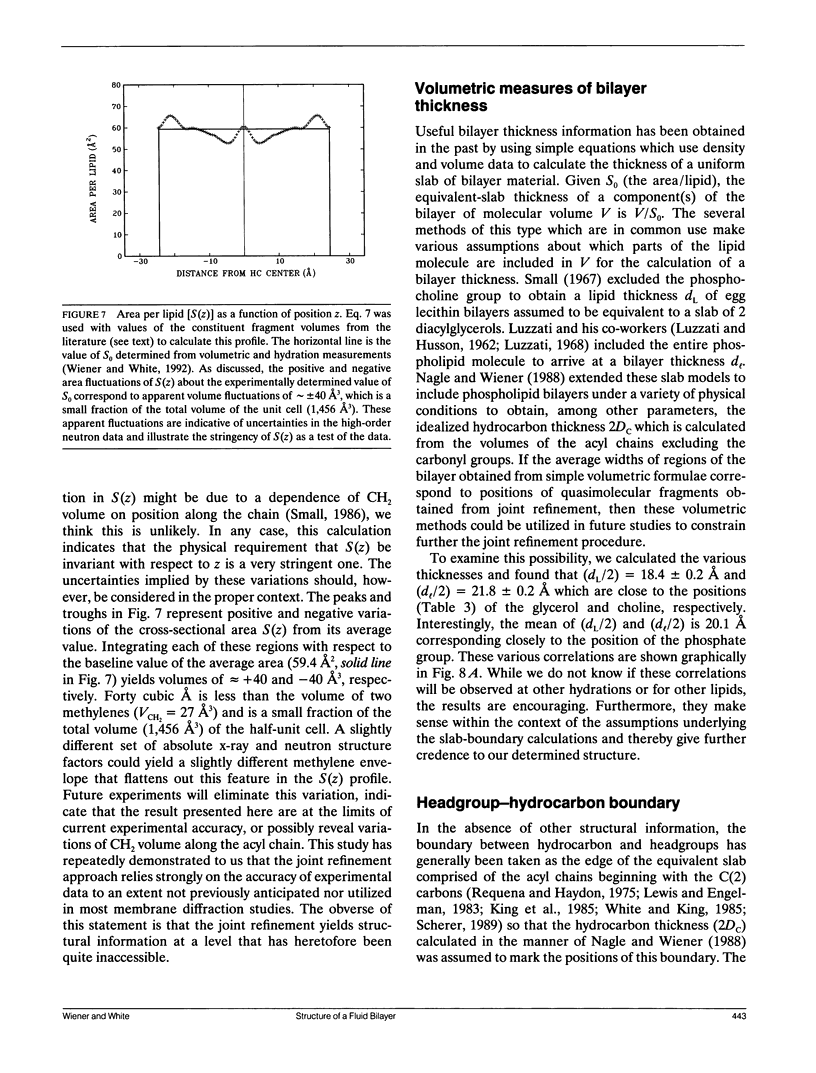
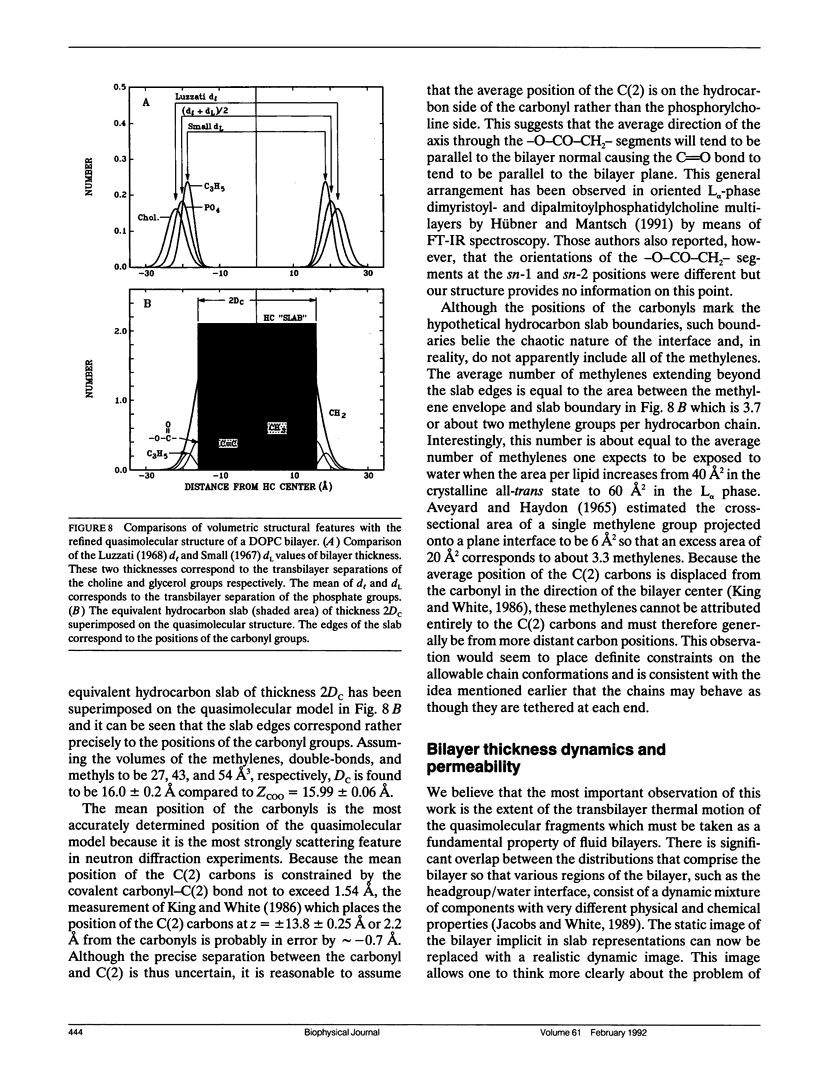
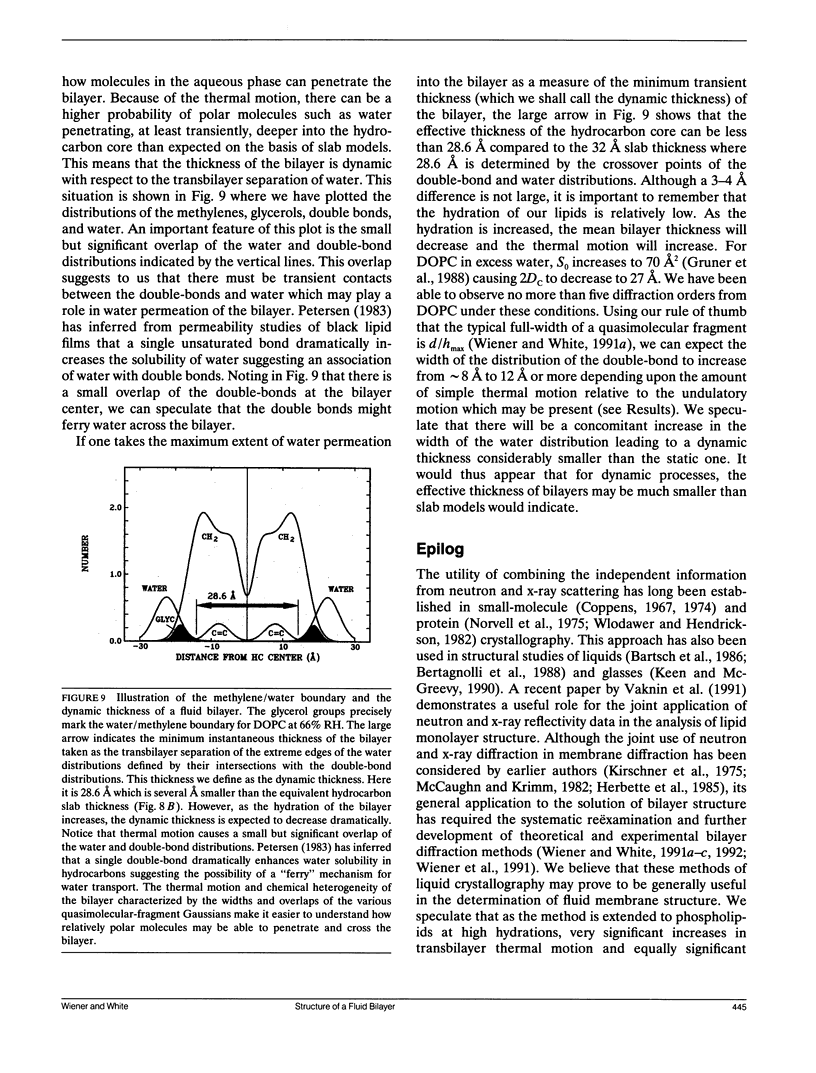
Abstract
We present in this paper the complete structure of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) in the L alpha phase (66% RH, 23 degrees C) obtained by the joint refinement of neutron and x-ray lamellar diffraction data. The structural details obtained have previously required a large number of neutron diffraction experiments, using numerous specifically-deuterated phospholipid isomorphs (Büldt et al., 1978. Nature (Lond.). 271:182-184). The joint-refinement approach minimizes specific deuteration by utilizing independent neutron and x-ray data sets. The method yields a quasimolecular structure consisting of a series of multiatomic fragments that are each represented by one or several Gaussian distributions whose positions and widths can be determined to within 0.06 to 0.52 A exclusive of the methylene region. The image of DOPC at 66% RH (5.36 +/- 0.08 waters per lipid) is consistent with many aspects of bilayer structure previously determined by structural and spectroscopic studies. The most striking feature of the structure is the large amount of transbilayer thermal motion suggested by the widths and overlaps of the Gaussian envelopes of the quasimolecular fragments. We discuss the "dynamic bilayer thickness" which describes the minimum effective thickness of the hydrocarbon permeability barrier in terms of the thermal motion of the water. A gradient of thermal motion exists that increases in either direction away from the glycerol backbone which is the most constrained portion of the bilayer. The steric interactions between headgroups of apposed bilayers, expected at the hydration level of our experiments, are clearly revealed. A useful consequence of the quasimolecular structure is that average boundaries within bilayers calculated using composition and volumetric data and ad hoc assumptions can be related to the positions of the principal structural groups. Several measures of "bilayer thickness" in common use can be identified as the positions of the cholines for Luzzati's d1 (Luzzati and Husson. 1962. J. Cell Biol. 12:207-219) and the glycerols for Small's dL (Small. 1967. J. Lipid Res. 8:551-556). We do not know if these relations will be true at other hydrations or for other lipids. Of particular interest is the fact that the position of the carbonyl groups marks the average hydrocarbon/headgroup boundary. It must be emphasized, however, that this region of the bilayer must be generally characterized as one of tumultuous chemical heterogeneity because of the thermal motion of the bilayer.