Abstract
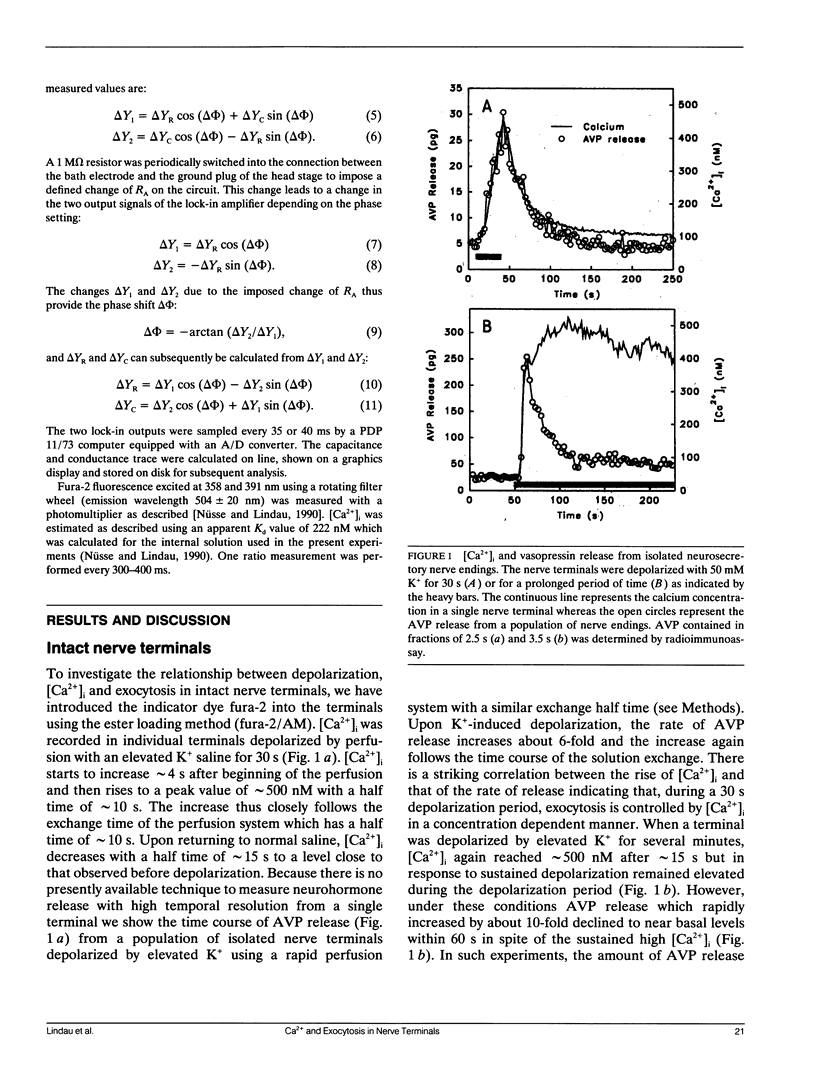
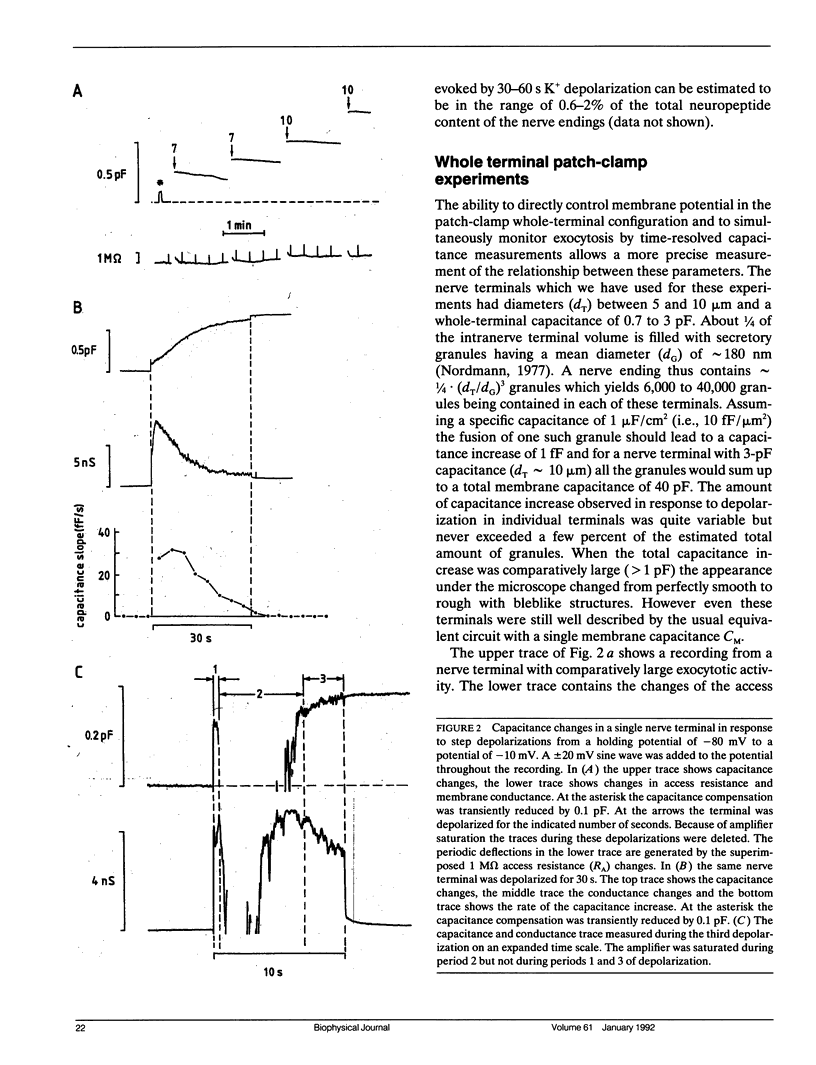
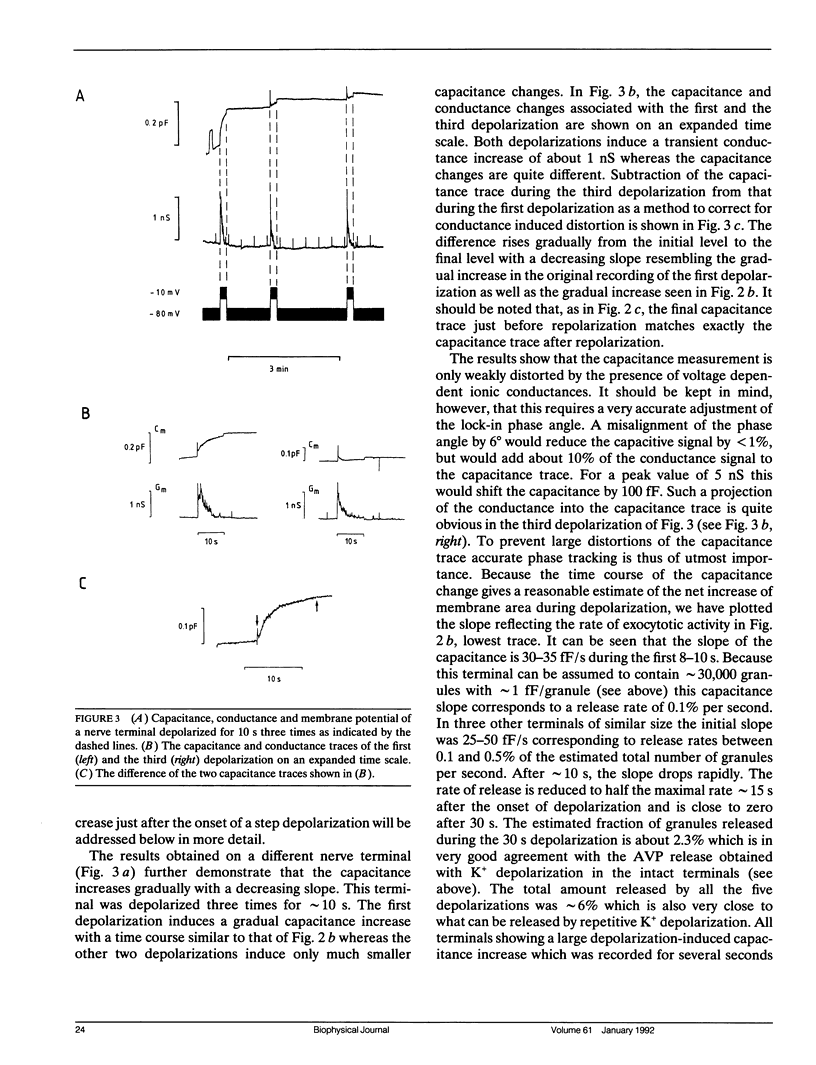
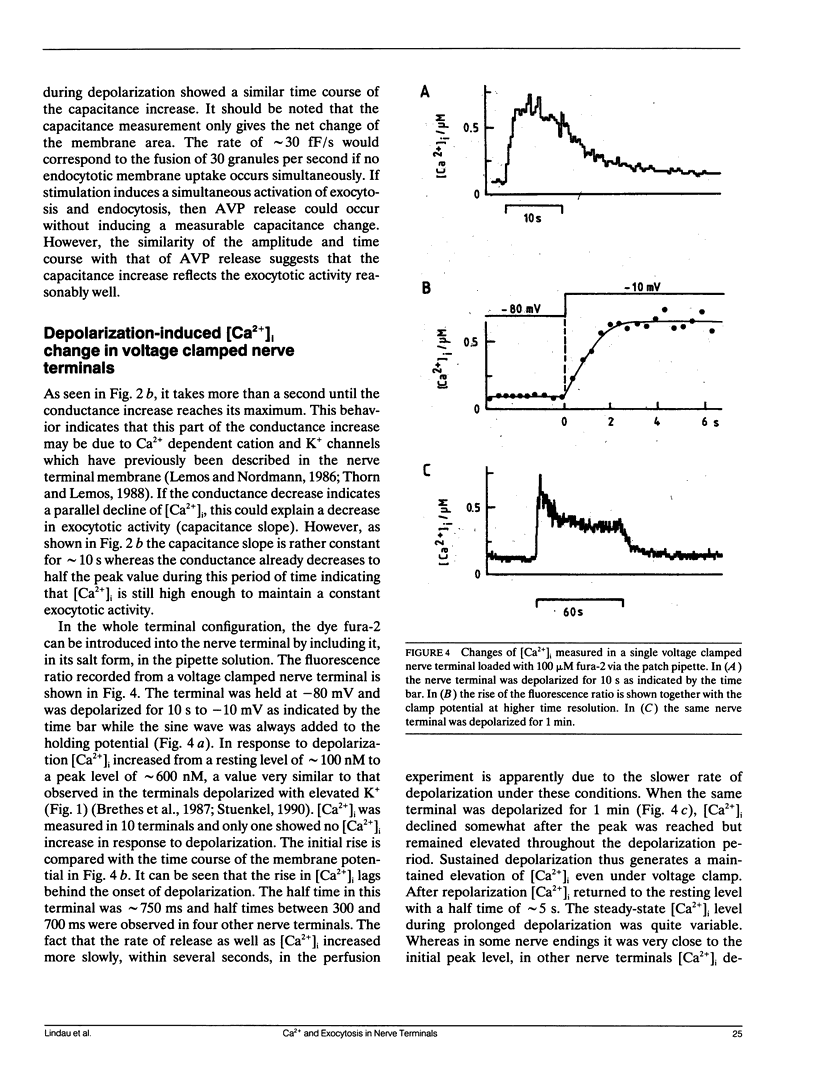
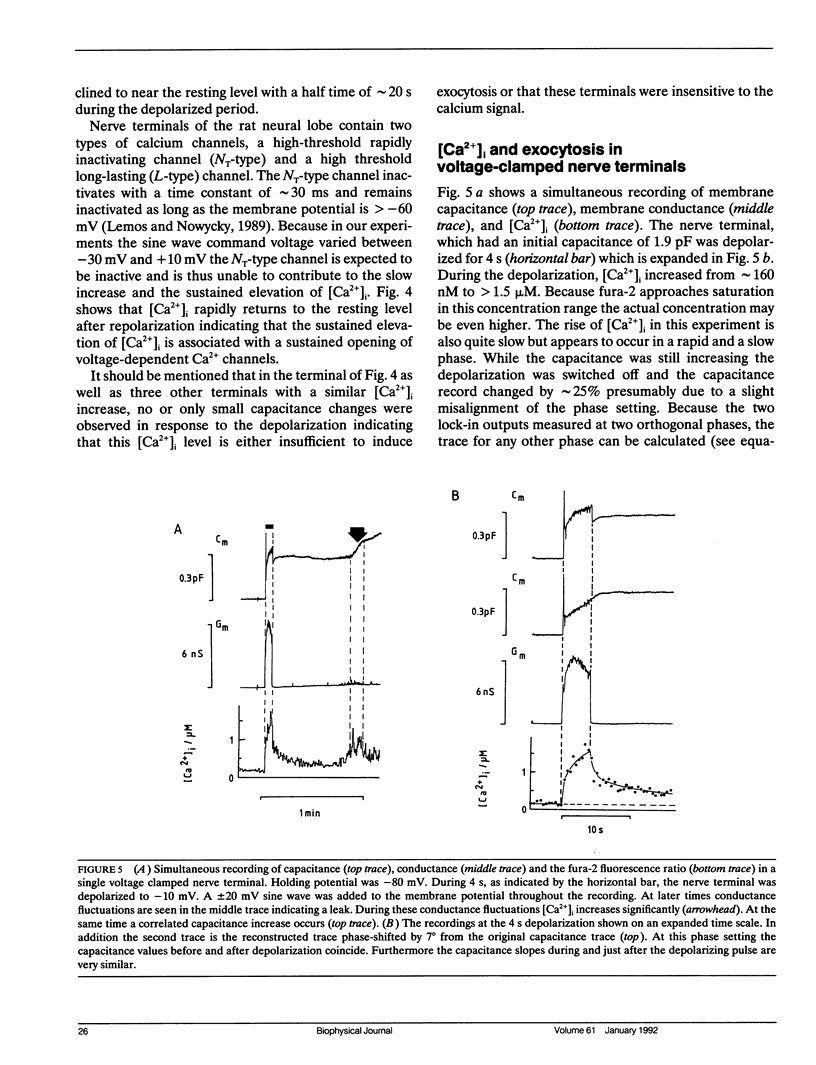
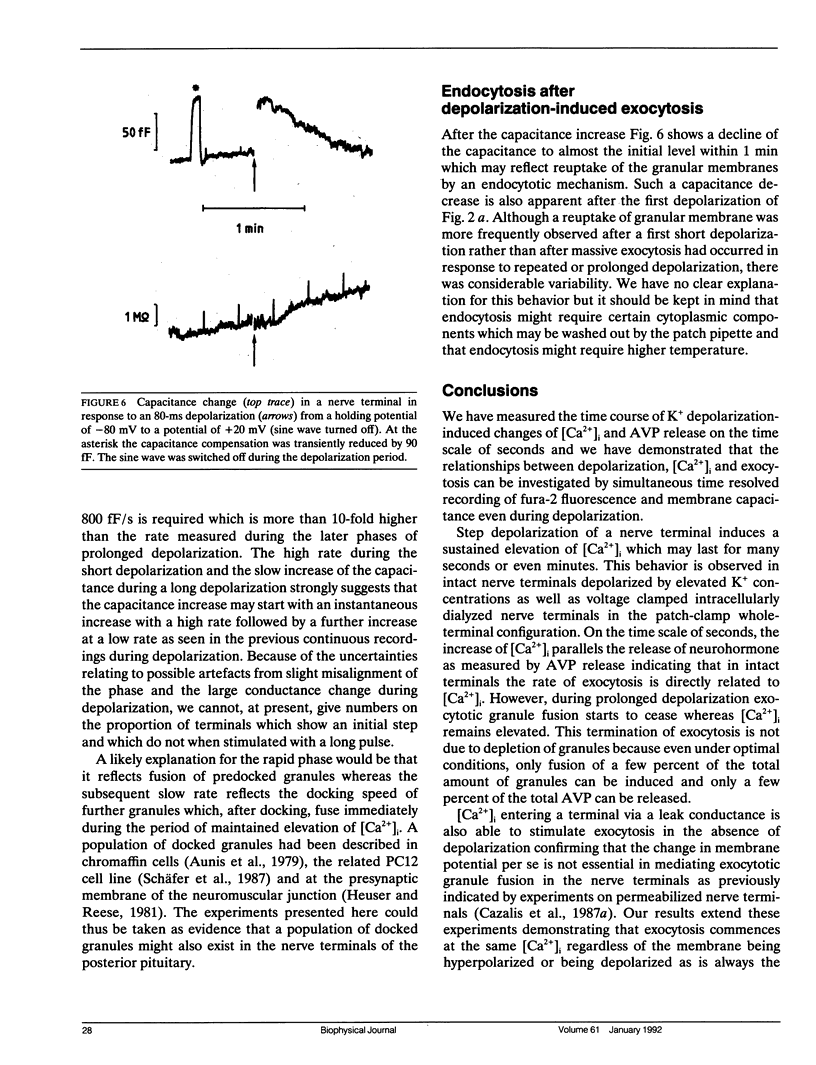
We have investigated the temporal relationship between depolarization, elevation of [Ca2+]i and exocytosis in single vertebrate neuroendocrine nerve terminals. The change of [Ca2+]i and vasopressin release were measured with a time resolution of less than 1 s in response to K(+)-induced depolarization. Exocytosis was also monitored in the whole-terminal patch-clamp configuration by time resolved capacitance measurements while [Ca2+]i was simultaneously followed by fura-2 fluorescence measurements. In intact as well as patch-clamped nerve terminals sustained depolarization leads to a sustained rise of [Ca2+]i. The rate of vasopressin release from intact nerve terminals rises in parallel with [Ca2+]i but then declines rapidly towards basal (t1/2 approximately 15 s) despite the maintained high [Ca2+]i indicating that only a limited number of exocytotic vesicles can be released. We demonstrate that in nerve terminals exocytosis can be followed during step depolarization