. Experiment. Biophys J. 1982 Jul;39(1):83–89. doi: 10.1016/S0006-3495(82)84493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. W. Mobility and diffusion in the plane of cell membrane. J Theor Biol. 1973 Jul;40(1):11–17. doi: 10.1016/0022-5193(73)90161-6. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Matrix control of protein diffusion in biological membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3576–3580. doi: 10.1073/pnas.78.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh E., Benveniste M., Prywes R., Felder S., Kam Z., Schlessinger J. Large deletions in the cytoplasmic kinase domain of the epidermal growth factor receptor do not affect its laternal mobility. J Cell Biol. 1986 Aug;103(2):327–331. doi: 10.1083/jcb.103.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle D. R., Evans E. A., Hochmuth R. M. Force relaxation and permanent deformation of erythrocyte membrane. Biophys J. 1983 Apr;42(1):91–98. doi: 10.1016/S0006-3495(83)84372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. The membrane skeleton of erythrocytes. A percolation model. Biophys J. 1990 Jun;57(6):1167–1177. doi: 10.1016/S0006-3495(90)82636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. The spectrin network as a barrier to lateral diffusion in erythrocytes. A percolation analysis. Biophys J. 1989 Jan;55(1):21–28. doi: 10.1016/S0006-3495(89)82776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Koppel D. E., Sheetz M. P. Modulation of membrane protein lateral mobility by polyphosphates and polyamines. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1457–1461. doi: 10.1073/pnas.77.3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Smith D. K., Palek J. Modulation of lateral mobility of band 3 in the red cell membrane by oxidative cross-linking of spectrin. Nature. 1982 Jun 3;297(5865):424–425. doi: 10.1038/297424a0. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Wu E. S., Webb W. W. Enhanced molecular diffusibility in muscle membrane blebs: release of lateral constraints. J Cell Biol. 1982 Jan;92(1):207–212. doi: 10.1083/jcb.92.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E., Agre P. Reductions of erythrocyte membrane viscoelastic coefficients reflect spectrin deficiencies in hereditary spherocytosis. J Clin Invest. 1988 Jan;81(1):133–141. doi: 10.1172/JCI113284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E., Hochmuth R. M. Mechanical equilibrium of thick, hollow, liquid membrane cylinders. Biophys J. 1987 Sep;52(3):391–400. doi: 10.1016/S0006-3495(87)83227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E. Temperature dependence of the yield shear resultant and the plastic viscosity coefficient of erythrocyte membrane. Implications about molecular events during membrane failure. Biophys J. 1982 Sep;39(3):273–278. doi: 10.1016/S0006-3495(82)84517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb W. W., Barak L. S., Tank D. W., Wu E. S. Molecular mobility on the cell surface. Biochem Soc Symp. 1981;(46):191–205. [PubMed] [Google Scholar]
- Wu E. S., Tank D. W., Webb W. W. Unconstrained lateral diffusion of concanavalin A receptors on bulbous lymphocytes. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4962–4966. doi: 10.1073/pnas.79.16.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]