Abstract
The oxidoreductase ERp57 is an integral component of the peptide loading complex of major histocompatibility complex (MHC) class I molecules, formed during their chaperone-assisted assembly in the endoplasmic reticulum. Misfolded MHC class I molecules or those denied suitable peptides are retrotranslocated and degraded in the cytosol. The presence of ERp57 during class I assembly suggests it may be involved in the reduction of intrachain disulfides prior to retrotranslocation. We have studied the ability of ERp57 to reduce MHC class I molecules in vitro. Recombinant ERp57 specifically reduced partially folded MHC class I molecules, whereas it had little or no effect on folded and peptide-loaded MHC class I molecules. Reductase activity was associated with cysteines at positions 56 and 405 of ERp57, the N-terminal residues of the active CXXC motifs. Our data suggest that the reductase activity of ERp57 may be involved during the unfolding of MHC class I molecules, leading to targeting for degradation.
Keywords: chaperone/endoplasmic reticulum/MHC class I/oxidoreductase ERp57/protein folding
Introduction
Major histocompatibility complex (MHC) class I molecules present short peptides to CD8+ T lymphocytes, permitting the detection and elimination of pathogen-infected cells. MHC class I molecules meet and bind peptides during assembly within the endoplasmic reticulum (ER) in an assembly process known to involve a series of interactions with the ER resident chaperones calnexin and calreticulin, the accessory molecule tapasin and the oxidoreductase ERp57 (Lehner and Trowsdale, 1998; Pamer and Cresswell, 1998). An early interaction of the MHC class I heavy chain with calnexin is normally rapidly replaced by the formation of a peptide loading complex containing calreticulin, ERp57, tapasin and the transporter associated with antigen processing (TAP) (Solheim et al., 1997; Diedrich et al., 2001). The MHC class I-specific accessory molecule tapasin is critical for the formation of this complex. Tapasin physically links MHC class I– chaperone complexes to TAP and is also involved in editing the peptide repertoire bound by MHC class I molecules (Lehner et al., 1998; Grandea and Van Kaer, 2001; Purcell et al., 2001).
ERp57 is a member of the protein disulfide isomerase (PDI) family, whose functions include disulfide bond oxidation, reduction and isomerization (Gilbert, 1997; High et al., 2000; Ellgaard and Helenius, 2001). The active cysteine residues are contained within two CXXC motifs located within N- and C-terminal thioredoxin-like domains (Hirano et al., 1995; Urade et al., 1997). ERp57 forms complexes with the ER chaperones calnexin and calreticulin (Oliver et al., 1997, 1999; High et al., 2000; Molinari and Helenius, 2000). These chaperone complexes possess specificity for monoglucosylated polypeptides, and it has been postulated that through consecutive rounds of deglucosylation and reglucosylation of non-native glycoprotein substrates, cycles of folding occur until a native protein conformation has been adopted (Elliott et al., 1997; Zapun et al., 1998; Oliver et al., 1999).
The association of ERp57 with MHC class I molecules and the TAP complex (Hughes and Cresswell, 1998; Lindquist et al., 1998; Morrice and Powis, 1998) and the detection of ERp57–MHC class I mixed disulfide intermediates (Lindquist et al., 2001) suggests that ERp57 is the main oxidoreductase involved in MHC class I assembly. Recently, ERp57 has also been shown to form a disulfide interaction with tapasin rather than MHC class I molecules when part of the peptide loading complex (Dick et al., 2002).
MHC class I misfolding during normal assembly, or due to deficiency in peptide supply by inhibition of the proteasome or TAP, or by intervention by viral proteins (Wiertz et al., 1996), results in retrotranslocation, deglycosylation of the heavy chain, ubiquitylation and degradation by the proteasome (Hughes et al., 1997; Shamu et al., 1999). Prior to the retrotranslocation step from the ER it is thought that MHC class I molecules must first undergo unfolding and reduction of disulfide bonds (Tortorella et al., 1998; Fagioli et al., 2001). To address whether ERp57 can directly reduce the disulfide bonds present in MHC class I molecules, we have developed a novel assay system to monitor the status of disulfide bonds in folding and folded MHC class I molecules in the presence of recombinant ERp57. Our results indicate that ERp57 can efficiently reduce a partially folded pool of MHC class I heavy chains, whereas folded MHC class I molecules are resistant to reduction. We also define, by site-directed mutagenesis, the cysteine residues that are critical in the reduction process.
Results
Monoclonal antibody HC10 recognizes oxidized and reduced pools of MHC class I
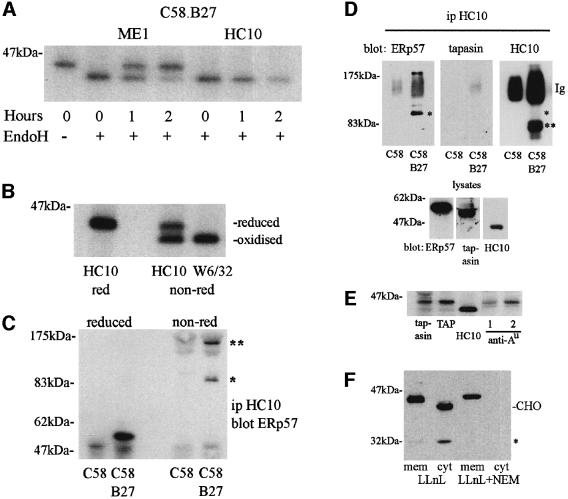
Monoclonal antibody (mAb) HC10 recognizes a pool of partially folded, non-β2-microglobulin (B2m) associated, human HLA-B and -C class I molecules (Stam et al., 1986). Rat C58 thymoma cells transfected with HLA-B*2705 (C58.B27) were pulse–chased, immunoprecipitated with HC10 and analysed by SDS–PAGE under reducing conditions. The HC10 reactive class I pool did not acquire endoglycosidase H (endo H) resistance, indicating that the HC10 reactive class I pool is predominantly located within the ER (Figure 1A). The HC10 signal also decreased over 2 h of chase. This loss of HC10 reactive material is indicative of class I molecules achieving fully folded status, but it is also likely to include a pool of molecules that fail to fold and are subsequently degraded (Raposo et al., 1995; Mear et al., 1999). In contrast, fully folded B27 molecules, as detected by mAb ME1, acquired endo H resistance, indicating exit from the ER and transit to the cell surface over the same time period (Figure 1A).
Fig. 1. MHC class I assembly in the ER. (A) C58.B27 cells were pulse radiolabelled, chased for the indicated times and B27 molecules immunoprecipitated with ME1 (folded B27) or HC10 (unfolded), followed by endo H digestion. (B) T3 cells were radiolabelled and MHC class I molecules immunoprecipitated with HC10 (unfolded) or W6/32 (folded). Samples were analysed reduced or non-reduced. (C) C58 or C58.B27 cells were lysed and immunoprecipitated with HC10. Samples were analysed reduced or non-reduced, followed by immunoblotting to reveal ERp57. (D) The experiment shown in (C) was repeated and immunoblotted for ERp57, tapasin and B27. Control blots of whole-cell lysates performed at the same time are also shown. (E) C58.B27 cells were radiolabelled, lysed in digitonin and immunoprecipitated with antibodies to tapasin, TAP, B27 (HC10) and rat RT1.Au (anti-Au), NR5/10 (lane labelled 1) and GN7/5.B11 (lane labelled 2). (F) C58.B27sv5 cells (C-terminally tagged B27) were incubated with LLnL with or without NEM, membrane and cytosol fractions were prepared and B27 molecules detected by immunoblotting with anti-tag mAb Pk. A cytosolic deglycosylated (CHO) B27 heavy chain product is indicated, as is a novel 32 kDa heavy chain breakdown fragment (*). Similar results were obtained with HC10, except for the absence of the 32 kDa product.
