Abstract
Rather than acting by modifying van der Waals or electrostatic double layer interactions or by directly bridging neighboring molecules, polyvalent ligands bound to DNA double helices appear to act by reconfiguring the water between macromolecular surfaces to create attractive long range hydration forces. We have reached this conclusion by directly measuring the repulsive forces between parallel B-form DNA double helices pushed together from the separations at which they have self organized into hexagonal arrays of parallel rods. For all of the wide variety of "condensing agents" from divalent Mn to polymeric protamines, the resulting intermolecular force varies exponentially with a decay rate of 1.4-1.5 A, exactly one-half that seen previously for hydration repulsion. Such behavior qualitatively contradicts the predictions of all electrostatic double layer and van der Waals force potentials previously suggested. It fits remarkably well with the idea, developed and tested here, that multivalent counterion adsorption reorganizes the water at discrete sites complementary to unadsorbed sites on the apposing surface. The measured strength and range of these attractive forces together with their apparent specificity suggest the presence of a previously unexpected force in molecular organization.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison S. A., Herr J. C., Schurr J. M. Structure of viral phi 29 DNA condensed by simple triamines: a light-scattering and electron-microscopy study. Biopolymers. 1981 Mar;20(3):469–488. doi: 10.1002/bip.1981.360200305. [DOI] [PubMed] [Google Scholar]
- Bloomfield V. A., Wilson R. W., Rau D. C. Polyelectrolyte effects in DNA condensation by polyamines. Biophys Chem. 1980 Jun;11(3-4):339–343. doi: 10.1016/0301-4622(80)87006-2. [DOI] [PubMed] [Google Scholar]
- Brenner S. L., McQuarrie D. A. Force balances in systems of cylindrical polyelectrolytes. Biophys J. 1973 Apr;13(4):301–331. doi: 10.1016/S0006-3495(73)85987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D. K., Gosule L. C., Schellman A. DNA condensation with polyamines. II. Electron microscopic studies. J Mol Biol. 1978 May 25;121(3):327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. DNA condensation with polyamines I. Spectroscopic studies. J Mol Biol. 1978 May 25;121(3):311–326. doi: 10.1016/0022-2836(78)90366-2. [DOI] [PubMed] [Google Scholar]
