Abstract
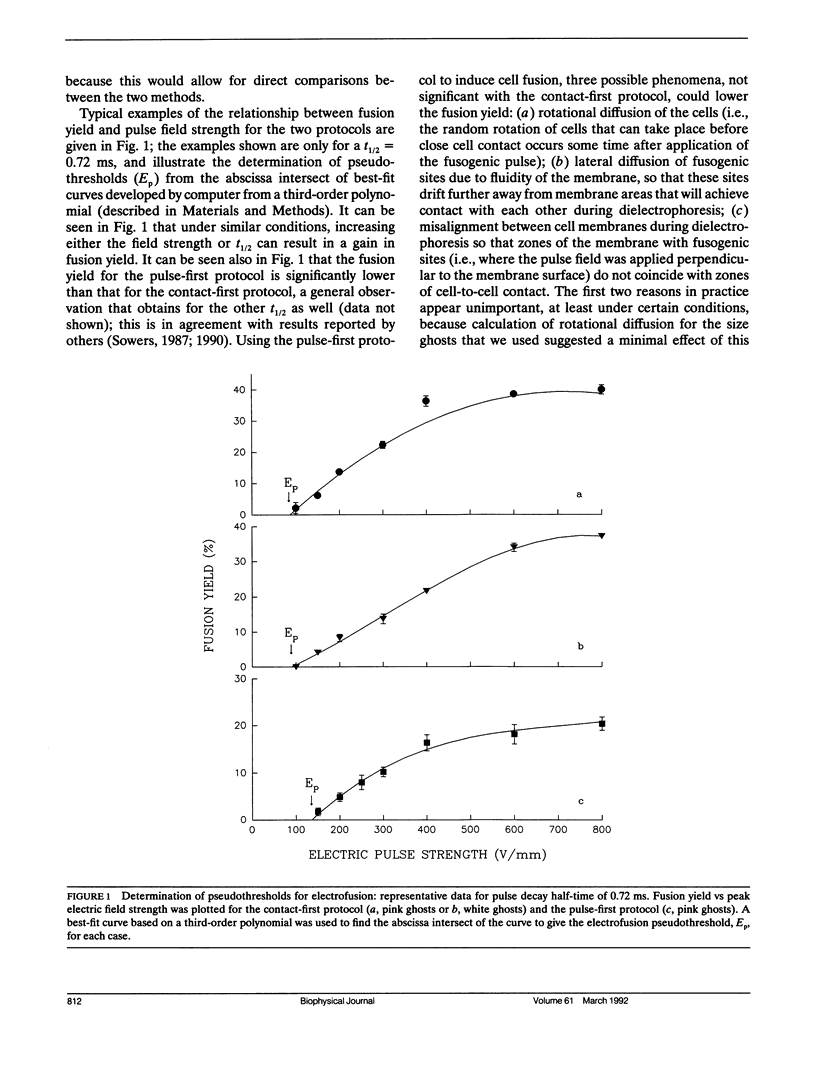
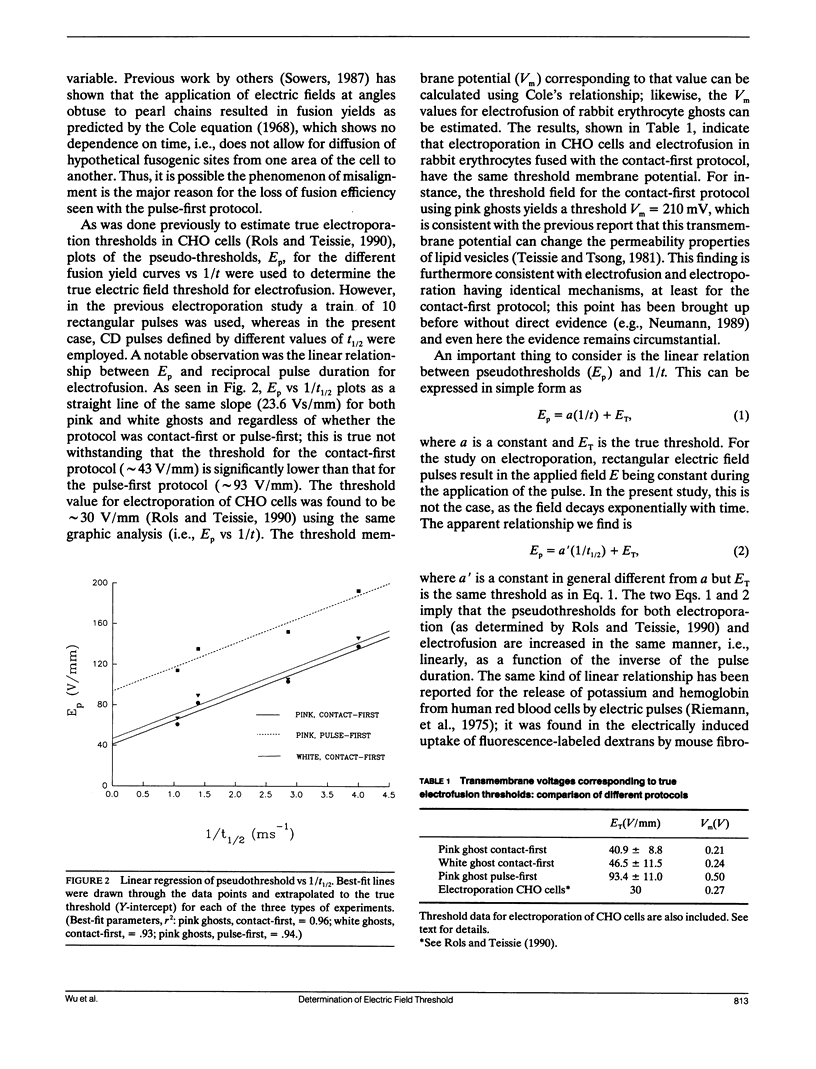
Rabbit erythrocyte ghosts were fused by means of electric pulses to determine the electrofusion thresholds for these membranes. Two protocols were used to investigate fusion events: contact-first, and pulse-first. Electrical capacitance discharge (CD) pulses were used to induce fusion. Plots of fusion yield vs peak field strength yielded curves that intersected the field strength axis at positive values (pseudothresholds) which depended on the protocol and decay half time of the pulses. It was found that plots of pseudothreshold vs reciprocal half time were linear for each protocol; when extrapolated to reciprocal half time = 0 (i.e., t----infinity), these lines intersected the ordinate at values of the field strength considered to be the true electrofusion thresholds. In this fashion, the contact-first protocol gave an electrofusion threshold of 46.5 +/- 11.5 V/mm for hemoglobin-free ghosts (white ghosts) and 40.9 +/- 8.8 V/mm for ghosts with fractional hemoglobin (pink ghosts), while the threshold for the pulse-first protocol applied to pink ghosts was determined to be 93.4 +/- 11.0 V/mm. Although the thresholds depended on the electrofusion protocol, plots of critical field strength vs reciprocal time had the same slopes, i.e., approximately 24 Vs/mm. The results suggest that the fusogenic state induced by an electric pulse in either the contact-first protocol or the pulse-first protocol (long-lived fusogenic state) may in fact share a common mechanism, if the two states are not actually identical.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowley J. M. Electrical breakdown of bimolecular lipid membranes as an electromechanical instability. Biophys J. 1973 Jul;13(7):711–724. doi: 10.1016/S0006-3495(73)86017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S. Electric field-induced breakdown of lipid bilayers and cell membranes: a thin viscoelastic film model. J Membr Biol. 1984;78(1):53–60. doi: 10.1007/BF01872532. