Abstract
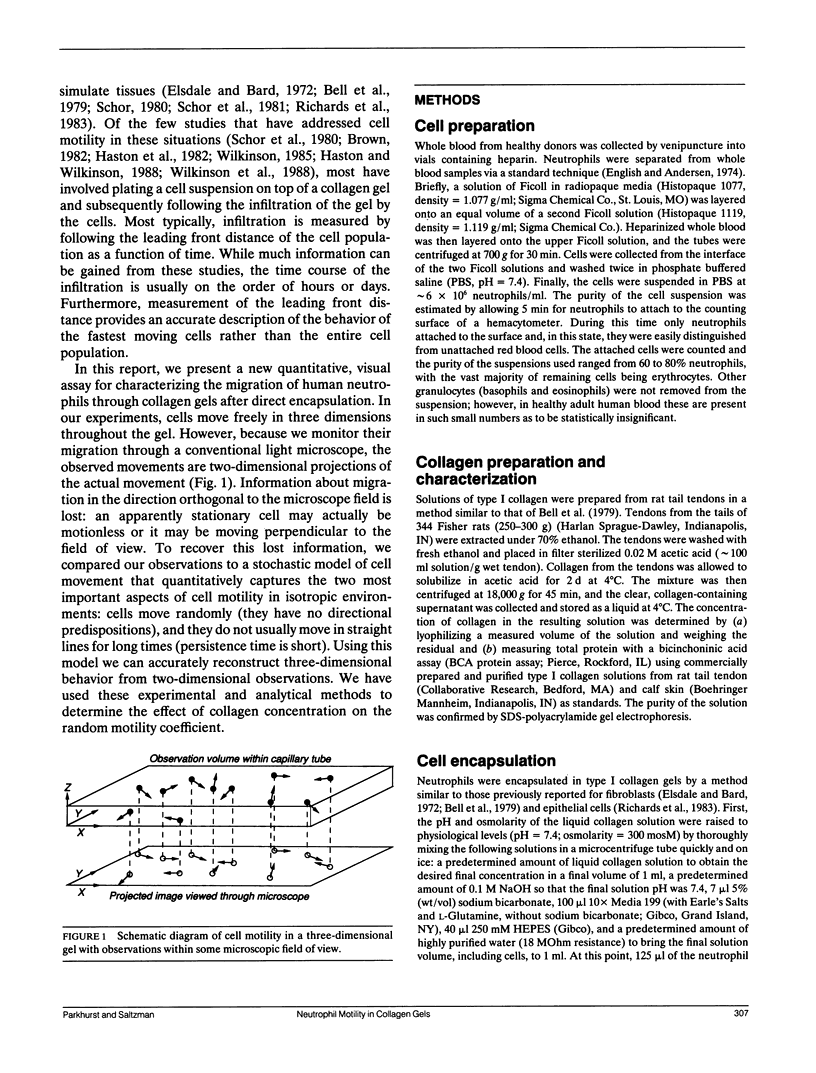
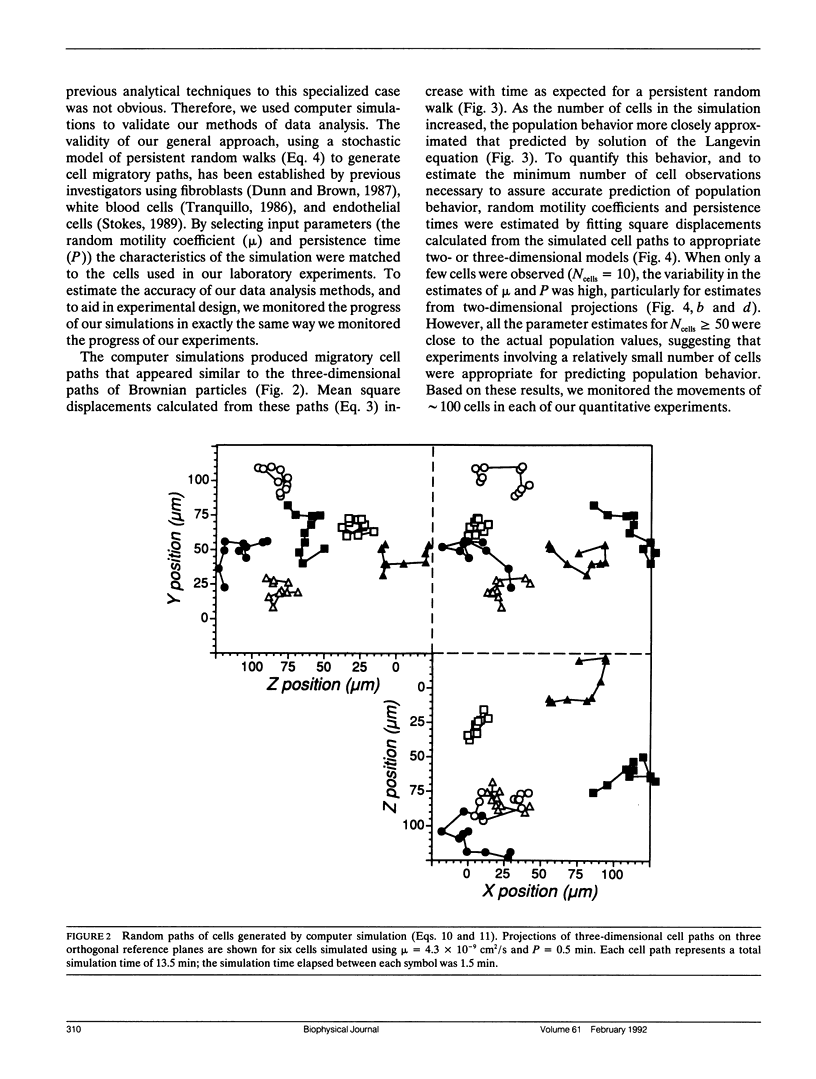
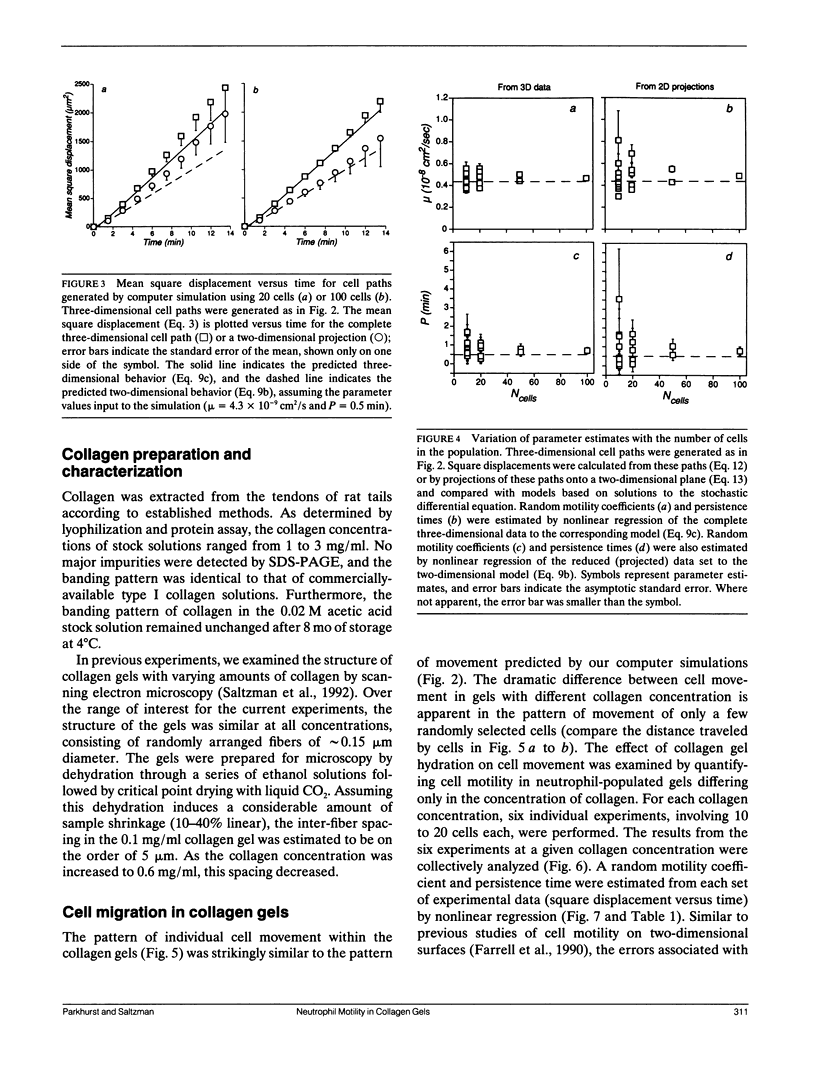
Leukocytes must migrate through tissues to fulfill their role in the immune response, but direct methods for observing and quantifying cell motility have mostly been limited to migration on two-dimensional surfaces. We have now developed methods for examining neutrophil movement in a three-dimensional gel containing 0.1 to 0.7 mg/ml rat tail tendon collagen. Neutrophil-populated collagen gels were formed within flat glass capillary tubes, permitting direct observation with light microscopy. By following the tracks of individual cells over a 13.5-min observation period and comparing them to a stochastic model of cell movement, we quantified cell speed within a given gel by estimating a random motility coefficient (mu) and persistence time (P). The random motility coefficient changed significantly with collagen concentration in the gel, varying from 1.6 to 13.3 x 10(-9) cm2/s, with the maximum occurring at a collagen gel concentration of 0.3 mg/ml. The methods described may be useful for studying tissue dynamics and for evaluating the mechanism of cell movement in three-dimensional gels of extracellular matrix (ECM) molecules.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R. B., Wilkinson P. C. A visual analysis of chemotactic and chemokinetic locomotion of human neutrophil leucocytes. Use of a new chemotaxis assay with Candida albicans as gradient source. Exp Cell Res. 1978 Jan;111(1):191–203. doi: 10.1016/0014-4827(78)90249-5. [DOI] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. F. Neutrophil granulocytes: adhesion and locomotion on collagen substrata and in collagen matrices. J Cell Sci. 1982 Dec;58:455–467. doi: 10.1242/jcs.58.1.455. [DOI] [PubMed] [Google Scholar]
