Abstract
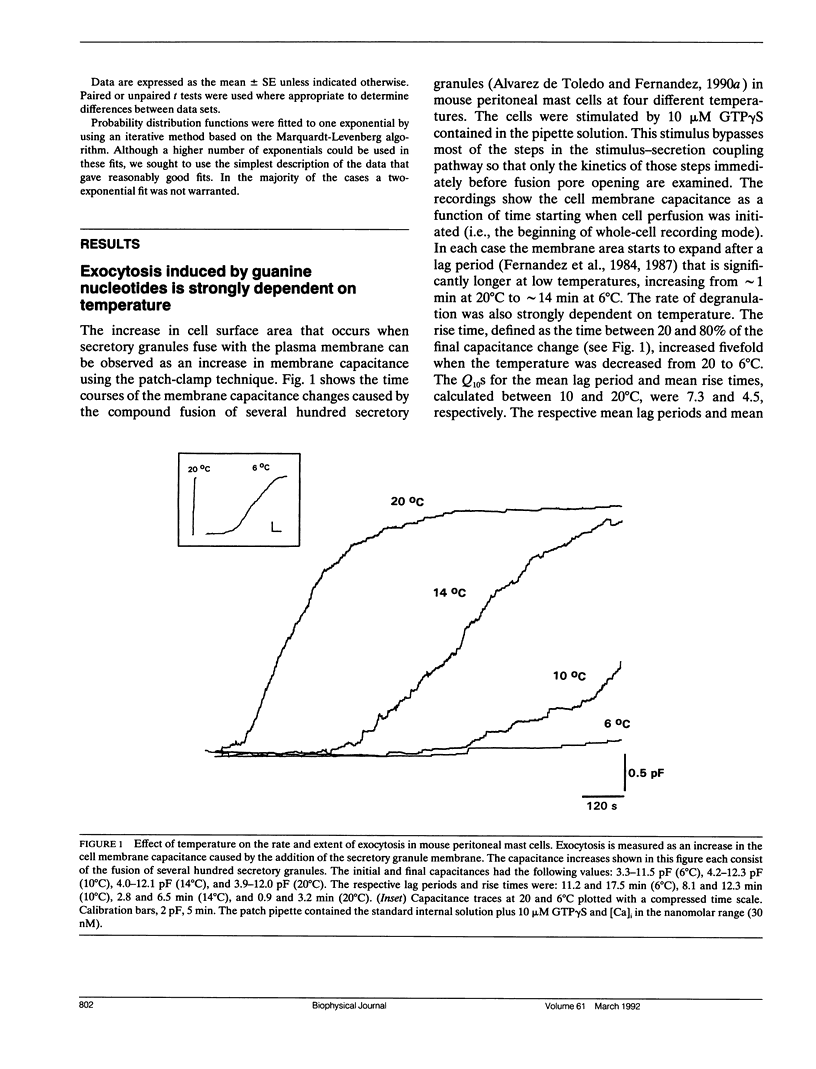
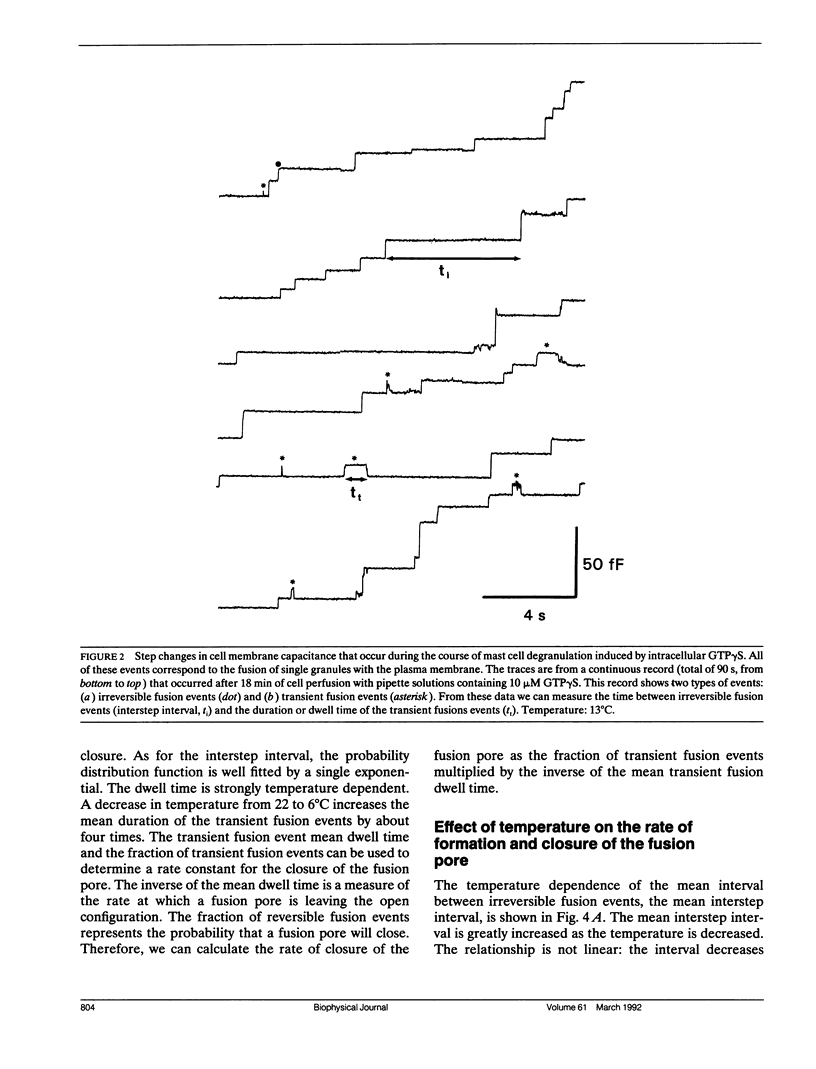
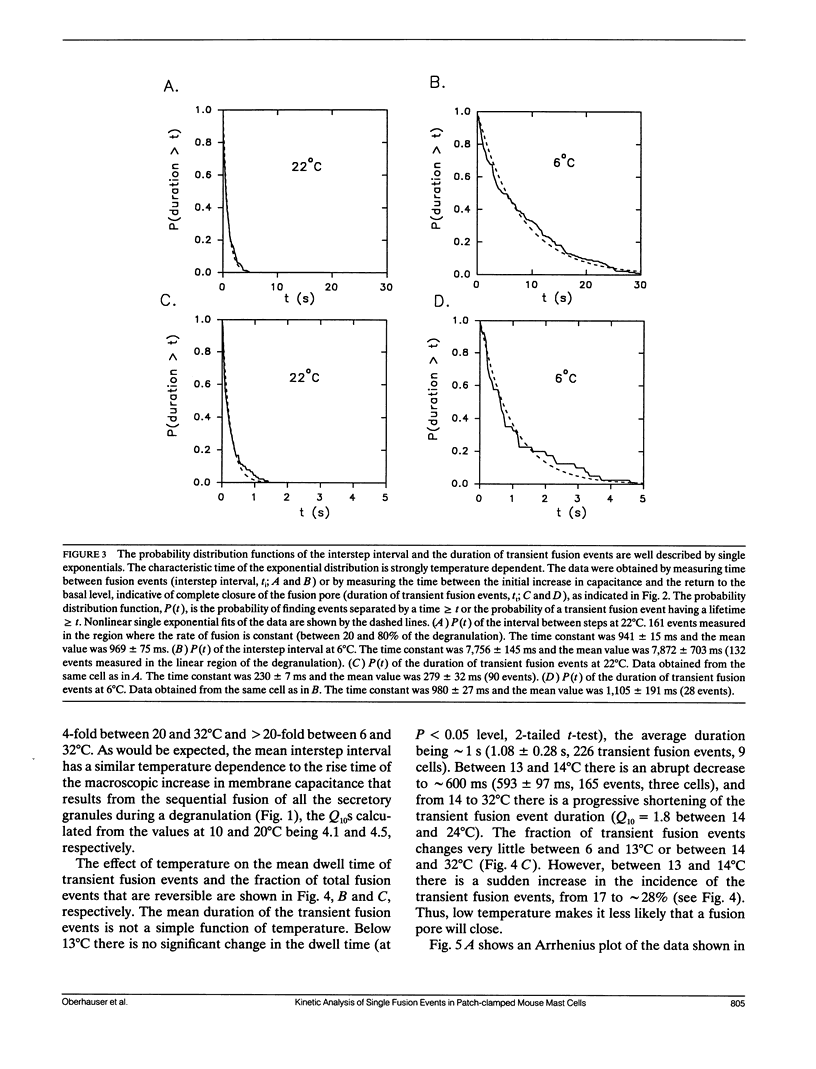
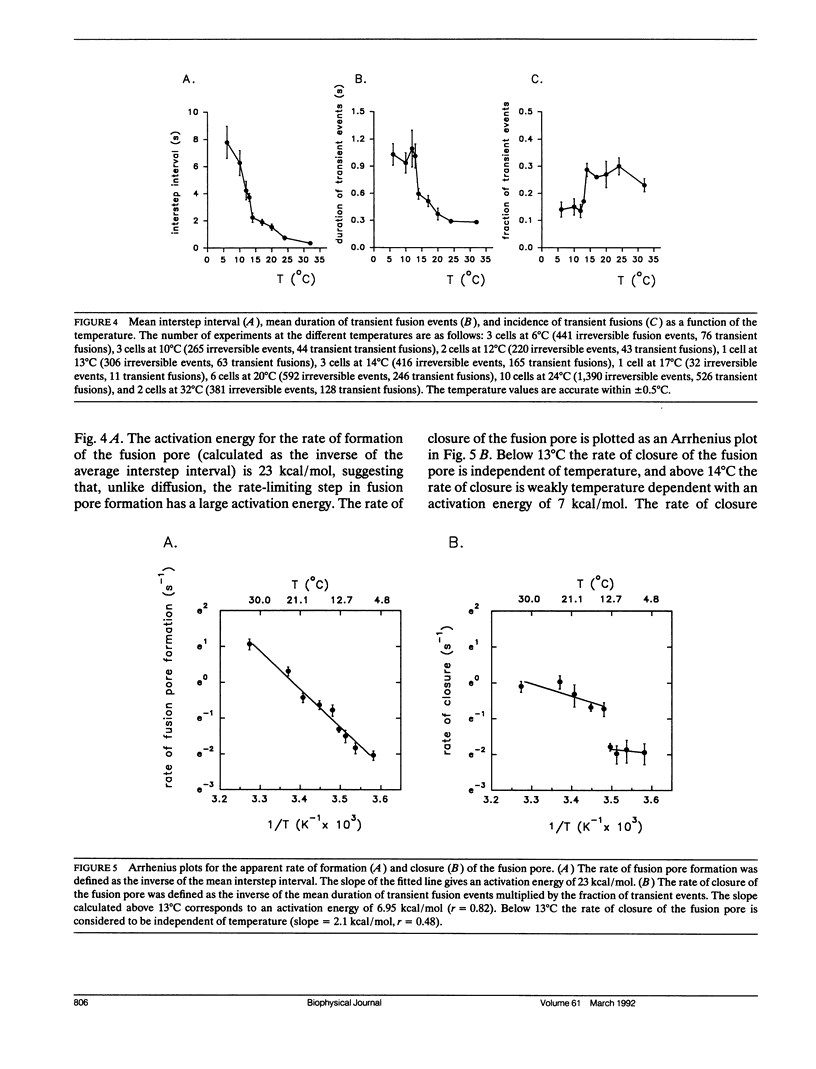
The earliest event in exocytosis is the formation of a fusion pore, an aqueous channel that connects the lumen of a secretory granule with the extracellular space. We can observe the formation of individual fusion pores and their subsequent dilation or closure by measuring the changes in the admittance of patch-clamped mast cells during GTP gamma S-stimulated exocytotic fusion. To investigate the molecular structure of the fusion pore, we have studied the temperature dependency of the rate constants for fusion pore formation and closure. An Arrhenius plot of the rate of fusion pore formation shows a simple linear relationship with an apparent activation energy of 23 kcal/mol. In contrast, the Arrhenius plot of the rate of closure of the fusion pore is discontinuous, with the break at approximately 13 degrees C. Above the break point, the rate of closure has a weak temperature dependence (7 kcal/mol), whereas below 13 degrees C the rate of closure is temperature independent. This type of temperature dependency is characteristic of events that depend on diffusion in a lipid phase that undergoes a fluid-solid phase transition. We propose that the formation of the fusion pore is regulated by the conformational change of a molecular structure with a high activation energy, whereas the closure of the fusion pore is regulated by lipids that become phase separated at 13 degrees C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albon N., Sturtevant J. M. Nature of the gel to liquid crystal transition of synthetic phosphatidylcholines. Proc Natl Acad Sci U S A. 1978 May;75(5):2258–2260. doi: 10.1073/pnas.75.5.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Exocytosis. Annu Rev Physiol. 1990;52:607–624. doi: 10.1146/annurev.ph.52.030190.003135. [DOI] [PubMed] [Google Scholar]
