Abstract
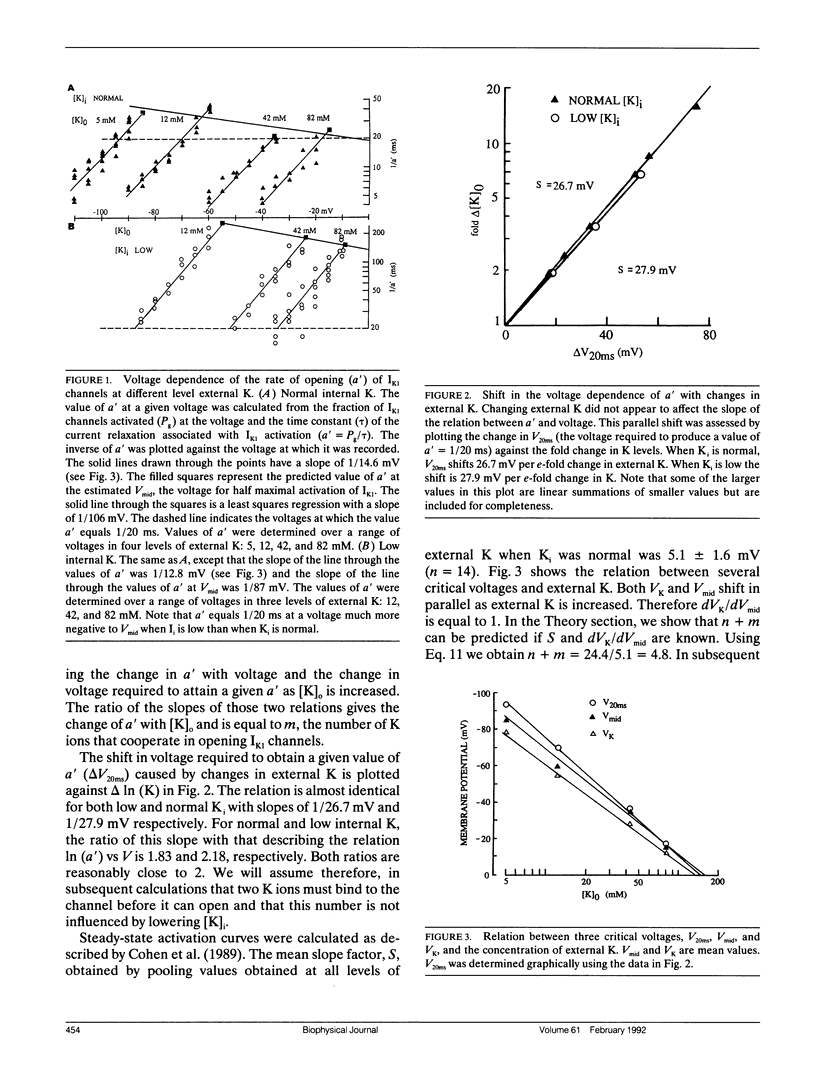
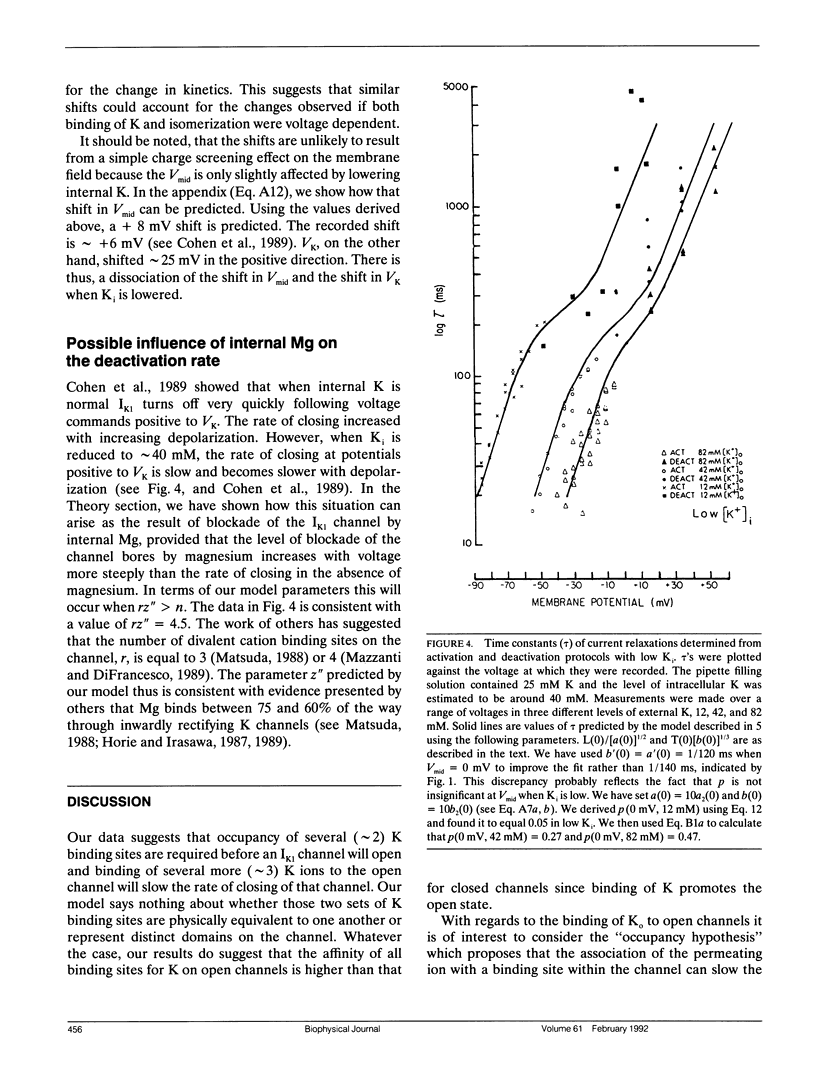
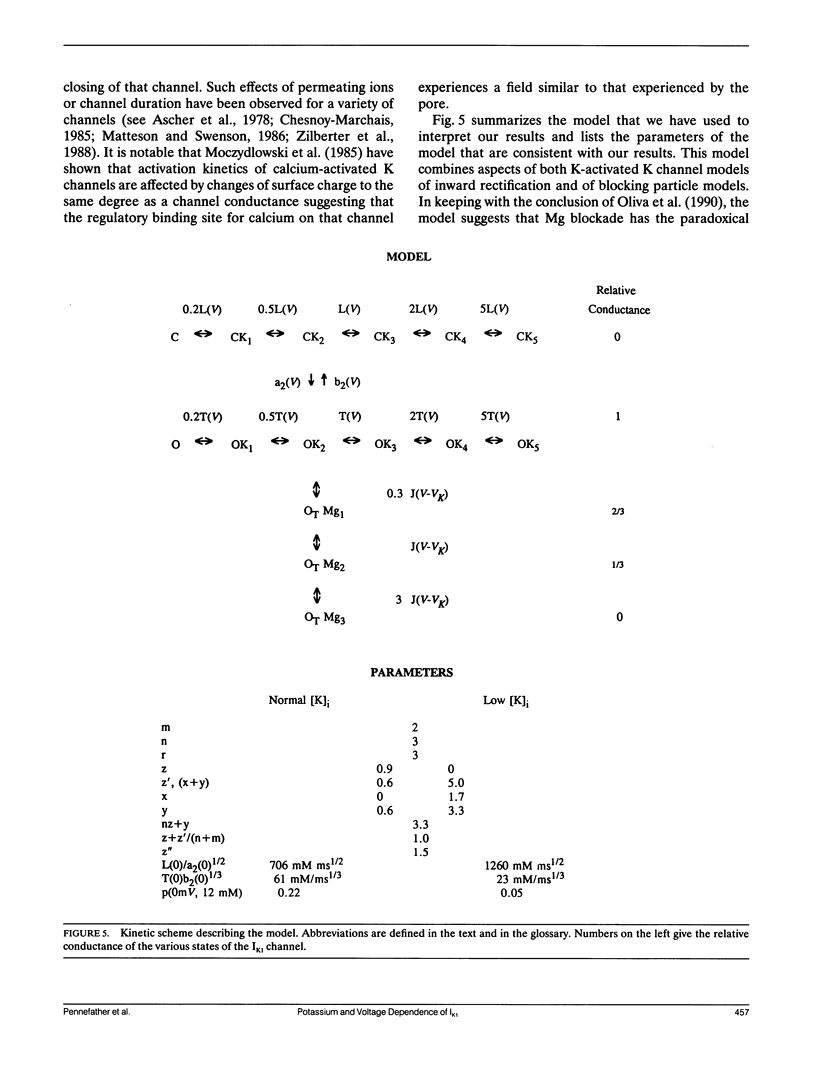
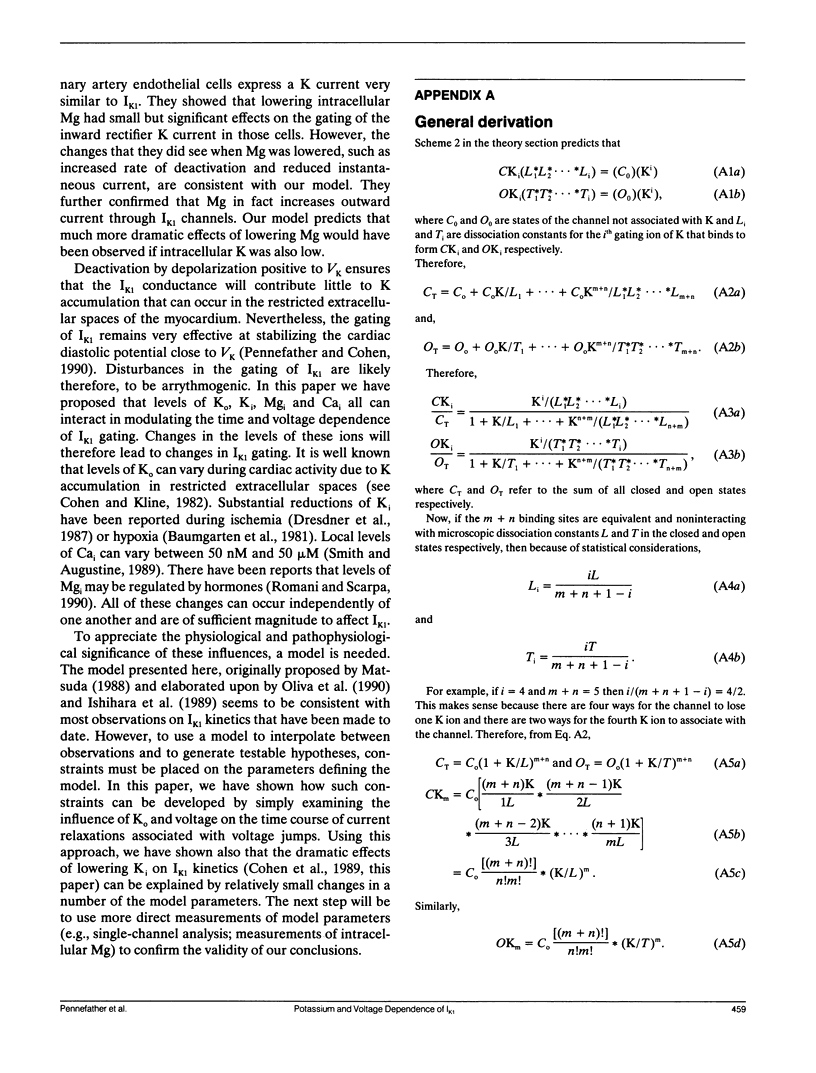
Using various voltage clamp protocols, we have examined the activation and deactivation kinetics of IK1 recorded in dissociated myocytes obtained from canine purkinje fibers. Exponential current relaxations following step changes of the membrane potential were characterized at several different K levels (5, 12, 42, and 82 mM) and several voltages (K reversal potential +/- 40 mV). We have interpreted our data according to a K-activated, K-channel model of IK1 gating. Our data suggests that at least two binding sites for extracellular K must be occupied before the channel opens and occupancy of about three more higher affinity sites for K on the open channel will slow the closing of that channel. In our model, the voltage dependency of gating arises from a combination of three voltage dependent steps: (a) isomerization between open and closed states, (b) binding of K, and (c) occupancy of the channel by internal Mg. Lowering internal K to 40 mM causes major changes in the voltage and K dependence of IK1 gating. However, these changes could be accounted for in our model by relatively small (approximately 20 to 30 mV) shifts in the voltage dependence of several of the steps that govern gating. Our data further suggest that there is an interaction between both extracellular and intracellular K levels and the ability of intracellular Mg to block the IK1 channel.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J Gen Physiol. 1969 Nov;54(5):553–575. doi: 10.1085/jgp.54.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten C. M., Cohen C. J., McDonald T. F. Heterogeneity of intracellular potassium activity and membrane potential in hypoxic guinea pig ventricle. Circ Res. 1981 Nov;49(5):1181–1189. doi: 10.1161/01.res.49.5.1181. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Induction and removal of inward-going rectification in sheep cardiac Purkinje fibres. J Physiol. 1982 Jun;327:285–308. doi: 10.1113/jphysiol.1982.sp014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy-Marchais D. Kinetic properties and selectivity of calcium-permeable single channels in Aplysia neurones. J Physiol. 1985 Oct;367:457–488. doi: 10.1113/jphysiol.1985.sp015835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani S., Krasne S., Miyazaki S., Hagiwara S. A model for anomalous rectification: electrochemical-potential-dependent gating of membrane channels. J Membr Biol. 1978 Dec 15;44(2):103–134. doi: 10.1007/BF01976035. [DOI] [PubMed] [Google Scholar]
- Cohen I. S., DiFrancesco D., Mulrine N. K., Pennefather P. Internal and external K+ help gate the inward rectifier. Biophys J. 1989 Jan;55(1):197–202. doi: 10.1016/S0006-3495(89)82792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Kline R. K+ fluctuations in the extracellular spaces of cardiac muscle. Evidence from the voltage clamp and extracellular K+ - selective microelectrodes. Circ Res. 1982 Jan;50(1):1–16. [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresdner K. P., Kline R. P., Wit A. L. Intracellular K+ activity, intracellular Na+ activity and maximum diastolic potential of canine subendocardial Purkinje cells from one-day-old infarcts. Circ Res. 1987 Jan;60(1):122–132. doi: 10.1161/01.res.60.1.122. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Ten Eick R. E. Characterization of the inward-rectifying potassium current in cat ventricular myocytes. J Gen Physiol. 1988 Apr;91(4):593–615. doi: 10.1085/jgp.91.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Rectification of muscarinic K+ current by magnesium ion in guinea pig atrial cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H210–H214. doi: 10.1152/ajpheart.1987.253.1.H210. [DOI] [PubMed] [Google Scholar]