- Buettner H. M., Lauffenburger D. A., Zigmond S. H. Measurement of leukocyte motility and chemotaxis parameters with the Millipore filter assay. J Immunol Methods. 1989 Sep 29;123(1):25–37. doi: 10.1016/0022-1759(89)90026-4. [DOI] [PubMed] [Google Scholar]
- Cunningham C. C., Stossel T. P., Kwiatkowski D. J. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science. 1991 Mar 8;251(4998):1233–1236. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- Cutler J. E., Munoz J. J. A simple in vitro method for studies on chemotaxis. Proc Soc Exp Biol Med. 1974 Nov;147(2):471–474. doi: 10.3181/00379727-147-38367. [DOI] [PubMed] [Google Scholar]
- DiMilla P. A., Barbee K., Lauffenburger D. A. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991 Jul;60(1):15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. A., Brown A. F. A unified approach to analysing cell motility. J Cell Sci Suppl. 1987;8:81–102. doi: 10.1242/jcs.1987.supplement_8.5. [DOI] [PubMed] [Google Scholar]
- Dunn G. A. Characterising a kinesis response: time averaged measures of cell speed and directional persistence. Agents Actions Suppl. 1983;12:14–33. doi: 10.1007/978-3-0348-9352-7_1. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Farrell B. E., Daniele R. P., Lauffenburger D. A. Quantitative relationships between single-cell and cell-population model parameters for chemosensory migration responses of alveolar macrophages to C5a. Cell Motil Cytoskeleton. 1990;16(4):279–293. doi: 10.1002/cm.970160407. [DOI] [PubMed] [Google Scholar]
- Folkman J. Toward an understanding of angiogenesis: search and discovery. Perspect Biol Med. 1985 Autumn;29(1):10–36. doi: 10.1353/pbm.1985.0049. [DOI] [PubMed] [Google Scholar]