Under non-reducing SDS–PAGE the same pool of HC10 reactive MHC class I molecules shown in Figure 1A resolves as two distinct species with different gel mobilities (Figure 1B). The upper band represents class I molecules with no intrachain disulfides and shares similar mobility to fully reduced HC10 reactive molecules (Tector et al., 1997). The lower band represents a partially folded oxidized class I species containing disulfide bonds and shares similar mobility to fully folded class I molecules, as detected by conformation-specific antibodies such as mAb W6/32. Similar results were obtained in C58.B27 cells using HC10 and ME1 mAbs and with HC10 in the B2m-deficient cell line Daudi (data not shown). Thus, mAb HC10 can be used to detect pools of partially folding oxidized and reduced class I molecules in the ER.
ERp57 forms a mixed disulfide intermediate with HLA-B27
We have previously shown that ERp57 associates with rat MHC class I molecules in the C58 cell line (Morrice and Powis, 1998). To determine whether ERp57 actively participates in the assembly of B27 molecules in these cells, we looked for the formation of a mixed disulfide intermediate (Lindquist et al., 2001). C58.B27 cells, pretreated with N-ethylmaleimide (NEM) to trap transient intermediates and inhibit post-lysis disulfide bond formation, were lysed in NP-40 containing lysis buffer, which disrupts non-disulfide interactions, and immunoprecipitated with HC10. Western blotting with anti-ERp57 antisera revealed a species under non-reducing conditions of mol. wt ∼95–100 kDa, the correct weight to be a complex of ERp57 (∼56 kDa) and B27 class I heavy chain (∼43 kDa), as shown in Figure 1C (single asterisk). An unidentified higher molecular weight species was also detected (Figure 1C, double asterisks), whose weight was difficult to determine due to non-reduced immunoglobulin distorting this region of the gel. Both these high molecular weight complexes are disrupted on reduction to reveal the presence of ERp57.
In light of the recent observation that ERp57 is disulfide-bonded to tapasin in the peptide-loading complex (Dick et al., 2002), we investigated the possible presence of tapasin as part of our B27–ERp57 complex. The experiment shown in Figure 1C was repeated and the membrane sequentially immunoblotted for the presence of ERp57, tapasin and B27. As shown in Figure 1D, we readily detected the species previously noted with ERp57 antisera, comprising an ∼100 kDa species (single asterisk) and a larger species. However, in both this experiment and several others we failed to detect any tapasin-conjugated species. Immunoblotting with HC10 revealed the faint presence of the B27–ERp57 conjugate (single asterisk), but the signal was swamped by the presence of cross-reactivity on the HC10 immunoglobulin used in the original immunoprecipitation (labelled Ig), and also by the presence of HC10-reactive B27 homodimers (double asterisk) with mol. wt ∼83 kDa (Allen et al., 1999). Simultaneous immunoblotting of whole-cell lysates confirmed the presence of ERp57, tapasin and B27. Taken together these data indicate that the partially folded pool of B27 molecules isolated by HC10 can undertake disulfide interactions with ERp57, but not with tapasin.
We also determined whether, when expressed in rat C58 cells, B27 exhibited a tapasin-independent phenotype previously noted by expression in the tapasin-deficient 721.220 cell line (Peh et al., 1998). Digitonin lysates of radiolabelled C58.B27 cells were immunoprecipitated with anti-murine tapasin or anti-rat TAP antibodies. As shown in Figure 1E, the level of B27 interaction with tapasin and TAP was low in comparison with the endogenous rat MHC class I allele RT1.Au expressed by C58 cells, indicating that B27 is tapasin-independent in these cells.
NEM has previously been shown to inhibit the degradation of MHC class I heavy chains in Daudi cells, suggesting that reduction occurs prior to retrotranslocation (Tortorella et al., 1998). We therefore tested whether NEM inhibited the retrotranslocation of B27 molecules in C58.B27 cells. Cells expressing B27 epitope-tagged at the C-terminus were treated with the proteasome inhibitor N-acetyl-Leu-Leu-norleucinal (LLnL) with or without NEM, and membrane and cytosol fractions prepared. In the presence of LLnL, a deglycosylated (Hughes et al., 1997) B27 heavy chain was detected in the cytosolic fraction (Figure 1F). We also detected a previously unreported MHC heavy breakdown product of ∼32 kDa (single asterisk). The appearance of the cytosolic heavy chain products was inhibited by the additional presence of NEM, indicating that the redox status of components in the ER is critical to the degradation process of MHC class I molecules.
Recombinant ERp57 reduces MHC class I heavy chains in vitro
The ability of HC10 to detect partially folded and oxidized class I molecules, as shown in Figure 1B, and the formation of B27–ERp57 disulfide-bonded intermediates (Figure 1C and D) led us to ask whether we could demonstrate ERp57-mediated reduction of MHC class I molecules in vitro. Recombinant rat ERp57 was therefore expressed in bacteria and purified to homogeneity (Figure 2A). To determine activity, the purified product was tested for its ability to reduce and precipitate insulin (Hirano et al., 1995). Recombinant ERp57 displayed reductase activity (Figure 2B), but was less efficient than PDI, an observation previously noted for ERp57 in comparison with both PDI and thioredoxin (Srivastava et al., 1993; Bourdi et al., 1995; Hirano et al., 1995).
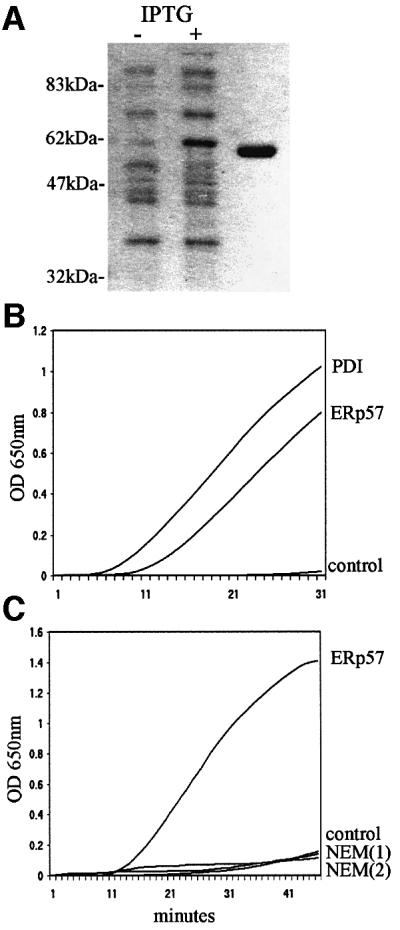
Fig. 2. Purification and activity of recombinant ERp57. (A) Escherichia coli expressing histidine-tagged rat ERp57 were cultured in the presence (+) or absence (–) of isopropyl β-d-thiogalactopyranoside (IPTG). Recombinant ERp57 was purified on Ni-agarose, thrombin cleaved to remove the histidine tag, and further purified by anion exchange chromotography (last lane). (B) Insulin reduction. ERp57 and PDI, both at 0.3 µM, were mixed with 1 mg/ml insulin and DTT (0.32 mM), and the OD650 measured at 60-s intervals. Control sample included insulin with DTT alone. (C) NEM inhibition of ERp57 activity. Insulin reduction assays were peformed with ERp57 (1 µM) in the presence of 1 mM NEM [NEM(1)], or by including 1 mM NEM in an ERp57 pre-activation step prior to addition to the insulin [NEM(2)]. Control reaction included insulin and DTT alone.