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Sowers A. E. A delay in membrane fusion: lag times observed by fluorescence microscopy of individual fusion events induced by an electric field pulse. Biochemistry. 1990 Sep 11;29(36):8337–8344. doi: 10.1021/bi00488a020. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977 Aug 4;268(5619):438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- Kubiniec R. T., Liang H., Hui S. W. Effects of pulse length and pulse strength on transfection by electroporation. Biotechniques. 1990 Jan;8(1):16–20. [PubMed] [Google Scholar]
- Liang H., Purucker W. J., Stenger D. A., Kubiniec R. T., Hui S. W. Uptake of fluorescence-labeled dextrans by 10T 1/2 fibroblasts following permeation by rectangular and exponential-decay electric field pulses. Biotechniques. 1988 Jun;6(6):550-2, 554, 556-8. [PubMed] [Google Scholar]
- Montané M. H., Dupille E., Alibert G., Teissié J. Induction of a long-lived fusogenic state in viable plant protoplasts permeabilized by electric fields. Biochim Biophys Acta. 1990 May 9;1024(1):203–207. doi: 10.1016/0005-2736(90)90227-f. [DOI] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann F., Zimmermann U., Pilwat G. Release and uptake of haemoglobin and ions in red blood cells induced by dielectric breakdown. Biochim Biophys Acta. 1975 Jul 3;394(3):449–462. doi: 10.1016/0005-2736(75)90296-5. [DOI] [PubMed] [Google Scholar]
- Rols M. P., Teissié J. Electropermeabilization of mammalian cells. Quantitative analysis of the phenomenon. Biophys J. 1990 Nov;58(5):1089–1098. doi: 10.1016/S0006-3495(90)82451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurich P., Zimmermann U., Mischel M., Lamprecht I. Membrane fusion and deformation of red blood cells by electric fields. Z Naturforsch C. 1980 Nov-Dec;35(11-12):1081–1085. doi: 10.1515/znc-1980-11-1236. [DOI] [PubMed] [Google Scholar]