- Herskovits T. T., Brahms J. Structural investigations on DNA-protamine complexes. Biopolymers. 1976 Apr;15(4):687–706. doi: 10.1002/bip.1976.360150408. [DOI] [PubMed] [Google Scholar]
- Horn R. G., Israelachvili J. N., Marra J., Parsegian V. A., Rand R. P. Comparison of forces measured between phosphatidylcholine bilayers. Biophys J. 1988 Dec;54(6):1185–1186. doi: 10.1016/S0006-3495(88)83055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornyshev AA, Leikin S. Fluctuation theory of hydration forces: The dramatic effects of inhomogeneous boundary conditions. Phys Rev A Gen Phys. 1989 Dec 1;40(11):6431–6437. doi: 10.1103/physreva.40.6431. [DOI] [PubMed] [Google Scholar]
- Leikin S, Rau DC, Parsegian VA. Measured entropy and enthalpy of hydration as a function of distance between DNA double helices. Phys Rev A. 1991 Oct 15;44(8):5272–5278. doi: 10.1103/physreva.44.5272. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Fuller N. L., Rau D. C. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 1986;127:400–416. doi: 10.1016/0076-6879(86)27032-9. [DOI] [PubMed] [Google Scholar]
- Plum G. E., Bloomfield V. A. Equilibrium dialysis study of binding of hexammine cobalt(III) to DNA. Biopolymers. 1988 Jun;27(6):1045–1051. doi: 10.1002/bip.360270611. [DOI] [PubMed] [Google Scholar]
- Prouty M. S., Schechter A. N., Parsegian V. A. Chemical potential measurements of deoxyhemoglobin S polymerization. Determination of the phase diagram of an assembling protein. J Mol Biol. 1985 Aug 5;184(3):517–528. doi: 10.1016/0022-2836(85)90298-0. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Gruner S. M., Parsegian V. A. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 1990 Jan 9;29(1):76–87. doi: 10.1021/bi00453a010. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N., Parsegian V. A., Rau D. C. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988 Oct 4;27(20):7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- Rand R. P. Interacting phospholipid bilayers: measured forces and induced structural changes. Annu Rev Biophys Bioeng. 1981;10:277–314. doi: 10.1146/annurev.bb.10.060181.001425. [DOI] [PubMed] [Google Scholar]