- Gail M. H., Boone C. W. The locomotion of mouse fibroblasts in tissue culture. Biophys J. 1970 Oct;10(10):980–993. doi: 10.1016/S0006-3495(70)86347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haston W. S., Shields J. M., Wilkinson P. C. Lymphocyte locomotion and attachment on two-dimensional surfaces and in three-dimensional matrices. J Cell Biol. 1982 Mar;92(3):747–752. doi: 10.1083/jcb.92.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haston W. S., Wilkinson P. C. Visual methods for measuring leukocyte locomotion. Methods Enzymol. 1988;162:17–38. doi: 10.1016/0076-6879(88)62060-x. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Holifield B., Jacobson K. Analysis of lateral redistribution of a plasma membrane glycoprotein-monoclonal antibody complex [corrected]. J Cell Biol. 1988 Feb;106(2):329–343. doi: 10.1083/jcb.106.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger D. Measurement of phenomenological parameters for leukocyte motility and chemotaxis. Agents Actions Suppl. 1983;12:34–53. doi: 10.1007/978-3-0348-9352-7_2. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Radomsky M. L., Whaley K. J., Cone R. A., Saltzman W. M. Macromolecules released from polymers: diffusion into unstirred fluids. Biomaterials. 1990 Nov;11(9):619–624. doi: 10.1016/0142-9612(90)90018-l. [DOI] [PubMed] [Google Scholar]
- Saltzman W. M., Parsons-Wingerter P., Leong K. W., Lin S. Fibroblast and hepatocyte behavior on synthetic polymer surfaces. J Biomed Mater Res. 1991 Jun;25(6):741–759. doi: 10.1002/jbm.820250605. [DOI] [PubMed] [Google Scholar]
- Sato M., Schwarz W. H., Pollard T. D. Dependence of the mechanical properties of actin/alpha-actinin gels on deformation rate. 1987 Feb 26-Mar 4Nature. 325(6107):828–830. doi: 10.1038/325828a0. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Allen T. D., Harrison C. J. Cell migration through three-dimensional gels of native collagen fibres: collagenolytic activity is not required for the migration of two permanent cell lines. J Cell Sci. 1980 Dec;46:171–186. doi: 10.1242/jcs.46.1.171. [DOI] [PubMed] [Google Scholar]
