Abstract
Biochemical studies have shown that Smad7 blocks signal transduction of transforming growth factor β (TGFβ); however, its in vivo functions are largely unknown. To determine the functions of Smad7, we have expressed Smad7 in transgenic mice, utilizing a keratin K5 promoter (K5.Smad7). K5.Smad7 mice exhibited pathological changes in multiple tissues and died within 10 days after birth. These mice were born with open eyelids and corneal defects, significantly delayed and aberrant hair follicle morphogenesis, and hyperproliferation in the epidermis and other stratified epithelia. Furthermore, K5.Smad7 mice developed severe thymic atrophy and massive thymocyte death, suggesting that Smad signaling in thymic epithelia is essential for thymocyte survival. Interestingly, in addition to a reduction in Smad phosphorylation, the protein levels of the receptors for TGFβ, activin and bone morphogenetic protein were significantly decreased in the affected tissues of K5.Smad7 mice. Our study provides evidence that Smad7 is a potent in vivo inhibitor for signal transduction of the TGFβ superfamily during development and maintenance of homeostasis of multiple epithelial tissues.
Keywords: epidermis/eye/hair follicles/TGFβ signaling/thymus
Introduction
The transforming growth factor β (TGFβ) superfamily regulates cell growth and differentiation, tissue remodeling, immune response and angiogenesis (Böttinger et al., 1997; Letterio and Roberts, 1997). This family includes three major subfamilies: TGFβs, activins and bone morphogenetic proteins (BMPs). The major signaling transducers for the TGFβ superfamily are Smads, which are classified as receptor-specific Smads (R-Smads), a co-Smad (Smad4), and inhibitory Smads. Among R-Smads, Smad1, Smad5 and Smad8 are responsible for BMP signaling, and Smad2 and Smad3 are responsible for TGFβ and activin signaling (for a recent review see Miyazono et al., 2001). When a ligand of the TGFβ superfamily binds to its receptor complex, the type II receptor phosphorylates and thereby activates the type I receptor kinase. The activated type I receptor phosphorylates R-Smads, which form heteromeric complexes with Smad4. The Smad complex translocates into the nucleus to regulate transcription. The inhibitory Smads, Smad6 and Smad7, block signaling by competing with R-Smads for interaction with the activated type I receptors, thereby inhibiting R-Smad phosphorylation (Hayashi et al., 1997; Imamura et al., 1997; Nakao et al., 1997) by competing with Smad4 for heteromeric complex formation with phosphorylated R-Smads (Hata et al., 1998) or by recruiting ubiquitin ligases to the activated receptor to induce its degradation via proteosomal and lysosomal pathways (Kavsak et al., 2000; Ebisawa et al., 2001).
The functions of Smad7 may be far more complicated than acting simply as an inhibitor of TGFβ signaling, which was initially depicted from the biochemical studies. In addition to interacting with TGFβ signaling, Smad7 inhibits signal transduction of activin and BMP (Casellas and Brivanlou, 1998). Moreover, Smad7 has been shown to increase TGFβ-mediated apoptosis in epithelial cells (Landström et al., 2000; Lallemand et al., 2001), and to cooperate with TGFβ in inhibiting adipocyte differentiation (Choy et al., 2000).
In contrast to R-Smads and Smad4, which are highly expressed in normal epithelial tissues but frequently mutated in cancers, Smad7 is expressed at very low levels in normal epithelial tissues, but is overexpressed in certain cancers (Kleeff et al., 1999; Boulay et al., 2001; He et al., 2001). Additionally, Smad7 overexpression has been observed in inflammatory bowel disease (Monteleone et al., 2001), and in damaged podocytes in progressive glomerulosclerosis in mice (Schiffer et al., 2001). These studies suggest that overexpression of Smad7 may play a pathological role under certain circumstances. To determine the functions of Smad7 overexpression in vivo, we have expressed Smad7 in transgenic mice, utilizing a truncated bovine keratin K5 promoter. This promoter targets gene expression at the highest level in the epidermis and hair follicles (Ramirez et al., 1994). It also targets gene expression in other stratified and pseudo-stratified squamous epithelia (Ramirez et al., 1994), and to a less extent, in thymic epithelia (Robles et al., 1996). K5.Smad7 mice exhibited severe defects in multiple epithelial tissues, including significantly delayed and aberrant hair follicle formation, a marked hyperplasia in the epidermis and other stratified epithelia, aberrant development of the eyelid and cornea, and severe thymic atrophy. We provide evidence that these phenotypes result primarily from blocking one or more signaling pathways of TGFβ, activin and BMP in the affected tissues.