by capacitance measurements. The capacitance increase starts instantaneously whereas [Ca2+]i rises with a half time of several hundred milliseconds. An instantaneous steep capacitance increase is followed by a slow increase with a slope of 25-50 fF/s indicating the sequential fusion of predocked and cytoplasmic vesicles. During depolarization the capacitance slope declines to zero with a similar time course as the vasopressin release indicating a decrease in exocytotic activity. Depolarization per se in the absence of a sufficient rise of [Ca2+]i does not induce exocytosis but elevation of [Ca2+]i in the absence of depolarization is as effective as in its presence. The experiments suggest that a rapid rise of [Ca2+]i in a narrow region beneath the plasma membrane induces a burst of exocytotic activity preceding the elevation of bulk [Ca2+]i in the whole nerve terminal.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Aunis D., Hesketh J. E., Devilliers G. Freeze-fracture study of the chromaffin cell during exocytosis: evidence for connections between the plasma membrane and secretory granules and for movements of plasma membrane-associated particles. Cell Tissue Res. 1979 Apr 12;197(3):433–441. doi: 10.1007/BF00233568. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. 1987 Aug 27-Sep 2Nature. 328(6133):814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Brethes D., Dayanithi G., Letellier L., Nordmann J. J. Depolarization-induced Ca2+ increase in isolated neurosecretory nerve terminals measured with fura-2. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1439–1443. doi: 10.1073/pnas.84.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. Hormone release from isolated nerve endings of the rat neurohypophysis. J Physiol. 1987 Sep;390:55–70. doi: 10.1113/jphysiol.1987.sp016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. Requirements for hormone release from permeabilized nerve endings isolated from the rat neurohypophysis. J Physiol. 1987 Sep;390:71–91. doi: 10.1113/jphysiol.1987.sp016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985 Dec;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanithi G., Martin-Moutot N., Barlier S., Colin D. A., Kretz-Zaepfel M., Couraud F., Nordmann J. J. The calcium channel antagonist omega-conotoxin inhibits secretion from peptidergic nerve terminals. Biochem Biophys Res Commun. 