To determine whether ERp57 could affect the status of disulfides in partially folded MHC class I molecules, we mixed together HC10 reactive class I molecules with recombinant ERp57. C58.B27 and Daudi cells were metabolically labelled, lysed in NP-40 buffer and immunoprecipitated with HC10. Pre-activated ERp57 was added for 1 h, followed by non-reducing SDS– PAGE analysis. Control or mock-treated samples retained the lower disulfide-containing band; however, in the presence of ERp57, reduction of the lower band was observed in both C58.B27 and Daudi samples, with an increase in the density of the upper band (Figure 3A and B). No smaller molecular weight species were detected by SDS–PAGE or mass spectrometry, indicating that in this system ERp57 was not acting as a protease. The kinetics of ERp57-dependent reduction of the oxidized MHC class I heavy chain were similar to that of insulin, with much of the reduction being complete within 40 min (Figure 3C). Addition of alkylating agents such as NEM or iodoacetamide during either the assay or during the preactivation of ERp57 inhibited both insulin (Figure 2C) and MHC class I reduction (not shown), indicating that the reductase activity was mediated through cysteine residues.
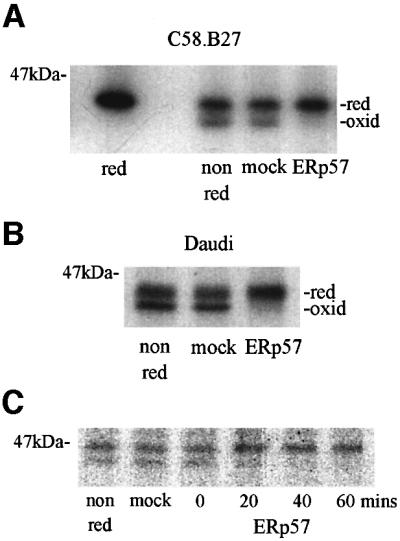
Fig. 3. ERp57 reduction of partially folded MHC class I molecules. (A) HC10 immunoprecipitates from radiolabelled C58.B27 cells were incubated in the presence of ERp57 (1 µM) or in assay buffer alone (mock). Control samples were analysed reduced (red) or non-reduced (non red) for comparison. (B) HC10 immunoprecipitates from radiolabelled Daudi cells were incubated with 1 µM ERp57, or mock-treated in assay buffer alone (mock), or analysed non-reduced as indicated. (C) Time course of ERp57-mediated reduction of MHC class I heavy chains. HC10 immunoprecipitates of radiolabelled C58.B27 cells were incubated with 1 µM ERp57 for the indicated times. Non-reduced and mock-treated controls were as described in (A) and (B).
Folded and peptide-loaded MHC class I molecules are resistant to ERp57 reduction
The above data indicated that partially folded MHC class I molecules were suitable substrates for reduction by ERp57. We next asked whether fully folded MHC class I molecules could also be substrates for ERp57. BB7.2 (anti-HLA-A2 specific) immunoprecipitates from T3 cells (TAP-deficient human T2 cells expressing rat TAP1 and TAP2) and ME1 immunoprecipitates from C58.B27 were incubated with recombinant ERp57 and analysed by non-reducing SDS–PAGE. BB7.2-isolated A2 molecules showed essentially no reduction, apart from a minor population (Figure 4A, arrow). Similarly, ME1-isolated B27 molecules showed no reduction in the presence of ERp57 (data not shown). The minor population of reduced A2 molecules could be derived from two possible sources, either an empty population of folded A2 molecules awaiting peptide loading or a population loaded with weak affinity peptides that may dissociate during the course of the assay, thus permitting ERp57-mediated reduction. To address this issue we utilized the ability of defined synthetic peptide epitopes to stabilize MHC class I molecules when added to detergent lysates treated at 37°C. Under such conditions, peptide-free MHC class I molecules dissociate, allowing the subsequent immunoprecipitation of MHC class I molecules loaded with a defined peptide (Figure 4B). We therefore incubated radiolabelled lysates of T3 cells with the A2 binding peptide LLDVPTAAV and immunoprecipitated peptide-loaded A2 molecules. These were then incubated with recombinant ERp57 and analysed by non-reducing SDS–PAGE. Under these conditions ERp57 did not reduce peptide-loaded A2 molecules, whereas non-peptide-loaded A2 molecules again showed a small population susceptible to reduction (Figure 4C).
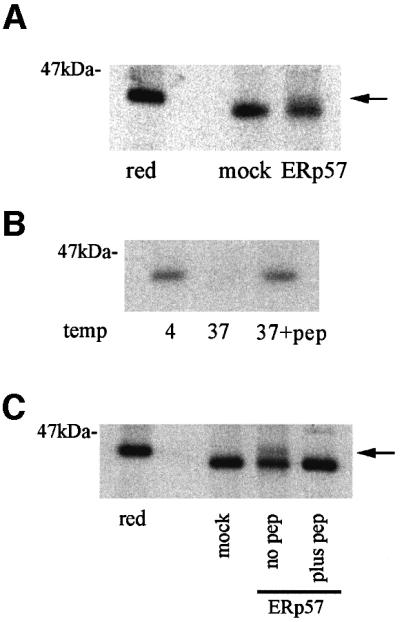
Fig. 4. ERp57 is inefficient at reducing folded and peptide-loaded MHC class I molecules. (A) BB7.2 HLA-A2 immunoprecipitates from radiolabelled T3 cells were incubated with 1 µM ERp57, or mock- treated with assay buffer alone and analysed non-reduced. A minor population of reduced heavy chains is indicated by an arrow. (B) Peptide stabilization of A2 molecules. Lysates of radiolabelled T3 cells were incubated at 4°C, or at 37°C in the presence or absence of 50 µM A2 binding peptide LLDVPTAAV, followed by immunoprecipitation with BB7.2. (C) BB7.2 immunoprecipitates from radiolabelled T3 lysates as shown in (A), and a peptide stabilized population (plus pep) as indicated in (B) were incubated with 1 µM ERp57, or mock-treated with assay buffer alone. The arrow indicates a minor population of reduced A2 heavy chains detected in the non-peptide stabilized sample (no pep).