Results
Gross phenotype of K5.Smad7 transgenic mice
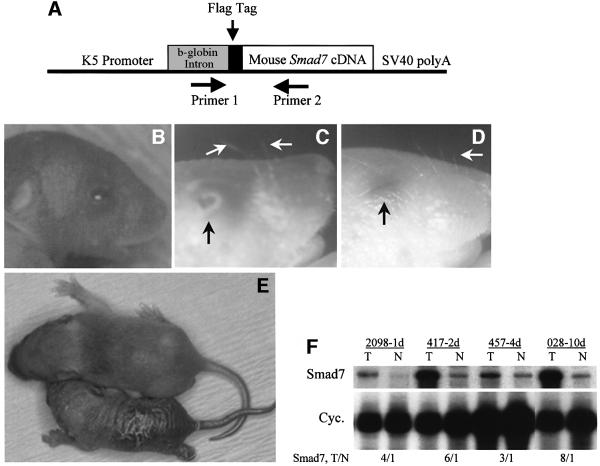
The Smad7 cDNA with a 5′ flag epitope was inserted into a targeting vector based on a truncated keratin K5 promoter (van Hogerlinden et al., 1999; Figure 1A). K5.Smad7 transgenic mice were born with open eyelids and protruding eyeballs (Figure 1B). By day 3, the eyelids in K5.Smad7 mice began to migrate towards the center (Figure 1C). K5.Smad7 mice had sparse and curly vibrissae (Figure 1C), in comparison with non-transgenic littermates, which possessed straight and dense vibrissae (Figure 1D). By 7–10 days, when non-transgenic littermates grew the first coat, K5.Smad7 pups exhibited a thickened, wrinkled and hyperkeratotic skin, and delayed hair growth (Figure 1E). The eyelids of K5.Smad7 mice closed at this stage. Although the size and weight of K5.Smad7 pups were similar to their littermates at birth, they showed retarded growth (Figure 1E) and typically died within 3 days after birth. The data in this study were collected from 15 transgenic founders derived from microinjections, and 20 transgenic pups generated from two mosaic founders that occasionally gave birth to pups positive for transgene expression. Out of these 35 transgenic pups, only four pups survived until 10 days of age. All phenotypic pups were confirmed to express the Smad7 transgene. Figure 1F shows examples of Smad7 expression in K5.Smad7 transgenic and control skins, detected by RNase protection assays (RPA). Smad7 transgene expression was elevated 2–7-fold above endogenous Smad7 levels. The phenotype severity correlated well with the transgene expression levels.
Fig. 1. Schematic of K5.Smad7 transgene (A), gross phenotypes (B–E) and transgene expression levels (F) of K5.Smad7 mice. Primer 1 and Primer 2 were used for genotyping. (B) A 1-day-old K5.Smad7 pup (2098–1d) showing an open eyelid. (C) A microscopic photo of a 3-day-old K5.Smad7 pup (2098–3d) showing a partially open eyelid (black arrow), as well as sparse and curly vibrissae (white arrows). (D) A 3-day-old normal pup showing a closed eyelid (black arrow), and straight and dense vibrissae (white arrow). (E) A 1-week-old K5.Smad7 pup (417–7d, bottom) versus a normal littermate (top). (F) RPA for expression of Smad7 in mouse skin. The numbers on top of the lanes are the ear tag numbers of the mothers. The ages are also indicated (d, days). Smad7 expression in transgenic mouse skin (T) represents both the transgene and endogenous gene expression. T/N: the ratios of Smad7 expression in transgenic versus normal skin, which were normalized with cyclophilin (Cyc).