- Rau D. C., Lee B., Parsegian V. A. Measurement of the repulsive force between polyelectrolyte molecules in ionic solution: hydration forces between parallel DNA double helices. Proc Natl Acad Sci U S A. 1984 May;81(9):2621–2625. doi: 10.1073/pnas.81.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Parsegian V. A. Direct measurement of forces between linear polysaccharides xanthan and schizophyllan. Science. 1990 Sep 14;249(4974):1278–1281. doi: 10.1126/science.2144663. [DOI] [PubMed] [Google Scholar]
- Rau D. C., Parsegian V. A. Direct measurement of temperature-dependent solvation forces between DNA double helices. Biophys J. 1992 Jan;61(1):260–271. doi: 10.1016/S0006-3495(92)81832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Lohman T. M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978 May;11(2):103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
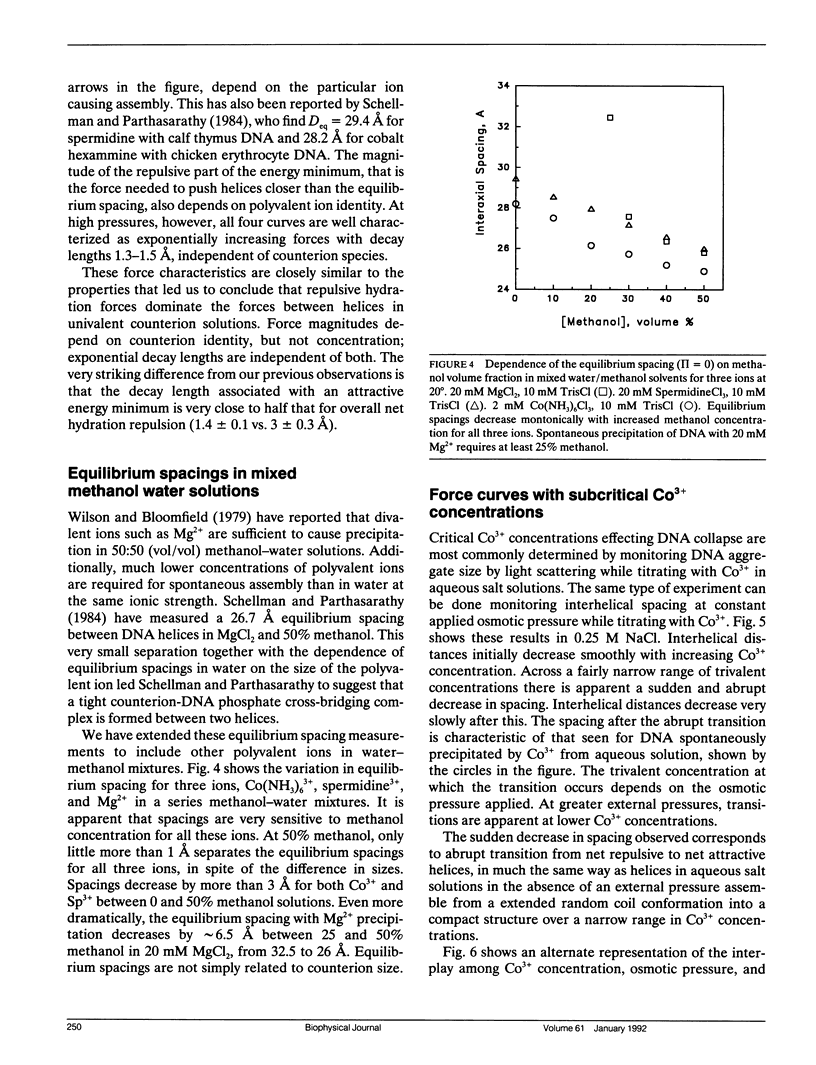
- Schellman J. A., Parthasarathy N. X-ray diffraction studies on cation-collapsed DNA. J Mol Biol. 1984 May 25;175(3):313–329. doi: 10.1016/0022-2836(84)90351-6. [DOI] [PubMed] [Google Scholar]
- Suau P., Subirana J. A. X-ray diffraction studies of nucleoprotamine structure. J Mol Biol. 1977 Dec 25;117(4):909–926. doi: 10.1016/s0022-2836(77)80005-3. [DOI] [PubMed] [Google Scholar]
- Suwalsky M., Traub W. A comparative x-ray study of a nucleoprotamine and DNA complexes with polylysine and polyarginine. Biopolymers. 1972;11(11):2223–2231. doi: 10.1002/bip.1972.360111103. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J Mol Biol. 1980 Dec 25;144(4):431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Monomolecular condensation of lambda-DNA induced by cobalt hexamine. Biopolymers. 1983 Jun;22(6):1595–1620. doi: 10.1002/bip.360220612. [DOI] [PubMed] [Google Scholar]
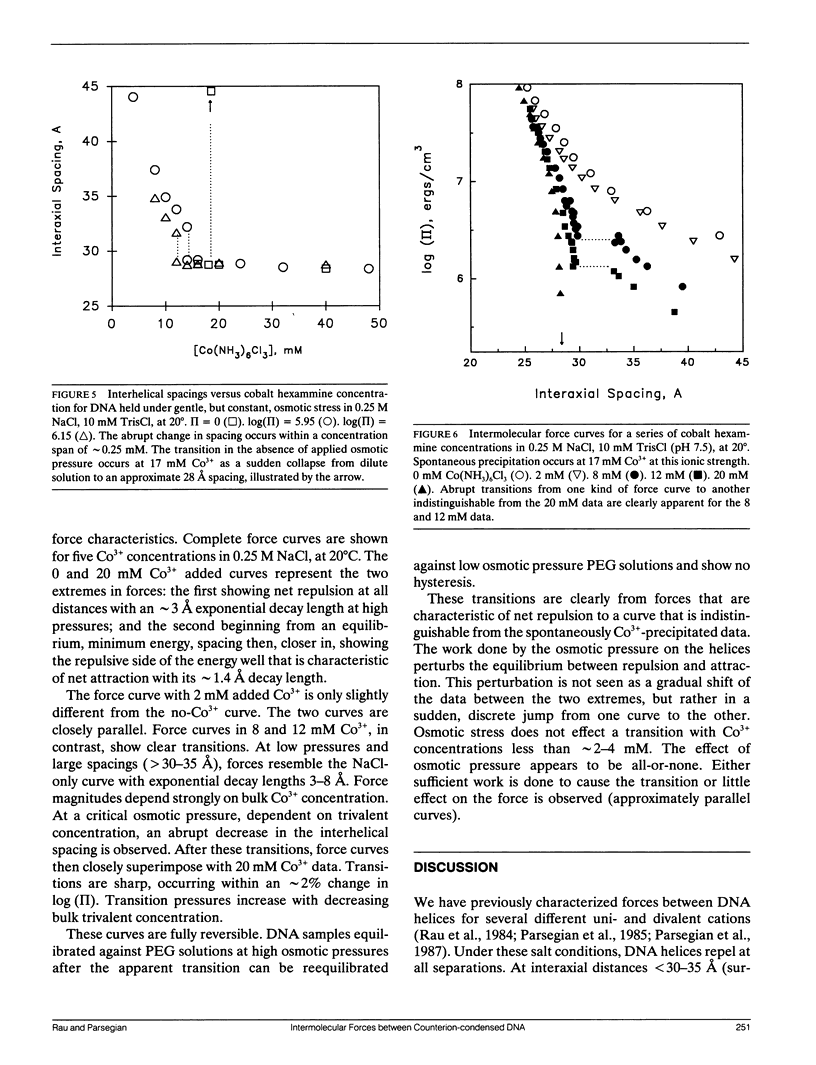
- Wilson R. W., Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979 May 29;18(11):2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Parsegian V. A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986 Sep 4;323(6083):36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]