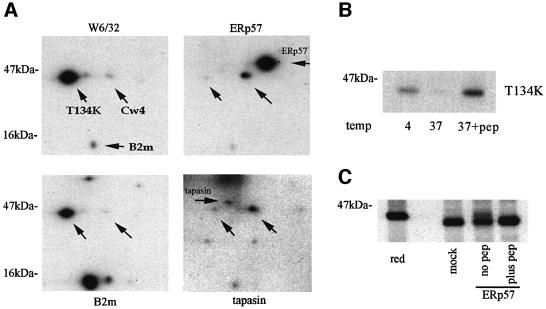
We also investigated a mutant A2 class I molecule containing a threonine to lysine amino acid change at position 134 (T134K) in the α2 domain. T134K rapidly transits to the cell surface with a sub-optimal selection of peptides, and whilst it can present synthetic peptides when added exogenously, it is inefficient at presenting endogenously produced epitopes (Lewis et al., 1996). T134K forms only weak or transient interactions with calreticulin (Lewis and Elliott, 1998) and, as demonstrated by immunoprecipitation and two-dimensional gel analysis (Figure 5A), also fails to interact with ERp57 and tapasin, despite being the major assembled product recognized by W6/32 and anti-human B2m antibodies. Thus, T134K appears to circumvent the quality control event(s) in the ER, allowing us to test whether such a mutant would be more susceptible to ERp57-mediated reduction. Immuno precipitated and peptide-loaded T134K molecules (Figure 5B) as described above were tested for reduction by recombinant ERp57. Non-peptide-loaded molecules were partially reduced, whereas peptide-loaded molecules were completely resistant (Figure 5C). Taken together these results suggest that MHC class I molecules, once having attained a fully folded conformation and bound peptide, are not substrates for reduction by ERp57.
Fig. 5. T134K mutant A2 molecules form weak interactions with ERp57 and tapasin, but display similar resistance to ERp57 reduction. (A) Two- dimensional gel analysis of T134K immunoprecipitations. C1R-T134K cells were radiolabelled and immunoprecipitated with W6/32, anti-human B2m, anti-ERp57 and anti-tapasin. Upward pointing arrows indicated the positions of T134K molecules and the endogenous HLA-Cw4 molecule. Horizontal arrows indicate the position of B2m, ERp57 and tapasin as indicated. T134K is the major assembled MHC class I species in these cells (left panels). A short pre-clearing step results in the detection of T134K/B2m complexes that dissociate under longer incubation times. In comparison with Cw4, T134K forms a weak interaction with ERp57 and tapasin (right panels). Short labelling times result in the detection of the MHC class I molecules and the relevant chaperone being immunoprecipitated, and not the full peptide-loading complex. (B) Peptide stabilization of T134K molecules. Lysates of radiolabelled C1R-T134K cells were incubated at 4°C, or at 37°C in the presence or absence of 50 µM A2 binding peptide LLDVPTAAV, followed by immunoprecipitation with BB7.2. (C) BB7.2 immunoprecipitates from radiolabelled C1R-T134K lysates, and a peptide stabilized population (plus pep) were incubated with 1 µM ERp57, or mock-treated with assay buffer alone. The arrow indicates a minor population of reduced T134K heavy chains detected in the non-peptide stabilized sample (no pep).
Association of ERp57 reductase activity with Cys56 and Cys405 in CXXC motifs
The CXXC active site motifs are characteristic of oxidoreductases, and ERp57 contains two such motifs (Hirano et al., 1995; Urade et al., 1997). To determine which of the Cys residues were active in the reduction of partially folded MHC class I molecules, we mutated both of the N-terminal Cys residues in each of the motifs (Cys56 and Cys405) and the C-terminal Cys residues (Cys59 and Cys408) to generate (SXXC)2 and (CXXS)2 mutants, respectively, as outlined in Figure 6A. Both mutants were purified and tested for their ability to reduce insulin. The (SXXC)2 mutant retained essentially no reductase activity, whereas the (CXXS)2 mutant retained a diminished activity in comparison with wild-type ERp57 (Figure 6B). When tested for their ability to reduce partially folded B27 molecules, a similar pattern of results was obtained: (SXXC)2 showed no activity whereas (CXXS)2 demonstrated reduced activity (Figure 6C). Mixing the motif mutants with wild-type ERp57 had no inhibitory effect on it (data not shown). These data further confirm that the reductase activity of ERp57 on partially folded MHC class I molecules is mediated by the cysteines in the CXXC motifs. Furthermore, we have identified that retention of the N-terminal pair of cysteine residues alone can maintain limited reductase activity.
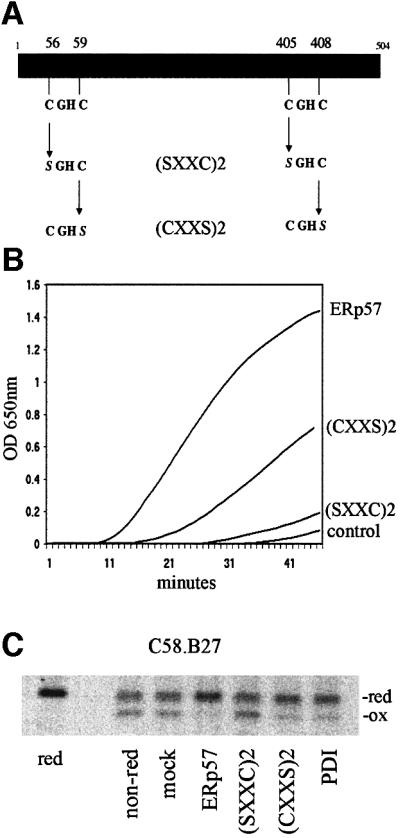
Fig. 6. ERp57 CXXC motif mutations affect reductase activity. (A) Schematic diagram of ERp57 indicating the location of the CXXC motifs and the generation of (SXXC)2 and (CXXS)2 mutants. (B) ERp57, (CXXS)2 and (SXXC)2 were incubated at 1 µM with insulin and DTT. The optical density was determined at 60-s intervals. Control sample included insulin with DTT alone. (C) Reductase activity of CXXC motif mutants and PDI on partially folded MHC class I molecules. HC10 immunoprecipitates from radiolabelled C58.B27 cells were incubated with 1 µM each of ERp57, (SXXC)2, (CXXS)2 or PDI. Control samples were treated with assay buffer alone (mock), or analysed reduced (red) or non reduced (non-red).
As a control for ERp57 reductase activity, we also included the ER oxidoreductase PDI in several experiments. To our surprise, despite PDI being a more active reductase in the insulin assay (Figure 2B), it was less able to reduce partially folded MHC heavy chains in comparison with ERp57 (Figure 6C). This occurred both when ERp57 and PDI were used at equivalent concentrations and when retested at concentrations demonstrating equivalent insulin reduction (not shown). When ERp57 and PDI were mixed together, efficient reduction was observed, indicating that PDI did not have an inhibitory effect on ERp57 activity (data not shown). This suggests that ERp57 has some as yet undetermined substrate selectivity in addition to that imposed upon it by interaction with its partner polypeptides calnexin and calreticulin.