Smad7 transgene expression results in delayed hair follicle morphogenesis and progressive epidermal hyperplasia
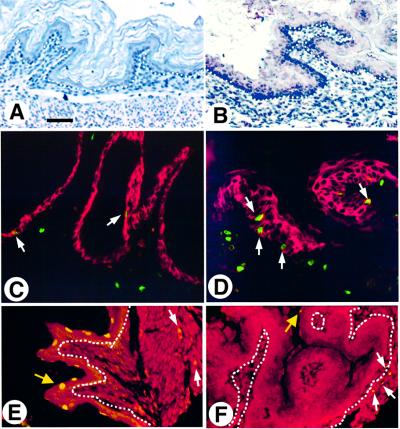
Neonatal K5.Smad7 epidermis exhibited a normal differentiation pattern. Remarkably, hair morphogenesis in K5.Smad7 skin was significantly delayed. In skins of 1-day-old non-transgenic mice, >50% of the hair follicles had formed an outer root sheath (ORS) and a dermal papilla (Figure 2A). However, hair follicles in 1-day-old K5.Smad7 skin were at a developmental stage comparable with wild-type E16 to E17 mouse embryos (Figure 2B). Keratin K6 was detected in the ORS of the hair follicle in normal neonatal skin (Figure 2C). In contrast, K6 was not expressed in the hair follicles even in 2-day-old K5.Smad7 skin (Figure 2D). By day 10, hair follicles in non-transgenic mice further matured and the hair shaft had formed (Figure 1E). Melanin was deposited in the pre-cortex and hair shaft of the hair follicles (Figure 2E). K5.Smad7 hair follicles did not show an obvious structure of the hair shaft (Figure 2F). Additionally, hair follicles in K5.Smad7 mice became disorientated and disorganized, and melanin was deposited irregularly (Figure 2F). At this stage, K5.Smad7 mice developed obvious hyperplasia in the epidermis (Figure 2F, H and J versus E, G and I). Immunohistochemistry using an antibody specific for the flag epitope of the Smad7 transgene confirmed that the Smad7 transgene was expressed in the epidermis and hair follicles in the transgenic skin (Figure 2H). Bromodeoxy uridine (BrdU) labeling in non-transgenic epidermis was restricted to the basal layer, and the labeling index in 10-day-old non-transgenic epidermis was 11.6 ± 5.2 cells/mm epidermis (Figure 2I, n = 4). However, BrdU labeling in K5.Smad7 epidermis increased to 50.6 ± 17 cells/mm epidermis (n = 4, p <0.01), with the positive labeling cells in both basal and suprabasal layers (Figure 2J). This result indicates that the proliferative compartment was expanded in K5.Smad7 epidermis.
Fig. 2. K5.Smad7 pups exhibit delayed and aberrant hair morphogenesis and epidermal hyperproliferation. One-day-old skins from a non-transgenic mouse (A) and a K5.Smad7 pup (2098–1d) (B) showing delayed hair follicle development in K5.Smad7 skin. (C and D) Immunofluorescence staining for keratin K6 (green/yellow) in the skin of a 1-day-old non-transgenic pup (C), but absent in a 2-day-old transgenic pup (417–2d) (D). Immunostaining for K14 (red) highlights the epidermis and hair follicles. (E and F) Hematoxylin and eosin staining for the skin from 10-day-old wild-type (E) and K5.Smad7 (028–10d) (F) mice. The arrows in (E) point to melanin deposits in the hair shaft (upper) and pre-cortex (lower). Hair follicles in K5.Smad7 skin (F) were irregularly shaped and disoriented. Dense and irregular melanin deposits are pointed in K5.Smad7 follicles (arrows). (G and H) Immunohistochemical staining for Smad7 transgene expression in the skin of a 10-day-old control (G) and K5.Smad7 (028–10d) (H) mice, using a flag antibody. Smad7 transgene expression was detected in the epidermis and hair follicles in the transgenic skin (brown in H), but not in control skin (G). (I and J) BrdU labeling (green/yellow) in the skin of a 10-day-old control (I) and K5.Smad7 (028–10d) (J) mice. The counterstain in red is keratin K14, which highlights the epidermis and hair follicles. The bar in (A) represents 50 µm for (A–H), and 30 µm for (I and J).
