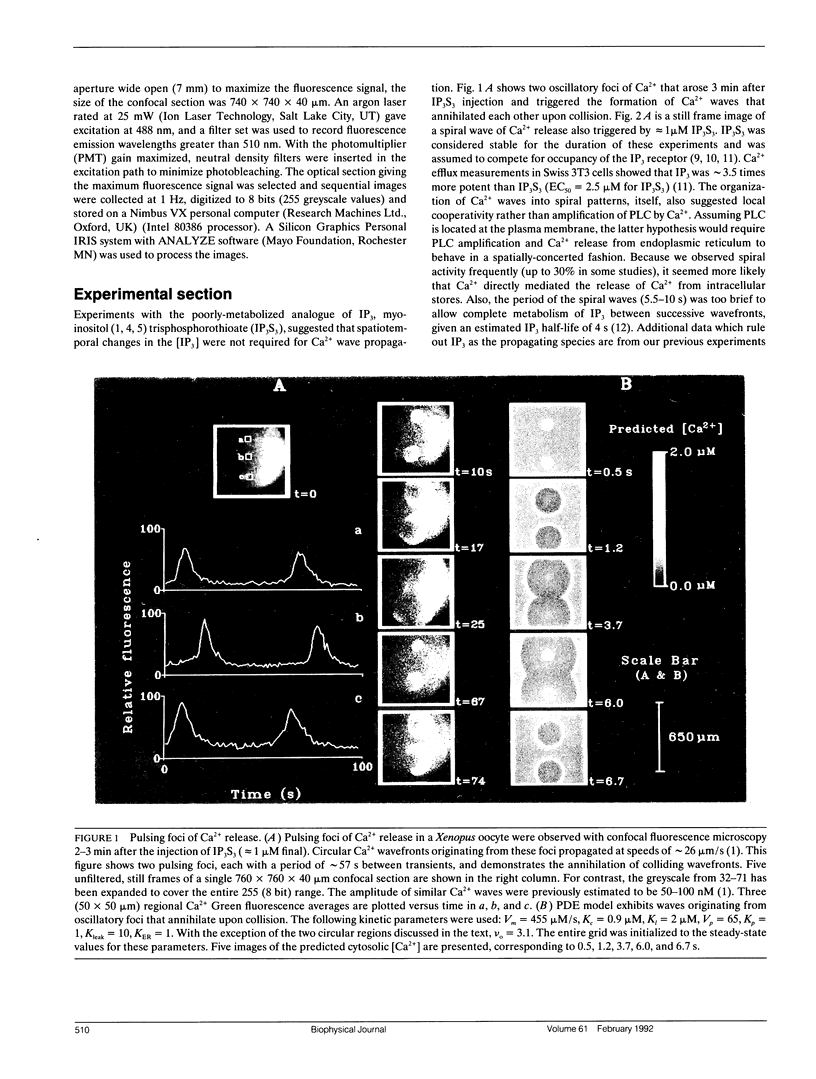
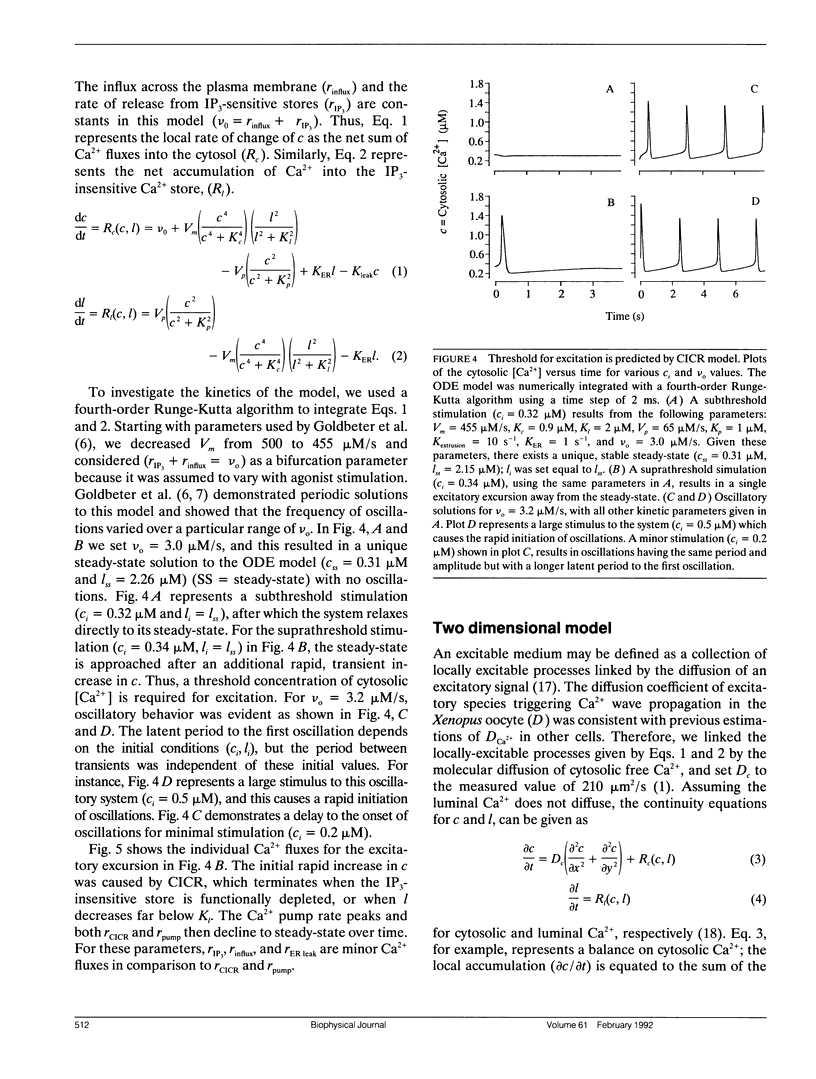
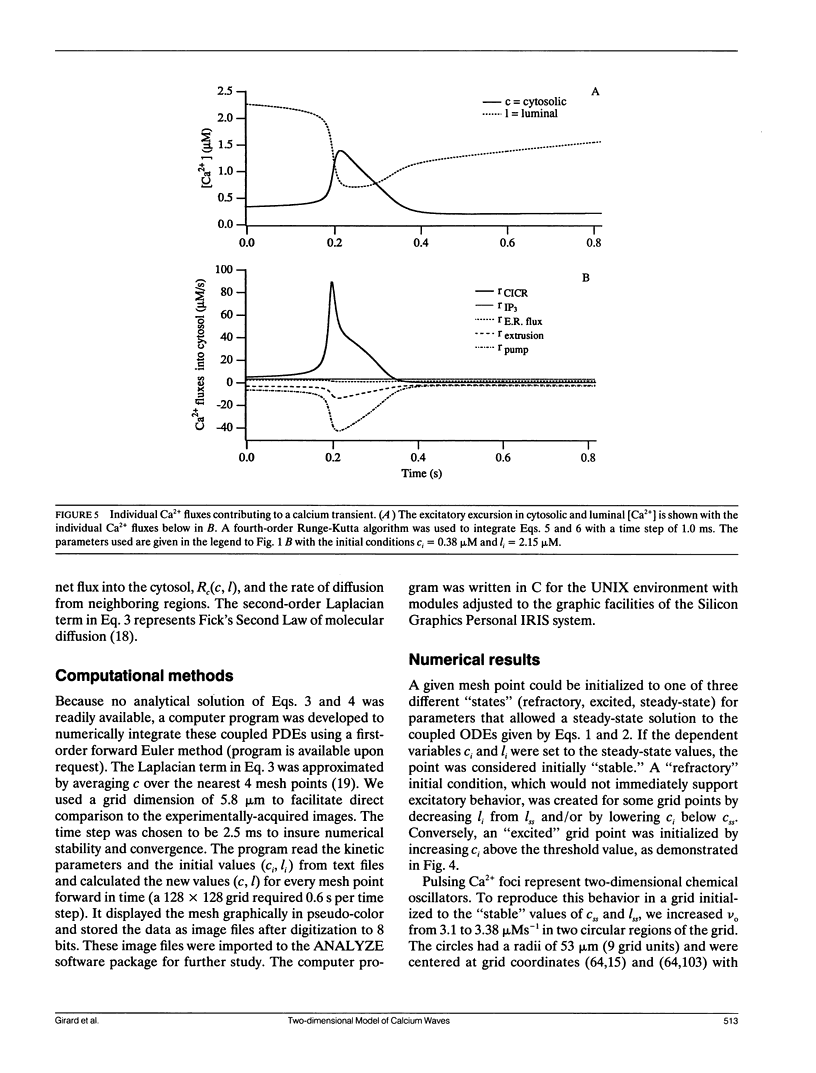
Abstract
Biological excitability enables the rapid transmission of physiological signals over distance. Using confocal fluorescence microscopy, we previously reported circular, planar, and spiral waves of Ca2+ in Xenopus laevis oocytes that annihilated one another upon collision. We present experimental evidence that the excitable process underlying wave propagation depends on Ca2+ diffusion and does not require oscillations in inositol (1,4,5)trisphosphate (IP3) concentration. Extending an existing ordinary differential equation (ODE) model of Ca2+ oscillations to two spatial dimensions, we develop a partial differential equation (PDE) model of Ca2+ excitability. The model assumes that cytosolic Ca2+ couples neighboring Ca2+ release sites. This simple PDE model qualitatively reproduces our experimental observations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backx P. H., de Tombe P. P., Van Deen J. H., Mulder B. J., ter Keurs H. E. A model of propagating calcium-induced calcium release mediated by calcium diffusion. J Gen Physiol. 