- Sowers A. E. Characterization of electric field-induced fusion in erythrocyte ghost membranes. J Cell Biol. 1984 Dec;99(6):1989–1996. doi: 10.1083/jcb.99.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers A. E. Low concentrations of macromolecular solutes significantly affect electrofusion yield in erythrocyte ghosts. Biochim Biophys Acta. 1990 Jun 27;1025(2):247–251. doi: 10.1016/0005-2736(90)90104-v. [DOI] [PubMed] [Google Scholar]
- Sowers A. E. The long-lived fusogenic state induced in erythrocyte ghosts by electric pulses is not laterally mobile. Biophys J. 1987 Dec;52(6):1015–1020. doi: 10.1016/S0006-3495(87)83294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar I. P., Neumann E. Stochastic model for electric field-induced membrane pores. Electroporation. Biophys Chem. 1984 May;19(3):211–225. doi: 10.1016/0301-4622(84)87003-9. [DOI] [PubMed] [Google Scholar]
- Teissie J., Rols M. P. Fusion of mammalian cells in culture is obtained by creating the contact between cells after their electropermeabilization. Biochem Biophys Res Commun. 1986 Oct 15;140(1):258–266. doi: 10.1016/0006-291x(86)91084-3. [DOI] [PubMed] [Google Scholar]
- Teissie J., Tsong T. Y. Electric field induced transient pores in phospholipid bilayer vesicles. Biochemistry. 1981 Mar 17;20(6):1548–1554. doi: 10.1021/bi00509a022. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Astumian R. D. Electroconformational coupling and membrane protein function. Prog Biophys Mol Biol. 1987;50(1):1–45. doi: 10.1016/0079-6107(87)90002-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electric field-mediated fusion and related electrical phenomena. Biochim Biophys Acta. 1982 Nov 30;694(3):227–277. doi: 10.1016/0304-4157(82)90007-7. [DOI] [PubMed] [Google Scholar]