K5.Smad7 transgenic mice develop epithelial hyperplasia in the upper digestive tract
In addition to epidermal hyperplasia, K5.Smad7 mice exhibited epithelial hyperplasia in the tongue, oral cavity, esophagus (data not shown) and forestomach, where Smad7 transgene expression was also detected (Figure 3B). This phenotype may cause problems during suckling, leading to perinatal lethality. The BrdU labeling index was 3.0 ± 1.6 cells/mm epithelium in non-transgenic neonatal forestomach (n = 3, Figure 3C); however, it increased to 19.0 ± 3.8 cells/mm epithelium in K5.Smad7 forestomach (Figure 3D, n = 3, p <0.01). In contrast, apoptotic cells, determined by Tdt (deoxynucleotidetransferase)-mediated dUTP nick-end labeling (TUNEL) assays, were frequently detected in non-transgenic epithelia of the forestomach (Figure 3E, 18.4 ± 8.5 cells/mm epithelium, n = 6), but were dramatically reduced to 1.5 ± 0.7 cells/mm epithelium in K5.Smad7 forestomach (Figure 3F, n = 6, p <0.01).
Fig. 3. Epithelial hyperplasia developed in K5.Smad7 forestomach (457–4d). (A and B) Immunohistochemical staining with the flag antibody is negative in the forestomach epithelium of a non-transgenic neonatal mouse (A), and positive in the epithelium of a K5.Smad7 forestomach [brown in (B)]. The dotted white lines in (A) and (B) indicate the basal layer of the epithelium. Note that the epithelium in K5.Smad7 forestomach was significantly hyperplastic (B). (C and D) BrdU labeling (green) in the epithelium of the forestomach (arrows) shows a low labeling rate in control forestomach (C), but is increased in K5.Smad7 forestomach (D). The counterstain in red is K14, which highlights the epithelium. (E and F) TUNEL assay. The counterstain in red is propidium iodide. Yellow arrows point to examples of apoptotic cells (yellow) in the epithelium, which were obvious in control forestomach (E), but were few in transgenic forestomach (F). The dotted lines in (E) and (F) highlight the basal layer of the epithelium. White arrows in (E) and (F) point to apoptotic cells in the stroma, indicating that apoptosis was only blocked in the epithelium of the transgenic forestomach. The bar in (A) represents 100 µm for (A–D), and 50 µm for (E and F).