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akutsu H., Nagamori T. Conformational analysis of the polar head group in phosphatidylcholine bilayers: a structural change induced by cations. Biochemistry. 1991 May 7;30(18):4510–4516. doi: 10.1021/bi00232a020. [DOI] [PubMed] [Google Scholar]
- Braach-Maksvytis V. L., Cornell B. A. Chemical shift anisotropies obtained from aligned egg yolk phosphatidylcholine by solid-state 13C nuclear magnetic resonance. Biophys J. 1988 May;53(5):839–843. doi: 10.1016/S0006-3495(88)83163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büldt G., Gally H. U., Seelig A., Seelig J., Zaccai G. Neutron diffraction studies on selectively deuterated phospholipid bilayers. Nature. 1978 Jan 12;271(5641):182–184. doi: 10.1038/271182a0. [DOI] [PubMed] [Google Scholar]
- Büldt G., Gally H. U., Seelig J., Zaccai G. Neutron diffraction studies on phosphatidylcholine model membranes. I. Head group conformation. J Mol Biol. 1979 Nov 15;134(4):673–691. doi: 10.1016/0022-2836(79)90479-0. [DOI] [PubMed] [Google Scholar]
- Coppens P. Comparative X-Ray and Neutron Diffraction Study of Bonding Effects in s-Triazine. Science. 1967 Dec 22;158(3808):1577–1579. doi: 10.1126/science.158.3808.1577. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Tate M. W., Kirk G. L., So P. T., Turner D. C., Keane D. T., Tilcock C. P., Cullis P. R. X-ray diffraction study of the polymorphic behavior of N-methylated dioleoylphosphatidylethanolamine. Biochemistry. 1988 Apr 19;27(8):2853–2866. doi: 10.1021/bi00408a029. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Herbette L., DeFoor P., Fleischer S., Pascolini D., Scarpa A., Blasie J. K. The separate profile structures of the functional calcium pump protein and the phospholipid bilayer within isolated sarcoplasmic reticulum membranes determined by X-ray and neutron diffraction. Biochim Biophys Acta. 1985 Jul 11;817(1):103–122. doi: 10.1016/0005-2736(85)90073-2. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. B., Mason R., Thomas K. M., Shipley G. G. Structural chemistry of 1,2 dilauroyl-DL-phosphatidylethanolamine: molecular conformation and intermolecular packing of phospholipids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3036–3040. doi: 10.1073/pnas.71.8.