Discussion
The oxidoreductase ERp57 is a component of the MHC class I molecule assembly complex. In digitonin containing cell lysates, a complex of calreticulin, ERp57, tapasin, MHC class I and TAP can be detected readily (Hughes and Cresswell, 1998; Lindquist et al., 1998; Morrice and Powis, 1998), and ERp57–MHC class I interactions have also been observed in an in vitro semi-permeabilized cell system (Farmery et al., 2000). ERp57 is thought to form direct interactions with chaperones such as calnexin and calreticulin; therefore, its presence in the class I assembly complex could be maintained purely through these chaperone associations (Oliver et al., 1997, 1999). However, the presence of ERp57 in the TAP complex of cells lacking calreticulin (Gao et al., 2002), and the detection of covalently linked ERp57–tapasin complexes but not ERp57–-MHC class I complexes associated with TAP (Dick et al., 2002), suggests that ERp57 is recruited by tapasin at this latter stage of MHC class I assembly. Nevertheless, a disulfide-bonded intermediate of ERp57 with MHC class I has been reported (Lindquist et al., 2001), an observation that we have confirmed here for a human HLA allele (B*2705) assembling in a rat host cell line (Figure 1C). An explanation for these apparently contradictory observations may lie in the use of detection reagents. We and Lindquist et al. (2001) have used mAb HC10 to isolate ERp57–MHC class I complexes. Since HC10 only detects non-B2m-associated MHC class I heavy chains, which are unlikely to be the TAP-associated pool, the ERp57–MHC class I complexes we have detected probably represent both the earliest stage of MHC class I assembly, involving calnexin, and also molecules undergoing reduction prior to retrotranslocation. Thus it is likely that ERp57 plays a dual role in MHC class I assembly, being directly involved in the formation and/or breakage of disulfide bonds in MHC class I heavy chains early during biosynthesis, and then acting in conjunction with tapasin to regulate the peptide loading of B2m-associated MHC class I molecules as part of the TAP complex.
We also detected a higher molecular weight ERp57 disulfide-bonded intermediate by immunoprecipitation with HC10. This higher molecular weight complex may represent a transient complex of class I heavy chains with more than one ERp57 molecule or, alternatively, with other, as yet undefined, components of the early MHC class I assembly pathway. Experiments are ongoing to determine the components of this complex. Our data also indicate that B27 assembles in a tapasin-independent manner in C58 cells (Figure 1E). It may be of interest in the future to compare the nature of HC10 immunopurified complexes of tapasin-dependent and -independent MHC alleles.
During folding and assembly in the ER, non-B2m-associated MHC class I molecules can be detected in at least two different disulfide-bonded isoforms by the mAb HC10 (Figure 1B). We have shown here that one of these isoforms, containing disulfide bonds, can be reduced by recombinant ERp57. The migration of the HC10-reactive disulfide-containing product is similar to that of folded MHC class I molecules (Figure 1B), suggesting that it may have both α2 (Cys101–Cys164) and α3 (Cys203–Cys259) domain bonds formed. It has been suggested that these bonds are temporally distinct, with the conformation of the α1/α2 domain being dependent on peptide and B2m, while the α3 domain relies upon the appropriate redox potential (Ribaudo and Margulies, 1992; Tector et al., 1997). We are therefore currently investigating whether both disulfide bonds are equally susceptible to ERp57 activity.
In sharp contrast to our results obtained with the partially folded pool of MHC class I molecules recognized by HC10, we demonstrated that fully folded MHC class I molecules are resistant to ERp57-mediated reduction. A small population of folded molecules were reduced (Figure 4A), but following loading with a synthetic peptide epitope no reduction was detectable (Figure 4C). The small population that was reduced by ERp57 may arise from the short metabolic labelling times used in these experiments, which may reveal MHC class I molecules that are essentially fully folded but lacking peptides of suitable affinity to survive the assay conditions at 37°C. Our results therefore suggest that even MHC molecules in the latter stages of assembly, once unfolding has been initiated, may still become susceptible to ERp57-mediated reduction. Similar observations obtained with mutant A2 T134K molecules (Figure 5C) strongly suggest that inappropriate peptide loading can also expose MHC class I molecules to ERp57 reductase activity. Further more, the poor association of T134K molecules with the peptide-loading complex suggest that the critical disulfide bond formation of MHC class I molecules occurs prior to the peptide loading and editing functions mediated by tapasin and the TAP complex.
The use of mAbs detecting conformation-dependent epitopes in these experiments raised concerns that steric hinderance in the immunoprecipitates prevented access by ERp57. However, we have also obtained similar results in experiments using folded class I molecules isolated with antibodies recognizing the cytoplasmic tail, thus limiting steric hinderance effects (data not shown). Furthermore, we have also determined that antibodies appear to be very poor substrates for ERp57-mediated reduction under the assay conditions used here (data not shown).
Using site-directed mutagenesis we identified that mutant ERp57 polypeptides containing (CXXS)2 motifs retained diminished reductase activity, whereas (SXXC)2 mutants were inactive. The partial reductase activity associated with the (CXXS)2 mutant again could reflect a population of class I molecules that are undergoing isomerization rather than full reduction. Similarly, (CXXS)2 PDI yeast mutants exhibited no in vitro reductase or oxidase activity, but retained isomerase activity (Laboissiere et al., 1995; Walker et al., 1996) and could rescue inviable cells with a chromosomal deletion of PDI (LaMantia and Lennarz, 1993). No structural data are available as yet for ERp57; however, by comparison with the data available for PDI (Kemmink et al., 1997), it can be predicted that the C-terminal cysteine residue of each CXXC motif is inaccessible compared with the N-terminal cysteine, thus allowing the latter to undergo restricted thiol-dependent activities.
Our experimental assay system described here utilizes low concentrations of dithiothreitol (DTT). Thus, cycles of reduction appear to require the presence of a reducing agent or a redox environment that maintains ERp57 in a reduced state. This raises the intriguing question of how the predominantly oxidizing environment of the ER (Hwang et al., 1992) can integrate oxidase, isomerase and reductase activities. In the case of PDI, cycles of oxidative folding activity are regulated by Ero1p (Frand and Kaiser, 1998, 1999). The ERp57 redox state may be maintained in a similar manner to PDI, although no evidence has yet been obtained to suggest that ERp57 utilizes Ero1 polypeptides, and no other candidate regulatory polypeptide(s) has yet been identified. Glutathione in the ER has been shown to serve as a net reductant (Cuozzo and Kaiser, 1999), which may provide the appropriate redox conditions for ERp57 to perform isomerase/reductase activities. Since calnexin and calreticulin have been demonstrated to enhance ERp57-mediated protein folding of RNase B (Zapun et al., 1998), it would be of interest to determine how these chaperones affect the reductase activity that we have described. The apparently relatively stable interaction of tapasin with ERp57 in the TAP complex also raises the intriguing possibility of tapasin regulating ERp57 function (Dick et al., 2002).