- Almers W., Tse F. W. Transmitter release from synapses: does a preassembled fusion pore initiate exocytosis? Neuron. 1990 Jun;4(6):813–818. doi: 10.1016/0896-6273(90)90134-2. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G., Fernandez J. M. Compound versus multigranular exocytosis in peritoneal mast cells. J Gen Physiol. 1990 Mar;95(3):397–409. doi: 10.1085/jgp.95.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de Toledo G., Fernandez J. M. Patch-clamp measurements reveal multimodal distribution of granule sizes in rat mast cells. J Cell Biol. 1990 Apr;110(4):1033–1039. doi: 10.1083/jcb.110.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de Toledo G., Fernandez J. M. The events leading to secretory granule fusion. Soc Gen Physiol Ser. 1988;43:333–344. [PubMed] [Google Scholar]
- Boheim G., Hanke W., Eibl H. Lipid phase transition in planar bilayer membrane and its effect on carrier- and pore-mediated ion transport. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3403–3407. doi: 10.1073/pnas.77.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. 1987 Aug 27-Sep 2Nature. 328(6133):814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Heuser J. E. Arrest of membrane fusion events in mast cells by quick-freezing. J Cell Biol. 1980 Aug;86(2):666–674. doi: 10.1083/jcb.86.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P., McIntyre J. O., Eibl H., Fleischer S. Activation of D-beta-hydroxybutyrate apodehydrogenase using molecular species of mixed fatty acyl phospholipids. J Biol Chem. 1983 Jan 10;258(1):208–214. [PubMed] [Google Scholar]