K5.Smad7 mice exhibit abnormalities in eye development
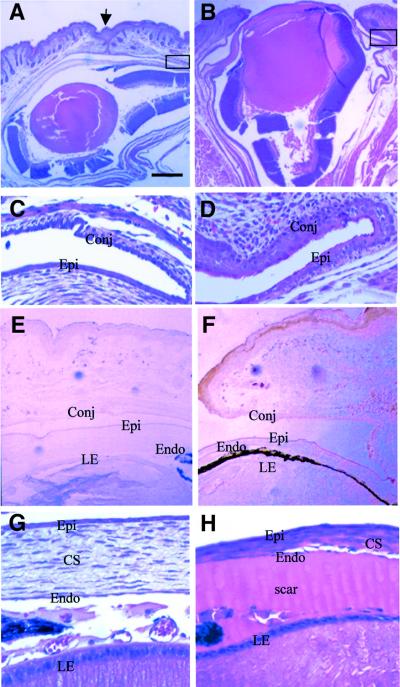
The eyelids in non-transgenic pups begin to fuse at E16.5, and remain closed until 12–14 days after birth. Histological analyses of the eyes of K5.Smad7 mice confirmed that the eyelids were open at birth (Figure 4B). In comparison with non-transgenic mice (Figure 4C), the conjunctiva and lateral corneal epithelium were hyperplastic in K5.Smad7 mice (Figure 4D). This is mainly due to increased proliferation in the corneal and conjunctival epithelia, whereas apoptotic rates in these regions were not altered (data not shown). Immunohistochemical staining utilizing the flag antibody confirmed that the Smad7 transgene was expressed in the epidermis and the conjunctival epithelium of the eyelid, and the corneal epithelium of transgenic eyes (Figure 4F). In addition to abnormalities in these affected tissues, some tissues in the eye exhibited pathological alterations where the Smad7 transgene was not expressed. For instance, the corneal stroma was thinner in K5.Smad7 eyes than in wild-type eyes (Figure 4H). The anterior chamber of transgenic eyes was filled with acidophilic scar (Figure 4B and H), which did not stain for β- or γ-crystallin (data not shown). The lens epithelium appeared thinner and less organized in K5.Smad7 eyes (Figure 4H) in comparison with non-transgenic eyes (Figure 4G).
Fig. 4. K5.Smad7 mice exhibit defects in eye development. The sections of K5.Smad7 eye shown in this figure are from 2098–1d. (A and B) Histology of 1-day-old wild-type (A) and K5.Smad7 (B) eyes. The eyelid was fused (arrow) in the wild-type eye, but open in K5.Smad7 eye. (C and D) Higher magnification for the regions in the black boxes in (A) and (B), respectively, showing conjunctival and corneal hyperplasia in K5.Smad7 eye (D) in comparison with that in the normal eye in (C). (E and F) Immunohistochemical staining for the flag epitope of the Smad7 transgene, negative in the control eye (E), but positive in the epidermis, conjunctival and corneal epithelia (F). (G and H) Higher magnification for the cornea and lens epithelium. The lens epithelium and corneal stroma appeared thinner and less organized in K5.Smad7 eye (H), in comparison with a non-transgenic eye (G). The anterior chamber was filled with acidophilic scar in K5.Smad7 eye (H). Epi, corneal epithelium; Conj, conjunctival epithelium; CS, corneal stroma; Endo, corneal endothelium; LE, lens epithelium. The bar in (A) represents 150 µm for (A and B), 15 µm for (C, D, G and H), and 60 µm for (E and F).
K5.Smad7 mice exhibit severe thymic atrophy
T lymphocyte development begins with double negative CD4–CD8– immature cells in the thymus. These cells progress to double positive CD4+CD8+ cells, which compose the majority of the cortex. A select population of differentiated thymocytes emerges as mature single positive CD4+ or CD8+ cells in the medulla of the thymus. The thymi of K5.Smad7 neonates were ∼1/3 the size of those of non-transgenic littermates. Histology of neonatal K5.Smad7 thymi revealed that most cells in the cortex contained condensed nuclei (Figure 5B), which were apoptotic cells, as confirmed by TUNEL assays (data not shown). Immunofluorescence staining confirmed that the Smad7 transgene was expressed in K5 positive and negative thymic epithelial cells (Figure 5D–F), but not in thymocytes (Figure 5F). This result indicates that the K5 vector targets expression to more thymic epithelial cells than the endogenous K5 promoter. The wild-type thymus grows rapidly after birth, and by day 10, the cortex and medulla were distinct (Figure 5G). However, the size of 10-day-old K5.Smad7 thymi was ∼1/5 of wild-type thymi (Figure 5H). TUNEL assays show that normal thymi had only sporadic apoptotic cells in the cortex (Figure 5I), whereas >30% of the thymocytes in the cortex of K5.Smad7 thymi underwent apoptosis (Figure 5J). Consistent with severe atrophy and massive apoptosis in the thymic cortex, the total number of live thymocytes was ∼50-fold lower in the K5.Smad7 mice than in control mice (Figure 5K). Flow cytometry analyses from three separate experiments revealed a depletion of thymocytes in K5.Smad7 thymi, primarily in the CD4+CD8+ population (Figure 5K). The total number of CD4–CD8+, CD4+CD8– and CD4–CD8– cells was also markedly reduced; however, the percentage of these cells was proportionally increased in K5.Smad7 thymi (Figure 5K).