Most studies of ERp57 in the MHC class I assembly pathway have focused on the role ERp57 may play in the production of a fully folded, peptide-loaded molecule for export to the cell surface. However, ER quality control mechanisms also target for degradation molecules that do not assemble correctly and fail to load with peptides. The retrotranslocation of MHC class I heavy chains into the cytoplasm is an integral part of this process (Hughes et al., 1997). NEM is capable of inhibiting this process (Tortorella et al., 1998), and whilst the non-specific nature of NEM action is likely to affect several components of the retrotranslocation process, it does indicate that inhibition of ERp57 activity may represent at least part of this process. We have shown in this study that ERp57 can act to reduce partially folded MHC molecules, which potentially would be subjected to quality control in the ER. ERp57 should therefore be detected where the process of MHC class I breakdown is initiated in the ER. Interest ingly, in the presence of proteasome inhibitors, immunofluorescent studies have indicated a clustering of MHC class I heavy chains into a subcellular compartment closely associated with the ER. This compartment also contains calnexin and calreticulin, but appears not to contain PDI (Kamhi-Nesher et al., 2001). The presence of calnexin, calreticulin and MHC class I heavy chains in this degradation-associated compartment would strongly suggest that ERp57 may also be present, placing it in the correct environment to perform the reductase activity we have described here.
Materials and methods
Cell lines and antibodies
The rat T-cell line C58 (Silva et al., 1983), human Daudi, human T3 cells (Momburg et al., 1992; T2 expressing rat TAP1 and TAP2) and C1R-T134K cells (Lewis et al., 1996) were maintained in RPMI 1640 (Gibco-BRL) supplemented with 5% fetal calf serum and 1 mg/ml G418 (PAA Laboratories) as required. C58.B27 was generated by electroporation (160 V, 975 µF) of C58 cells with a cDNA encoding HLA-B*2705 (kindly provided by Dr J.Taurog) cloned into vector pCR3 (Invitrogen). The C58.B27sv5 line expressing B27 epitope tagged at the C-terminus with the SV5 tag sequence SRGKPIPNPLLGLDST (Hanke et al., 1992) was generated using PCR primers incorporating the SV5 tag, cloned into pCR3 and electroporated as above.
mAb HC10 (Stam et al., 1986), recognizing unassembled HLA-B and -C molecules, was kindly provided by Drs J.Neefjes and P.Lehner; ME1 (Ellis et al., 1982), recognizing folded HLA-B molecules, was kindly provided by Dr J.Taurog; BB7.2 (Parham and Brodsky, 1981) recognizes folded HLA-A2 molecules; and W6/32 (Parham et al., 1979) recog nizes folded HLA-A, -B and -C molecules. Anti-human B2m was obtained from Serotec and rabbit anti-ERp57 was a gift from Dr N.Bulleid. Anti-human tapasin was a gift from Drs P.Lehner and P.Cresswell. Anti-murine tapasin (Harris et al., 2001) was a gift from Dr T.Hansen. mAb anti-RT1.Au antibodies NR5/10 and GN7/5.B11 were gifts from Dr G.Butcher. mAb Pk, recognizing the SV5 epitope tag, was a gift from Dr R.Randall. Anti-rat TAP antiserum was as described previously (Powis, 1997).
Metabolic labelling and immunoprecipitations
Methionine-starved cells were radiolabelled for between 5 and 10 min with 3.7MBq Trans label (ICN), and resuspended in lysis buffer [150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 mM NEM, 10 mM Tris pH 7.5] containing either 1% NP-40 or 1% digitonin (Sigma) as required. Immunoprecipitations were performed with the indicated antibodies and protein A/G–Sepharose (Sigma) as required (Powis, 1997). HC10 immunoisolates were washed with lysis buffer excluding NEM, and then used in ERp57 in vitro assays.
For pulse–chase analysis cells were labelled as above, returned to normal medium and samples lysed at the indicated timepoints. After immunoprecipitation the samples were incubated with 5 mU endo H (Roche) for 1 h at 37°C. For peptide stabilization assays, cells were labelled and lysed as above and incubated in the presence or absence of 50 µM A2 binding peptide LLDVPTAAV (Hill et al., 1995) for 1 h at 37°C. Control lysates were incubated on ice. Heat-treated lysates were then returned to ice and immunoprecipitated as above.
For the isolation of B27–ERp57 intermediates, C58 and C58.B27 cells were incubated in 10 mM NEM in phosphate-buffered saline on ice for 10 min prior to lysis in NP-40-containing buffer and immunoprecipitation with mAb HC10. SDS–PAGE under non-reducing and reducing conditions was perfomed on Bio-Rad mini-gel apparatus. Two-dimensional electrophoresis of immunoprecipitates was performed as described previously (King et al., 2000). Autoradiography was performed using Kodak Biomax MS1 film (Merck).
Cell fractionation
Cells were treated with or without 100 µM LLnL (Affiniti Research Products) and 1 mM NEM for 2 h at 37°C, pelleted and freeze-fractured three times on dry ice (Hughes et al., 1997). Samples were resuspended in buffer containing 10 mM Tris pH 7 and 50 µM LLnL and 1× mini-protease inhibitors (Roche) and spun at 2500 g for 3 min to remove nuclei. Supernatants were then spun at 50 000 g for 30 min and the subsequent membrane and supernatant fractions analysed by SDS–PAGE and western blotting. Western blotting was performed on BA85 nitrocellulose (Schleicher and Schuell) using Supersignal Femto chemiluminescent reagents (Pierce and Warriner).
Recombinant ERp57
Rat ERp57 cDNA lacking residues 1–20 of the signal sequence, was subcloned into pET15b vector with an N-terminal His6 tag (Novagen). ERp57 pET15b was transformed into BL21-competent bacteria. For protein purification, bacterial cultures were grown up and induced overnight at 25°C with 0.4 mM isopropyl β-d-thiogalactopyranoside (IPTG). Bacteria were lysed in BPER reagent (Pierce) according to manufacturer’s instructions and the lysate bound to Ni-NTA agarose (Qiagen) for 2 h at 4°C. The histidine tag was removed by sequential digestion of Ni-agarose beads with 2 U bovine/0.5 U human thrombin (Sigma) at room temperature for 1 h. Thrombin was removed by incubation with p-aminobenzamidine agarose beads (Sigma). Recom binant ERp57 was then dialysed overnight against 50 mM Tris–HCl pH 8 and further purified by high-performance liquid chromatography using anion exchange chromatography. ERp57-containing fractions were pooled and dialysed against 50 mM HEPES, pH 7.5, and stored at –80°C.
Insulin and MHC class I reduction assays
ERp57 reduction of insulin was performed essentially as described previously (Hirano et al., 1995). Briefly, insulin (Sigma) was prepared freshly at 1 mg/ml in assay buffer (100 mM KAc pH 7.5, 2 mM EDTA and 10 µM DTT). Insulin (50 µl) was mixed with ERp57 or PDI (Sigma) in a final volume of 10 µl assay buffer and then 2 µl of 10 mM DTT was added. The OD650 of the assay was read every minute.
For the MHC class I reduction assays, immunoprecipitates were resuspended in 10 µl assay buffer as above and then supplemented with a further 10 µl of buffer containing pre-activated ERp57 or PDI, or DTT alone. Pre-activation was performed by incubating ERp57 in assay buffer for 5 min at 37°C in the presence of 100 µM DTT. ERp57 reduction assays were performed at 37°C for 1 h. Samples were then analysed on non-reducing SDS–PAGE and autoradiography.