- Crowe J. H., Hoekstra F. A., Crowe L. M., Anchordoguy T. J., Drobnis E. Lipid phase transitions measured in intact cells with Fourier transform infrared spectroscopy. Cryobiology. 1989 Feb;26(1):76–84. doi: 10.1016/0011-2240(89)90035-7. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Lindau M., Eckstein F. Intracellular stimulation of mast cells with guanine nucleotides mimic antigenic stimulation. FEBS Lett. 1987 May 25;216(1):89–93. doi: 10.1016/0014-5793(87)80762-7. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner S. M. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M., Bers D. M. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim Biophys Acta. 1987 Aug 13;925(2):133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Hilton B. D., Woodward C. K. Nuclear magnetic resonance measurement of hydrogen exchange kinetics of single protons in basic pancreatic trypsin inhibitor. Biochemistry. 1978 Aug 8;17(16):3325–3332. doi: 10.1021/bi00609a024. [DOI] [PubMed] [Google Scholar]
- Hoffmann W., Sarzala M. G., Gomez-Fernandez J. C., Goni F. M., Restall C. J., Chapman D., Heppeler G., Kreutz W. Protein rotational diffusion and lipid structure of reconstituted systems of Ca2+-activated adenosine triphosphatase. J Mol Biol. 1980 Aug 5;141(2):119–132. doi: 10.1016/0022-2836(80)90380-0. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. J., Kleinfeld A. M., Hoover R. L., Klausner R. D. The concept of lipid domains in membranes. J Cell Biol. 1982 Jul;94(1):1–6. doi: 10.1083/jcb.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasne S., Eisenman G., Szabo G. Freezing and melting of lipid bilayers and the mode of action of nonactin, valinomycin, and gramicidin. Science. 1971 Oct 22;174(4007):412–415. doi: 10.1126/science.174.4007.412. [DOI] [PubMed] [Google Scholar]