Fig. 5. Thymic atrophy in K5.Smad7 mice. (A and B) Histology of the neonatal thymus from a non-transgenic mouse (A) and a K5.Smad7 mouse (2098–1d) (B). The lymphocytes in (B) exhibited condensed nuclei. (C–F) Immunofluorescence staining with a flag antibody (red) and a K5 antibody (green). The dark areas in these four panels were occupied by thymocytes. The Smad7 transgene co-localizes with endogenous K5 (yellow) or K5 negative cells (red) in the transgenic thymus (D), but not in the control littermate (C). (E) Single exposure for K5 expression in K5.Smad7 thymic epithelia. (F) Single exposure for the flag antibody staining showing the Smad7 transgene was expressed in thymic epithelia. (G and H) Histology of the thymus from a 10-day-old non-transgenic mouse (G) and a K5.Smad7 mouse (028–10d) (H). The cortex (c) and the medulla (m) have completely developed in the non-transgenic thymus (G). Note that the transgenic thymus displayed significant atrophy in the cortex (c in H). (I and J) TUNEL assay for (G) and (H). The counterstain in red is propidium iodide. Apoptotic cells (yellow) were sporadic in the non-transgenic thymus (I), but were massive in the cortex of the transgenic thymus (J). The dotted circle in H highlights the medulla. The bar in (A) represents 10 µm for (A and B), 30 µm for (C–F), 200 µm for (G and H), and 50 µm for (I and J). (K) Flow cytometry analysis of thymocyte profiles in 7-day-old wild-type (left) and K5.Smad7 (right) thymocytes. The percentage and the total number of live thymocytes in the four subpopulations of the thymus are indicated in each panel.
K5.Smad7 transgenic skin exhibits a reduction in receptor proteins of the TGFβ superfamily and decreased phosphorylation of Smads
To elucidate the molecular mechanisms by which Smad7 overexpression induces the observed phenotypes, we analyzed expression and activation of TGFβ signaling components. Since the Smad7 transgene is expressed at the highest level in the epidermis and hair follicles, we chose the skin for analysis. We examined the protein levels of the type I and II receptors of TGFβ, activin and BMP in day 1–10 skins of control and K5.Smad7 mice. Throughout all these stages, the type I and II receptors for TGFβ (TGFβRI and TGFβRII), activin (ActR-IA, ActR-IB, ActR-IIA and ActR-IIB) and BMP (BMPR-IA, BMPR-IB and BMPRII) were detected in the epidermis of non-transgenic mice, but were reduced to barely detectable levels (except for BMPR-IA) in K5.Smad7 epidermis. Figure 6 shows an example of this analysis in skins of day 7 mice. In addition to the epidermis, these receptors were also detected in non-transgenic hair follicles with various distributions. For instance, consistent with the previous report (Paus et al., 1997), TGFβRI was located in the inner root sheath (IRS), whereas TGFβRII was in the ORS (Figure 6). BMPR proteins were located in the ORS and matrix cells of the hair follicles, while ActR proteins were mainly located in ORS (Figure 6). The staining patterns of these receptors can be blocked by pre-incubating individual antibodies with the corresponding specific peptides. With the exception of BMPR-IA, all of these receptors were reduced to non- or barely detectable levels in K5.Smad7 skin (Figure 6). To further confirm the specificity of immunohistochemical staining and quantitate protein levels, western blot analyses were performed on protein extracts from control and K5.Smad7 skin, utilizing the above antibodies (Figure 7A). The keratin K14 antibody was used as a loading control to normalize the amount of protein from the epidermis and hair follicles of each sample. One specific band with the predicted molecular weight was detected with each antibody. Pre-incubating antibodies with the corresponding specific peptides eliminated these reactions. An exception was the BMPRII antibody, which yielded two bands: a predicted 110 kDa band, which represents the full-length BMPRII, and a 60 kDa band. Both bands can be blocked by the blocking peptide corresponding to the intracellular juxtamembranous part of BMPRII (Onishi et al., 1998). Therefore, the 60 kDa band probably represents a previously documented splice variant of BMPRII (Yamashita et al., 1996). Consistent with the staining patterns of immunohistochemistry with the exception of BMPR-IA, which remained unchanged in K5.Smad7 skin, all other receptor proteins were decreased in K5.Smad7 skin (Figure 7A). However, it is more apparent in western blot analysis than in immunohistochemistry that the sensitivity to Smad7-induced reduction varied: TGFβRI, TGFβRII, ActR-IIB and BMP-IB exhibited a complete loss in K5.Smad7 skin; ActR-IA and ActR-IB were completely lost in a higher expressor (028–10d) but only partially lost in a lower expressor (457–4d, Figure 7A and B); and ActR-IIA and BMPRII were partially lost in the skin from both lower and higher K5.Smad7 expressors (Figure 7A).
Fig. 6. Immunohistochemical staining for TGFβR, ActR and BMPR. Non-transgenic day 7 skin (NL) exhibits expression of all these receptor proteins throughout the entire epidermis. The TGFβRI is mainly located in the IRS (arrows), whereas TGFβRII is in the ORS (arrows) of the hair follicles. BMPR and ActR proteins are located in the ORS (an example is pointed by a black arrow in BMPR-IB) and matrix cells (an example is pointed by a white arrow in BMPR-IB) of the hair follicle. Significant reductions of all these receptors except BMPR-IA are seen in K5.Smad7 epidermis and hair follicles (417–7d). The bar in the first panel represents 50 µm for all normal sections, and 100 µm for all Smad7 sections. K5.Smad7 skin at earlier stages showed changes in staining patterns of these receptors similar to this figure (data not shown).
Fig. 7. Expression of TGFβR, ActR and BMPR (A and B), total and phosphorylated Smads (A), and c-myc and p21 (C) in normal (N) and transgenic (T) skins. The numbers on top of the lanes in each panel are the ear tag numbers of the mothers. The ages are also indicated (d, days). (A) Most antibodies yielded one specific band, except for BMPRII, which had two splicing variants, both of which can be blocked by the specific peptide, and Smad2P, which shows two close bands, presumably due to different phosphorylation status. With the exception of BMPR-IA, all of the receptors, as well as phosphorylated Smad1 (Smad1P) and Smad2 (Smad2P) showed a complete or partial loss in K5.Smad7 skin, depending on Smad7 transgene expression levels. Levels of Smad1, 2 and 4 were not altered in Smad7 transgenic skin. K14 was used as a loading control. (B) RPA for expression of receptor transcripts. The protein levels of each are shown in (A). Cyclophilin (Cyc) was used as a loading control. (C) RPA for expression of c-myc and p21 in mouse skin. T/N, the ratios of c-myc or p21 expression in transgenic versus normal skin, which were normalized with cyclophilin (Cyc).
To determine whether Smad7 induces reduction of these receptors at the protein or transcription level, transcripts of these receptors were examined in K5.Smad7 and control skins by RPA. K5.Smad7 skin did not exhibit reductions in these receptor transcripts in comparison with the skin of control littermates (Figure 7B).
Phosphorylated forms of Smad1 and Smad2, which represent activation of signaling of BMP and TGFβ/activin, respectively, were also examined in control and transgenic skin by western blot analysis. The total amount of Smad1, Smad2 and Smad4 proteins was not changed in K5.Smad7 transgenic skin, in comparison with non-transgenic skin (Figure 7A). However, K5.Smad7 transgenic skin exhibited complete or partial loss of phosphorylated Smad1 and Smad2, depending on the levels of Smad7 transgene expression (Figure 7A).