Acknowledgments
Acknowledgement
We thank Adam Benham for comments and suggestions.
References
- Allen R.L., O’Callaghan,C.A., McMichael,A.J. and Bowness,P. (1999) Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. J. Immunol., 162, 5045–5048. [PubMed] [Google Scholar]
- Bourdi M., Demady,D., Martin,J.L., Jabbour,S.K., Martin,B.M., George,J.W. and Pohl,L.R. (1995) cDNA cloning and baculovirus expression of the human liver endoplasmic reticulum P58: characterization as a protein disulfide isomerase isoform, but not as a protease or a carnitine acyltransferase. Arch. Biochem. Biophys., 323, 397–403. [DOI] [PubMed] [Google Scholar]
- Cuozzo J.W. and Kaiser,C.A. (1999) Competition between glutathione and protein thiols for disulphide-bond formation. Nature Cell Biol., 1, 130–135. [DOI] [PubMed] [Google Scholar]
- Dick T.P., Bangia,N., Peaper,D.R. and Cresswell,P. (2002) Disulfide bond isomerization and the assembly of MHC class I–peptide complexes. Immunity, 16, 87–98. [DOI] [PubMed] [Google Scholar]
- Diedrich G., Bangia,N., Pan,M. and Cresswell,P. (2001) A role for calnexin in the assembly of the MHC class I loading complex in the endoplasmic reticulum. J. Immunol., 166, 1703–1709. [DOI] [PubMed] [Google Scholar]
- Ellgaard L. and Helenius,A. (2001) ER quality control: towards an understanding at the molecular level. Curr. Opin. Cell Biol., 13, 431–437. [DOI] [PubMed] [Google Scholar]
- Elliott J.G., Oliver,J.D. and High,S. (1997) The thiol-dependent reductase ERp57 interacts specifically with N-glycosylated integral membrane proteins. J. Biol. Chem., 272, 13849–13855. [DOI] [PubMed] [Google Scholar]
- Ellis S.A., Taylor,C. and McMichael,A. (1982) Recognition of HLA-B27 and related antigen by a monoclonal antibody. Hum. Immunol., 5, 49–59. [DOI] [PubMed] [Google Scholar]
- Fagioli C., Mezghrani,A. and Sitia,R. (2001) Reduction of interchain disulfide bonds precedes the dislocation of Ig-μ chains from the endoplasmic reticulum to the cytosol for proteasomal degradation. J. Biol. Chem., 276, 40962–40967. [DOI] [PubMed] [Google Scholar]
- Farmery M.R., Allen,S., Allen,A.J. and Bulleid,N.J. (2000) The role of ERp57 in disulfide bond formation during the assembly of major histocompatibility complex class I in a synchronized semipermeabilized cell translation system. J. Biol. Chem., 275, 14933–14938. [DOI] [PubMed] [Google Scholar]
- Frand A.R. and Kaiser,C.A. (1998) The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell, 1, 161–170. [DOI] [PubMed] [Google Scholar]
- Frand A.R. and Kaiser,C.A. (1999) Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol. Cell, 4, 469–477. [DOI] [PubMed] [Google Scholar]
- Gao B., Adhikari,R., Howarth,M., Nakamura,K., Gold,M.C., Hill,A.B., Knee,R., Michalak,M. and Elliott,T. (2002) Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity, 16, 99–109. [DOI] [PubMed] [Google Scholar]
- Gilbert H.F. (1997) Protein disulfide isomerase and assisted protein folding. J. Biol. Chem., 272, 29399–29402. [DOI] [PubMed] [Google Scholar]
- Grandea A.G. III, and Van Kaer,L. (2001) Tapasin: an ER chaperone that controls MHC class I assembly with peptide. Trends Immunol., 22, 194–199. [DOI] [PubMed] [Google Scholar]
- Hanke T., Szawlowski,P. and Randall,R.E. (1992) Construction of solid matrix–antibody–antigen complexes containing simian immuno deficiency virus p27 using tag-specific monoclonal antibody and tag-linked antigen. J. Gen. Virol., 73, 653–660. [DOI] [PubMed] [Google Scholar]
- Harris M.R., Lybarger,L., Yu,Y.Y., Myers,N.B. and Hansen,T.H. (2001) Association of ERp57 with mouse MHC class I molecules is tapasin dependent and mimics that of calreticulin and not calnexin. J. Immunol., 166, 6686–6692. [DOI] [PubMed] [Google Scholar]
- High S., Lecomte,F.J., Russell,S.J., Abell,B.M. and Oliver,J.D. (2000) Glycoprotein folding in the endoplasmic reticulum: a tale of three chaperones? FEBS Lett., 476, 38–41. [DOI] [PubMed] [Google Scholar]
- Hill A., Worth,A., Elliott,T., Rowland-Jones,S., Brooks,J., Rickinson,A. and McMichael,A. (1995) Characterization of two Epstein–Barr virus epitopes restricted by HLA-B7. Eur. J. Immunol., 25, 18–24. [DOI] [PubMed] [Google Scholar]
- Hirano N., Shibasaki,F., Sakai,R., Tanaka,T., Nishida,J., Yazaki,Y., Takenawa,T. and Hirai,H. (1995) Molecular cloning of the human glucose-regulated protein ERp57/GRP58, a thiol-dependent reductase. Identification of its secretory form and inducible expression by the oncogenic transformation. Eur. J. Biochem., 234, 336–342. [DOI] [PubMed] [Google Scholar]
- Hughes E.A. and Cresswell,P. (1998) The thiol oxidoreductase ERp57 is a component of the MHC class I peptide-loading complex. Curr. Biol., 8, 709–712. [DOI] [PubMed] [Google Scholar]
- Hughes E.A., Hammond,C. and Cresswell,P. (1997) Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc. Natl Acad. Sci. USA, 94, 1896–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C., Sinskey,A.J. and Lodish,H.F. (1992) Oxidized redox state of glutathione in the endoplasmic reticulum. Science, 257, 1496–1502. [DOI] [PubMed] [Google Scholar]
- Kamhi-Nesher S., Shenkman,M., Tolchinsky,S., Fromm,S.V., Ehrlich,R. and Lederkremer,G.Z. (2001) A novel quality control compartment derived from the endoplasmic reticulum. Mol. Biol. Cell, 12, 1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmink J., Darby,N.J., Dijkstra,K., Nilges,M. and Creighton,T.E. (1997) The folding catalyst protein disulfide isomerase is constructed of active and inactive thioredoxin modules. Curr. Biol., 7, 239–245. [DOI] [PubMed] [Google Scholar]
- King A. et al. (2000) HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol., 30, 1623–1631. [DOI] [PubMed] [Google Scholar]
- Laboissiere M.C., Sturley,S.L. and Raines,R.T. (1995) The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J. Biol. Chem., 270, 28006–28009. [DOI] [PubMed] [Google Scholar]
- LaMantia M.L. and Lennarz,W.J. (1993) The essential function of yeast protein disulfide isomerase does not reside in its isomerase activity. Cell, 74, 899–908. [DOI] [PubMed] [Google Scholar]
- Lehner P.J. and Trowsdale,J. (1998) Antigen presentation: coming out gracefully. Curr. Biol., 8, R605–R608. [DOI] [PubMed] [Google Scholar]
