Abstract
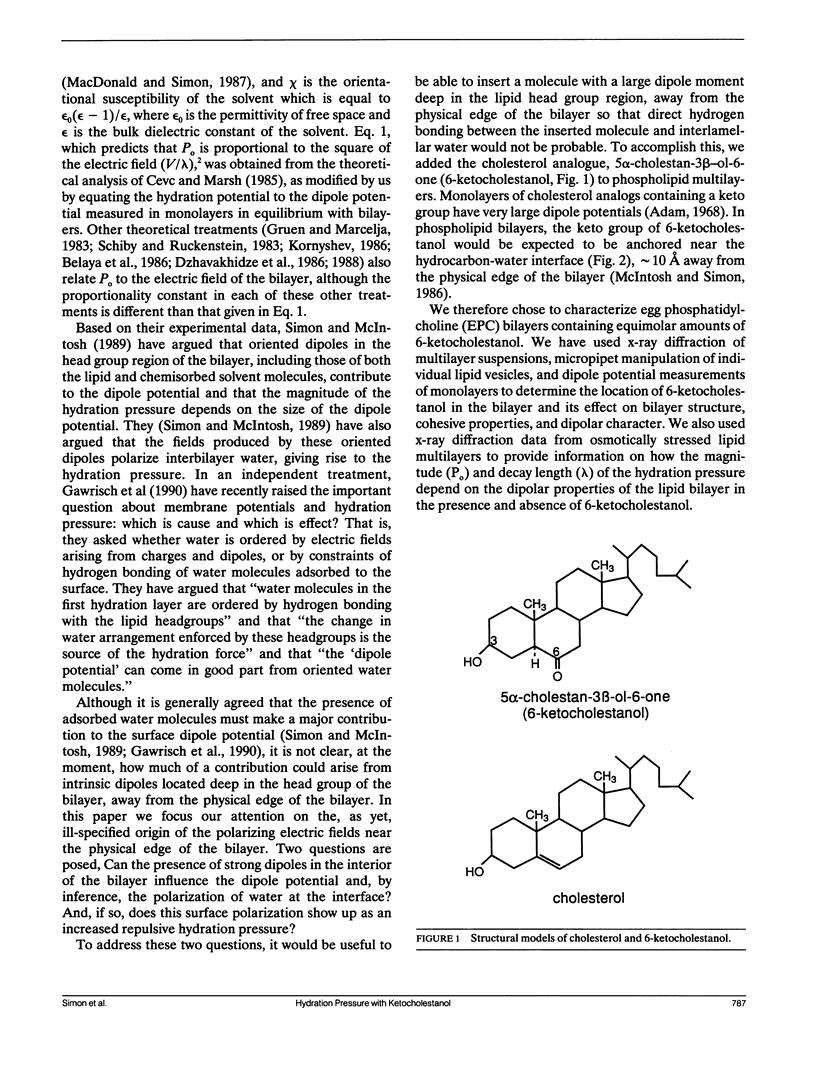
The effects of the cholesterol analog 5 alpha-cholestan-3 beta-ol-6-one (6-ketocholestanol) on bilayer structure, bilayer cohesive properties, and interbilayer repulsive pressures have been studied by a combination of x-ray diffraction, pipette aspiration, and dipole potential experiments. It is found that 6-ketocholestanol, which has a similar structure to cholesterol except with a keto moiety at the 6 position of the B ring, has quite different effects than cholesterol on bilayer organization and cohesive properties. Unlike cholesterol, 6-ketocholestanol does not appreciably modify the thickness of liquid-crystalline egg phosphatidylcholine (EPC) bilayers, and causes a much smaller increase in bilayer compressibility modulus than does cholesterol. These data imply that 6-ketocholestanol has both its hydroxyl and keto moieties situated near the water-hydrocarbon interface, thus making its orientation in the bilayer different from cholesterol's. The addition of equimolar 6-ketocholestanol into EPC bilayers increases the magnitude, but not the decay length, of the exponentially decaying repulsive hydration pressure between adjacent bilayers. Incorporation of equimolar 6-ketocholestanol into EPC monolayers increases the dipole potential by approximately 300 mV. These data are consistent with our previous observation that the magnitude of the hydration pressure is proportional to the square of the dipole potential. These results mean that 6-ketocholestanol, despite its location in the bilayer hydrocarbon region, approximately 10 A from the physical edge of the bilayer, modifies the organization of interlamellar water. We argue that the incorporation of 6-ketocholestanol into EPC bilayers increases the hydration pressure, at least in part, by increasing the electric field strength in the polar head group region.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S., Feldberg S., Nakadomari H., Levy S., McLaughlin S. Electrostatic interactions among hydrophobic ions in lipid bilayer membranes. Biophys J. 1978 Jan;21(1):35–70. doi: 10.1016/S0006-3495(78)85507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechinger B., Seelig J. Interaction of electric dipoles with phospholipid head groups. A 2H and 31P NMR study of phloretin and phloretin analogues in phosphatidylcholine membranes. Biochemistry. 1991 Apr 23;30(16):3923–3929. doi: 10.1021/bi00230a017. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Worthington C. R. Treatment of low angle x-ray data from planar and concentric multilayered structures. Biophys J. 1966 May;6(3):305–312. doi: 10.1016/S0006-3495(66)86658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. F., Seelig J. Influence of cholesterol on the polar region of phosphatidylcholine and phosphatidylethanolamine bilayers. Biochemistry. 1978 Jan 24;17(2):381–384. doi: 10.1021/bi00595a029. [DOI] [PubMed] [Google Scholar]