1989 May;93(5):963–977. doi: 10.1085/jgp.93.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Dupont G., Berridge M. J., Goldbeter A. Signal-induced Ca2+ oscillations: properties of a model based on Ca(2+)-induced Ca2+ release. Cell Calcium. 1991 Feb-Mar;12(2-3):73–85. doi: 10.1016/0143-4160(91)90010-c. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Gerhardt M., Schuster H., Tyson J. J. A cellular automation model of excitable media including curvature and dispersion. Science. 1990 Mar 30;247(4950):1563–1566. doi: 10.1126/science.2321017. [DOI] [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M. R., Flora J. S., Potter B. V. myo-Inositol phosphorothioates, phosphatase-resistant analogues of myo-inositol phosphates. Synthesis of DL-myo-inositol 1,4-bisphosphate and DL-myo-inositol 1,4-bisphosphorothioate. Biochem J. 1987 Sep 15;246(3):771–774. doi: 10.1042/bj2460771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intracellular diffusion in the presence of mobile buffers. Application to proton movement in muscle. Biophys J. 1990 Apr;57(4):717–721. doi: 10.1016/S0006-3495(90)82592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Takeshita S. Simulation of intracellular Ca2+ oscillation in a sympathetic neurone. J Theor Biol. 1981 Dec 21;93(4):1009–1031. doi: 10.1016/0022-5193(81)90352-0. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Berridge M. J., Brown K. D., Cooke A. M., Potter B. V. DL-myo-inositol 1,4,5-trisphosphorothioate mobilizes intracellular calcium in Swiss 3T3 cells and Xenopus oocytes. Biochem Biophys Res Commun. 1988 Jan 29;150(2):626–632. doi: 10.1016/0006-291x(88)90438-x. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y. Oscillatory Ca2+ signaling and its cellular function. New Biol. 1991 Jan;3(1):3–17. [PubMed] [Google Scholar]
- Willcocks A. L., Potter B. V., Cooke A. M., Nahorski S. R. Myo-inositol(1,4,5)trisphosphorothioate binds to specific [3H]inositol(1,4,5)trisphosphate sites in rat cerebellum and is resistant to 5-phosphatase. Eur J Pharmacol. 1988 Oct 11;155(1-2):181–183. doi: 10.1016/0014-2999(88)90420-7. [DOI] [PubMed] [Google Scholar]