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner W., Mantsch H. H. Orientation of specifically 13C=O labeled phosphatidylcholine multilayers from polarized attenuated total reflection FT-IR spectroscopy. Biophys J. 1991 Jun;59(6):1261–1272. doi: 10.1016/S0006-3495(91)82341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. E., White S. H. The nature of the hydrophobic binding of small peptides at the bilayer interface: implications for the insertion of transbilayer helices. Biochemistry. 1989 Apr 18;28(8):3421–3437. doi: 10.1021/bi00434a042. [DOI] [PubMed] [Google Scholar]
- King G. I., Jacobs R. E., White S. H. Hexane dissolved in dioleoyllecithin bilayers has a partial molar volume of approximately zero. Biochemistry. 1985 Aug 13;24(17):4637–4645. doi: 10.1021/bi00338a024. [DOI] [PubMed] [Google Scholar]
- King G. I., White S. H. Determining bilayer hydrocarbon thickness from neutron diffraction measurements using strip-function models. Biophys J. 1986 May;49(5):1047–1054. doi: 10.1016/S0006-3495(86)83733-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUZZATI V., HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962 Feb;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- McCaughan L., Krimm S. Biochemical profiles of membranes from x-ray and neutron diffraction. Biophys J. 1982 Feb;37(2):417–426. doi: 10.1016/S0006-3495(82)84687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Steric repulsion between phosphatidylcholine bilayers. Biochemistry. 1987 Nov 17;26(23):7325–7332. doi: 10.1021/bi00397a020. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Wiener M. C. Structure of fully hydrated bilayer dispersions. Biochim Biophys Acta. 1988 Jul 7;942(1):1–10. doi: 10.1016/0005-2736(88)90268-4. [DOI] [PubMed] [Google Scholar]
- Norvell J. C., Nunes A. C., Schoenborn B. P. Neutron diffraction analysis of myoglobin: structure of the carbon monoxide derivative. Science. 1975 Nov 7;190(4214):568–570. doi: 10.1126/science.1188354. [DOI] [PubMed] [Google Scholar]
- Scherer J. R. On the position of the hydro-phobic/philic boundary in lipid bilayers. Biophys J. 1989 May;55(5):957–964. doi: 10.1016/S0006-3495(89)82894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota E. B., Smith G. S., Safinya C. R., Plano R. J., Clark N. A. X-ray Scattering Studies of Aligned, Stacked Surfactant Membranes. Science. 1988 Dec 9;242(4884):1406–1409. doi: 10.1126/science.242.4884.1406. [DOI] [PubMed] [Google Scholar]
- Small D. M. Phase equilibria and structure of dry and hydrated egg lecithin. J Lipid Res. 1967 Nov;8(6):551–557. [PubMed] [Google Scholar]