1988 Oct 14;156(1):255–262. doi: 10.1016/s0006-291x(88)80833-7. [DOI] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Structural changes after transmitter release at the frog neuromuscular junction. J Cell Biol. 1981 Mar;88(3):564–580. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Konnerth A., Augustine G. J. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J. R., Nordmann J. J., Cooke I. M., Stuenkel E. L. Single channels and ionic currents in peptidergic nerve terminals. 1986 Jan 30-Feb 5Nature. 319(6052):410–412. doi: 10.1038/319410a0. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nordmann J. J. Ionic channels and hormone release from peptidergic nerve terminals. J Exp Biol. 1986 Sep;124:53–72. doi: 10.1242/jeb.124.1.53. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nowycky M. C. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989 May;2(5):1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- Lim N. F., Nowycky M. C., Bookman R. J. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990 Mar 29;344(6265):449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Lindau M. Time-resolved capacitance measurements: monitoring exocytosis in single cells. Q Rev Biophys. 1991 Feb;24(1):75–101. doi: 10.1017/s0033583500003279. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann J. J., Dayanithi G. Release of neuropeptides does not only occur at nerve terminals. Biosci Rep. 1988 Oct;8(5):471–483. doi: 10.1007/BF01121646. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J. Ultrastructural morphometry of the rat neurohypophysis. J Anat. 1977 Feb;123(Pt 1):213–218. [PMC free article] [PubMed] [Google Scholar]
- Nüsse O., Lindau M. GTP gamma S-induced calcium transients and exocytosis in human neutrophils. Biosci Rep. 1990 Feb;10(1):93–103. doi: 10.1007/BF01116857. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer T., Karli U. O., Schweizer F. E., Burger M. M. Docking of chromaffin granules--a necessary step in exocytosis? Biosci Rep. 1987 Apr;7(4):269–279. doi: 10.1007/BF01121448. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L. Effects of membrane depolarization on intracellular calcium in single nerve terminals. Brain Res. 1990 Oct 8;529(1-2):96–101. doi: 10.1016/0006-8993(90)90815-s. [DOI] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Thorn P. J., Wang X. M., Lemos J. R. A fast, transient K+ current in neurohypophysial nerve terminals of the rat. J Physiol. 1991 Jan;432:313–326. doi: 10.1113/jphysiol.1991.sp018386. [DOI] [PMC free article] [PubMed] [Google Scholar]