- Laidler K. J., Peterman B. F. Temperature effects in enzyme kinetics. Methods Enzymol. 1979;63:234–257. doi: 10.1016/0076-6879(79)63012-4. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Chapman D. The effects of temperature on biological membranes and their models. Symp Soc Exp Biol. 1987;41:35–52. [PubMed] [Google Scholar]
- Markin V. S., Kozlov M. M., Borovjagin V. L. On the theory of membrane fusion. The stalk mechanism. Gen Physiol Biophys. 1984 Oct;3(5):361–377. [PubMed] [Google Scholar]
- Monck J. R., Alvarez de Toledo G., Fernandez J. M. Tension in secretory granule membranes causes extensive membrane transfer through the exocytotic fusion pore. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7804–7808. doi: 10.1073/pnas.87.20.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Characterization of lipid domains in erythrocyte membranes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1364–1368. doi: 10.1073/pnas.88.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971 Nov;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Breckenridge L. J., Lee A. K., Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990 May;4(5):643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Tocanne J. F., Dupou-Cézanne L., Lopez A., Tournier J. F. Lipid lateral diffusion and membrane organization. FEBS Lett. 1989 Oct 23;257(1):10–16. doi: 10.1016/0014-5793(89)81774-0. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Melo E. C., Thompson T. E. Translational diffusion and fluid domain connectivity in a two-component, two-phase phospholipid bilayer. Biophys J. 1989 Nov;56(5):869–876. doi: 10.1016/S0006-3495(89)82733-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiel E., Edidin M. Micrometer-scale domains in fibroblast plasma membranes. J Cell Biol. 1987 Aug;105(2):755–760. doi: 10.1083/jcb.105.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]