- Strenk L. M., Westerman P. W., Doane J. W. A model of orientational ordering in phosphatidylcholine bilayers based on conformational analysis of the glycerol backbone region. Biophys J. 1985 Nov;48(5):765–773. doi: 10.1016/S0006-3495(85)83834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Vaknin D., Kjaer K., Als-Nielsen J., Lösche M. Structural properties of phosphatidylcholine in a monolayer at the air/water interface: Neutron reflection study and reexamination of x-ray reflection measurements. Biophys J. 1991 Jun;59(6):1325–1332. doi: 10.1016/S0006-3495(91)82347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H., Jacobs R. E., King G. I. Partial specific volumes of lipid and water in mixtures of egg lecithin and water. Biophys J. 1987 Oct;52(4):663–665. doi: 10.1016/S0006-3495(87)83259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H., King G. I. Molecular packing and area compressibility of lipid bilayers. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6532–6536. doi: 10.1073/pnas.82.19.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., King G. I., White S. H. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and neutron diffraction data. I. Scaling of neutron data and the distributions of double bonds and water. Biophys J. 1991 Sep;60(3):568–576. doi: 10.1016/S0006-3495(91)82086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., White S. H. Fluid bilayer structure determination by the combined use of x-ray and neutron diffraction. I. Fluid bilayer models and the limits of resolution. Biophys J. 1991 Jan;59(1):162–173. doi: 10.1016/S0006-3495(91)82208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., White S. H. Fluid bilayer structure determination by the combined use of x-ray and neutron diffraction. II. "Composition-space" refinement method. Biophys J. 1991 Jan;59(1):174–185. doi: 10.1016/S0006-3495(91)82209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., White S. H. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and neutron diffraction data. II. Distribution and packing of terminal methyl groups. Biophys J. 1992 Feb;61(2):428–433. doi: 10.1016/S0006-3495(92)81848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., White S. H. Transbilayer distribution of bromine in fluid bilayers containing a specifically brominated analogue of dioleoylphosphatidylcholine. Biochemistry. 1991 Jul 16;30(28):6997–7008. doi: 10.1021/bi00242a027. [DOI] [PubMed] [Google Scholar]
- Zaccai G., Büldt G., Seelig A., Seelig J. Neutron diffraction studies on phosphatidylcholine model membranes. II. Chain conformation and segmental disorder. J Mol Biol. 1979 Nov 15;134(4):693–706. doi: 10.1016/0022-2836(79)90480-7. [DOI] [PubMed] [Google Scholar]