- Ishihara K., Mitsuiye T., Noma A., Takano M. The Mg2+ block and intrinsic gating underlying inward rectification of the K+ current in guinea-pig cardiac myocytes. J Physiol. 1989 Dec;419:297–320. doi: 10.1113/jphysiol.1989.sp017874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985 Sep;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Effects of external and internal K+ ions on magnesium block of inwardly rectifying K+ channels in guinea-pig heart cells. J Physiol. 1991 Apr;435:83–99. doi: 10.1113/jphysiol.1991.sp018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Magnesium gating of the inwardly rectifying K+ channel. Annu Rev Physiol. 1991;53:289–298. doi: 10.1146/annurev.ph.53.030191.001445. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Matsuura H., Noma A. Triple-barrel structure of inwardly rectifying K+ channels revealed by Cs+ and Rb+ block in guinea-pig heart cells. J Physiol. 1989 Jun;413:139–157. doi: 10.1113/jphysiol.1989.sp017646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol. 1988 Mar;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Swenson R. P., Jr External monovalent cations that impede the closing of K channels. J Gen Physiol. 1986 May;87(5):795–816. doi: 10.1085/jgp.87.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Ca modulates outward current through IK1 channels. J Membr Biol. 1990 Jun;116(1):41–45. doi: 10.1007/BF01871670. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DiFrancesco D. Intracellular Ca modulates K-inward rectification in cardiac myocytes. Pflugers Arch. 1989 Jan;413(3):322–324. doi: 10.1007/BF00583549. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Alvarez O., Vergara C., Latorre R. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J Membr Biol. 1985;83(3):273–282. doi: 10.1007/BF01868701. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Latorre R. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J Gen Physiol. 1983 Oct;82(4):511–542. doi: 10.1085/jgp.82.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Freudenrich C. C., Levy L. A., London R. E., Lieberman M. Monitoring cytosolic free magnesium in cultured chicken heart cells by use of the fluorescent indicator Furaptra. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2981–2984. doi: 10.1073/pnas.86.8.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva C., Cohen I. S., Pennefather P. The mechanism of rectification of iK1 in canine Purkinje myocytes. J Gen Physiol. 1990 Aug;96(2):299–318. doi: 10.1085/jgp.96.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani A., Scarpa A. Hormonal control of Mg2+ transport in the heart. Nature. 1990 Aug 30;346(6287):841–844. doi: 10.1038/346841a0. [DOI] [PubMed] [Google Scholar]
- Saigusa A., Matsuda H. Outward currents through the inwardly rectifying potassium channel of guinea-pig ventricular cells. Jpn J Physiol. 1988;38(1):77–91. doi: 10.2170/jjphysiol.38.77. [DOI] [PubMed] [Google Scholar]
- Silver M. R., DeCoursey T. E. Intrinsic gating of inward rectifier in bovine pulmonary artery endothelial cells in the presence or absence of internal Mg2+. J Gen Physiol. 1990 Jul;96(1):109–133. doi: 10.1085/jgp.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R., Nakajima Y., Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985 Jun 6;315(6019):498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y., Burnashev N., Papin A., Portnov V., Khodorov B. Gating kinetics of ATP-sensitive single potassium channels in myocardial cells depends on electromotive force. Pflugers Arch. 1988 May;411(5):584–589. doi: 10.1007/BF00582382. [DOI] [PubMed] [Google Scholar]