- Cevc G., Marsh D. Hydration of noncharged lipid bilayer membranes. Theory and experiments with phosphatidylethanolamines. Biophys J. 1985 Jan;47(1):21–31. doi: 10.1016/S0006-3495(85)83872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel R. A., Bruckdorfer K. R., van Deenen L. L. Structural requirements of sterols for the interaction with lecithin at the air water interface. Biochim Biophys Acta. 1972 Jan 17;255(1):311–320. doi: 10.1016/0005-2736(72)90030-2. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Parsegian V. A. Thermal-mechanical fluctuations enhance repulsion between bimolecular layers. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7132–7136. doi: 10.1073/pnas.83.19.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys J. 1986 Feb;49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. P. Structural analysis of hydrated egg lecithin and cholesterol bilayers. I. X-ray diffraction. J Mol Biol. 1976 Jan 25;100(3):345–358. doi: 10.1016/s0022-2836(76)80067-8. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hauser H., Shipley G. G. Comparative structural aspects of cation binding to phosphatidylserine bilayers. Biochim Biophys Acta. 1985 Mar 14;813(2):343–346. doi: 10.1016/0005-2736(85)90251-2. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Herbette L., Marquardt J., Scarpa A., Blasie J. K. A direct analysis of lamellar x-ray diffraction from hydrated oriented multilayers of fully functional sarcoplasmic reticulum. Biophys J. 1977 Nov;20(2):245–272. doi: 10.1016/S0006-3495(77)85547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. B., Mason R., Thomas K. M., Shipley G. G. Structural chemistry of 1,2 dilauroyl-DL-phosphatidylethanolamine: molecular conformation and intermolecular packing of phospholipids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3036–3040. doi: 10.1073/pnas.71.8.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner M., Cafiso D., Marcelja S., McLaughlin S. Electrostatics of phosphoinositide bilayer membranes. Theoretical and experimental results. Biophys J. 1990 Feb;57(2):335–349. doi: 10.1016/S0006-3495(90)82535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A., McLaughlin A., McLaughlin S. The adsorption of divalent cations to phosphatidylglycerol bilayer membranes. Biochim Biophys Acta. 1981 Jul 20;645(2):279–292. doi: 10.1016/0005-2736(81)90199-1. [DOI] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P. Measurement and modification of forces between lecithin bilayers. Biophys J. 1977 May;18(2):209–230. doi: 10.1016/S0006-3495(77)85608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesslauer W., Cain J. E., Blasie J. K. X-ray diffraction studies of lecithin bimolecular leaflets with incorporated fluorescent probes. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1499–1503. doi: 10.1073/pnas.69.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. C., Simon S. A. Lipid monolayer states and their relationships to bilayers. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4089–4093. doi: 10.1073/pnas.84.12.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Holloway P. W. Determination of the depth of bromine atoms in bilayers formed from bromolipid probes. Biochemistry. 1987 Mar 24;26(6):1783–1788. doi: 10.1021/bi00380a042. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Cholesterol modifies the short-range repulsive interactions between phosphatidylcholine membranes. Biochemistry. 1989 Jan 10;28(1):17–25. doi: 10.1021/bi00427a004. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Interactions between charged, uncharged, and zwitterionic bilayers containing phosphatidylglycerol. Biophys J. 1990 Jun;57(6):1187–1197. doi: 10.1016/S0006-3495(90)82638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Range of the solvation pressure between lipid membranes: dependence on the packing density of solvent molecules. Biochemistry. 1989 Sep 19;28(19):7904–7912. doi: 10.1021/bi00445a053. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Repulsive interactions between uncharged bilayers. Hydration and fluctuation pressures for monoglycerides. Biophys J. 1989 May;55(5):897–904. doi: 10.1016/S0006-3495(89)82888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Steric repulsion between phosphatidylcholine bilayers. Biochemistry. 1987 Nov 17;26(23):7325–7332. doi: 10.1021/bi00397a020. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta. 1978 Oct 19;513(1):43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- Needham D., Nunn R. S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990 Oct;58(4):997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltauf F., Hauser H., Phillips M. C. Monolayer characteristics of some 1,2-diacyl, I-alkyl-2-acyl and 1,2-dialkyl phospholipids at the air-water interface. Biochim Biophys Acta. 1971 Dec 3;249(2):539–547. doi: 10.1016/0005-2736(71)90129-5. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Fuller N. L., Rau D. C. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 1986;127:400–416. doi: 10.1016/0076-6879(86)27032-9. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Rau D. C., Lee B., Parsegian V. A. Measurement of the repulsive force between polyelectrolyte molecules in ionic solution: hydration forces between parallel DNA double helices. Proc Natl Acad Sci U S A. 1984 May;81(9):2621–2625. doi: 10.1073/pnas.81.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Parsegian V. A. Direct measurement of forces between linear polysaccharides xanthan and schizophyllan. Science. 1990 Sep 14;249(4974):1278–1281. doi: 10.1126/science.2144663. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Seelig J., Macdonald P. M., Scherer P. G. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 1987 Dec 1;26(24):7535–7541. doi: 10.1021/bi00398a001. [DOI] [PubMed] [Google Scholar]
- Sharp K. A., Nicholls A., Fine R. F., Honig B. Reconciling the magnitude of the microscopic and macroscopic hydrophobic effects. Science. 1991 Apr 5;252(5002):106–109. doi: 10.1126/science.2011744. [DOI] [PubMed] [Google Scholar]
- Simon S. A., Fink C. A., Kenworthy A. K., McIntosh T. J. The hydration pressure between lipid bilayers. Comparison of measurements using x-ray diffraction and calorimetry. Biophys J. 1991 Mar;59(3):538–546. doi: 10.1016/S0006-3495(91)82270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Depth of water penetration into lipid bilayers. Methods Enzymol. 1986;127:511–521. doi: 10.1016/0076-6879(86)27041-x. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J., Latorre R. Influence of cholesterol on water penetration into bilayers. Science. 1982 Apr 2;216(4541):65–67. doi: 10.1126/science.7063872. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Magnitude of the solvation pressure depends on dipole potential. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9263–9267. doi: 10.1073/pnas.86.23.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L. Surface dipole moments of lipids at the argon-water interface. Similarities among glycerol-ester-based lipids. Biophys J. 1990 Jul;58(1):195–204. doi: 10.1016/S0006-3495(90)82365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Worcester D. L., Franks N. P. Structural analysis of hydrated egg lecithin and cholesterol bilayers. II. Neutrol diffraction. J Mol Biol. 1976 Jan 25;100(3):359–378. doi: 10.1016/s0022-2836(76)80068-x. [DOI] [PubMed] [Google Scholar]