- Schor S. L. Cell proliferation and migration on collagen substrata in vitro. J Cell Sci. 1980 Feb;41:159–175. doi: 10.1242/jcs.41.1.159. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M., Bazill G. W. The effects of fibronectin on the migration of human foreskin fibroblasts and Syrian hamster melanoma cells into three-dimensional gels of native collagen fibres. J Cell Sci. 1981 Apr;48:301–314. doi: 10.1242/jcs.48.1.301. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Turney S., Qian H., Elson E. L. Nanometre-level analysis demonstrates that lipid flow does not drive membrane glycoprotein movements. Nature. 1989 Jul 27;340(6231):284–288. doi: 10.1038/340284a0. [DOI] [PubMed] [Google Scholar]
- Tranquillo R. T., Lauffenburger D. A., Zigmond S. H. A stochastic model for leukocyte random motility and chemotaxis based on receptor binding fluctuations. J Cell Biol. 1988 Feb;106(2):303–309. doi: 10.1083/jcb.106.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. A visual study of chemotaxis of human lymphocytes using a collagen-gel assay. J Immunol Methods. 1985 Jan 21;76(1):105–120. doi: 10.1016/s0022-1759(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C., Lackie J. M., Haston W. S., Islam L. N. Effects of phorbol esters on shape and locomotion of human blood lymphocytes. J Cell Sci. 1988 Aug;90(Pt 4):645–655. doi: 10.1242/jcs.90.4.645. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C., Lackie J. M. The adhesion, migration and chemotaxis of leucocytes in inflammation. Curr Top Pathol. 1979;68:47–88. doi: 10.1007/978-3-642-67311-5_3. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977 Nov;75(2 Pt 1):606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]