- Lehner P.J., Surman,M.J. and Cresswell,P. (1998) Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity, 8, 221–231. [DOI] [PubMed] [Google Scholar]
- Lewis J.W. and Elliott,T. (1998) Evidence for successive peptide binding and quality control stages during MHC class I assembly. Curr. Biol., 8, 717–720. [DOI] [PubMed] [Google Scholar]
- Lewis J.W., Neisig,A., Neefjes,J. and Elliott,T. (1996) Point mutations in the alpha 2 domain of HLA-A2.1 define a functionally relevant interaction with TAP. Curr. Biol., 6, 873–883. [DOI] [PubMed] [Google Scholar]
- Lindquist J.A., Jensen,O.N., Mann,M. and Hammerling,G.J. (1998) ER-60, a chaperone with thiol-dependent reductase activity involved in MHC class I assembly. EMBO J., 17, 2186–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist J.A., Hammerling,G.J. and Trowsdale,J. (2001) ER60/ERp57 forms disulfide-bonded intermediates with MHC class I heavy chain. FASEB J., 15, 1448–1450. [DOI] [PubMed] [Google Scholar]
- Mear J.P., Schreiber,K.L., Munz,C., Zhu,X., Stevanovic,S., Rammensee,H.G., Rowland-Jones,S.L. and Colbert,R.A. (1999) Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J. Immunol., 163, 6665–6670. [PubMed] [Google Scholar]
- Molinari M. and Helenius,A. (2000) Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science, 288, 331–333. [DOI] [PubMed] [Google Scholar]
- Momburg F. et al. (1992) Proteasome subunits encoded by the major histocompatibility complex are not essential for antigen presentation. Nature, 360, 174–177. [DOI] [PubMed] [Google Scholar]
- Morrice N.A. and Powis,S.J. (1998) A role for the thiol-dependent reductase ERp57 in the assembly of MHC class I molecules. Curr. Biol., 8, 713–716. [DOI] [PubMed] [Google Scholar]
- Oliver J.D., van der Wal,F.J., Bulleid,N.J. and High,S. (1997) Interaction of the thiol-dependent reductase ERp57 with nascent glycoproteins. Science, 275, 86–88. [DOI] [PubMed] [Google Scholar]
- Oliver J.D., Roderick,H.L., Llewellyn,D.H. and High,S. (1999) ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Mol. Biol. Cell, 10, 2573–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E. and Cresswell,P. (1998) Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol., 16, 323–358. [DOI] [PubMed] [Google Scholar]
- Parham P. and Brodsky,F.M. (1981) Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum. Immunol., 3, 277–299. [DOI] [PubMed] [Google Scholar]
- Parham P., Barnstable,C.J. and Bodmer,W.F. (1979) Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J. Immunol., 123, 342–349. [PubMed] [Google Scholar]
- Peh C.A., Burrows,S.R., Barnden,M., Khanna,R., Cresswell,P., Moss,D.J. and McCluskey,J. (1998) HLA-B27-restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. Immunity, 8, 531–542. [DOI] [PubMed] [Google Scholar]
- Powis S.J. (1997) Major histocompatibility complex class I molecules interact with both subunits of the transporter associated with antigen processing, TAP1 and TAP2. Eur. J. Immunol., 27, 2744–2747. [DOI] [PubMed] [Google Scholar]
- Purcell A.W. et al. (2001) Quantitative and qualitative influences of tapasin on the class I peptide repertoire. J. Immunol., 166, 1016–1027. [DOI] [PubMed] [Google Scholar]
- Raposo G., van Santen,H.M., Leijendekker,R., Geuze,H.J. and Ploegh,H.L. (1995) Misfolded major histocompatibility complex class I molecules accumulate in an expanded ER–Golgi intermediate compartment. J. Cell Biol., 131, 1403–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribaudo R.K. and Margulies,D.H. (1992) Independent and synergistic effects of disulfide bond formation, β 2-microglobulin and peptides on class I MHC folding and assembly in an in vitro translation system. J. Immunol., 149, 2935–2944. [PubMed] [Google Scholar]
- Shamu C.E., Story,C.M., Rapoport,T.A. and Ploegh,H.L. (1999) The pathway of US11-dependent degradation of MHC class I heavy chains involves a ubiquitin-conjugated intermediate. J. Cell Biol., 147, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A., MacDonald,H.R., Conzelmann,A., Corthesy,P. and Nabholz,M. (1983) Rat X mouse T-cell hybrids with inducible specific cytolytic activity. Immunol. Rev., 76, 105–129. [DOI] [PubMed] [Google Scholar]
- Solheim J.C., Harris,M.R., Kindle,C.S. and Hansen,T.H. (1997) Prominence of β 2-microglobulin, class I heavy chain conformation and tapasin in the interactions of class I heavy chain with calreticulin and the transporter associated with antigen processing. J. Immunol., 158, 2236–2241. [PubMed] [Google Scholar]
- Srivastava S.P., Fuchs,J.A. and Holtzman,J.L. (1993) The reported cDNA sequence for phospholipase C alpha encodes protein disulfide isomerase, isozyme Q-2 and not phospholipase-C. Biochem. Biophys. Res. Commun., 193, 971–978. [DOI] [PubMed] [Google Scholar]
- Stam N.J., Spits,H. and Ploegh,H.L. (1986) Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J. Immunol., 137, 2299–2306. [PubMed] [Google Scholar]
- Tector M., Zhang,Q. and Salter,R.D. (1997) Beta 2-microglobulin and calnexin can independently promote folding and disulfide bond formation in class I histocompatibility proteins. Mol. Immunol., 34, 401–408. [DOI] [PubMed] [Google Scholar]
- Tortorella D., Story,C.M., Huppa,J.B., Wiertz,E.J., Jones,T.R., Bacik,I., Bennink,J.R., Yewdell,J.W. and Ploegh,H.L. (1998) Dislocation of type I membrane proteins from the ER to the cytosol is sensitive to changes in redox potential. J. Cell Biol., 142, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urade R., Oda,T., Ito,H., Moriyama,T., Utsumi,S. and Kito,M. (1997) Functions of characteristic Cys-Gly-His-Cys (CGHC) and Gln-Glu-Asp-Leu (QEDL) motifs of microsomal ER-60 protease. J. Biochem. (Tokyo), 122, 834–842. [DOI] [PubMed] [Google Scholar]
- Walker K.W., Lyles,M.M. and Gilbert,H.F. (1996) Catalysis of oxidative protein folding by mutants of protein disulfide isomerase with a single active-site cysteine. Biochemistry, 35, 1972–1980. [DOI] [PubMed] [Google Scholar]
- Wiertz E.J., Jones,T.R., Sun,L., Bogyo,M., Geuze,H.J. and Ploegh,H.L. (1996) The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell, 84, 769–779. [DOI] [PubMed] [Google Scholar]
- Zapun A., Darby,N.J., Tessier,D.C., Michalak,M., Bergeron,J.J. and Thomas,D.Y. (1998) Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J. Biol. Chem., 273, 6009–6012. [DOI] [PubMed] [Google Scholar]