K5.Smad7 transgenic skin exhibits an increase in c-myc expression and a decrease in p21waf1 expression
To determine whether blocking TGFβ/Smad signaling by Smad7 transgene expression affects expression of TGFβ transcription targets, we examined expression of the c-myc proto-oncogene, and p21, an inhibitor for cyclin-dependent kinases. TGFβ1 transcriptionally represses c-myc expression (Pietenpol et al., 1990) and increases p21 expression (Datto et al., 1995). We found that c-myc expression was 2- to 3-fold higher whereas p21 expression was 2- to 4-fold lower in K5.Smad7 transgenic skin compared with non-transgenic mice (Figure 7C).
Discussion
In this study, we have generated Smad7 transgenic mice utilizing a K5 promoter. Smad7 transgene expression results in severe pathological alterations in multiple epithelial tissues. In addition to extending in vitro studies that Smad7 blocks phosphorylation of R-Smads, we observed that Smad7 overexpression greatly reduces the protein levels of TGFβR, ActR and BMPR in the epidermis and hair follicles, where the Smad7 transgene is expressed at the highest level. Our study is consistent with biochemical studies showing that Smad7 recruits Smurf1 or Smurf2 for degradation of TGFβR proteins (Kavsak et al., 2000; Ebisawa et al., 2001). To determine whether the changes were a secondary effect brought by the delayed development in K5.Smad7 skin, we examined expression of these receptor proteins at several stages of development, and used K14 as a control to ensure that the protein extracts from keratinocytes were equally presented. All of these receptors (except BMPR-IA) were significantly reduced in K5.Smad7 skin at all stages examined, in comparison with either the age-matched or neonatal non-transgenic skin. In addition, these receptors exhibited differences in susceptibility to Smad7-induced reduction. For example, levels of BMPR-IA were unchanged, BMPRII was only partially lost, whereas TGFβR proteins were completely lost. This result suggests further that Smad7 overexpression directly induces reductions of certain receptor proteins of TGFβ, activin and BMP, thus, acting as a potent in vivo inhibitor of signal transduction by the TGFβ superfamily in certain epithelial tissues.
Smad7 overexpression perturbs hair follicle development and differentiation possibly via blocking signal transduction of TGFβ, activin and BMP
One of the most striking phenotypes that developed in K5.Smad7 mice was delayed hair follicle formation, which mimics the skin phenotype of mice null for TGFβ2 (Foitzik et al., 1999) or for activin βA (Matzuk et al., 1995). Consistently, K5.Smad7 transgenic skin showed downregulation of TGFβR and ActR, as well as decreased phosphorylation of Smad2, suggesting that Smad7 overexpression blocks both TGFβ and activin signaling. In addition to a significant delay in hair follicle neogenesis, Smad7 transgene expression results in delayed differentiation and aberrant formation of hair follicles. These phenotypes are similar to, but more severe than, those developed in transgenic mice overexpressing Noggin, a BMP neutralizer, in the matrix cells of hair follicles (Kulessa et al., 2000). In Noggin transgenic mice, although the hair follicles are formed, they fail to differentiate to form the hair shaft (Kulessa et al., 2000), suggesting that BMP signaling within hair follicle cells is essential for hair follicle differentiation. Since K5.Smad7 skin exhibited decreased levels of BMPR and phosphorylated Smad1, it is reasonable to believe that Smad7 transgene expression perturbs BMP signaling in hair follicle cells. In addition to BMP, TGFβ may directly regulate hair follicle differentiation. For instance, keratin K6, a marker for early differentiation of hair follicles, as well as for hyperproliferation in the interfollicular epidermis (Wang et al., 1997), is paradoxically overexpressed in the hypoproliferative epidermis of TGFβ1 transgenic mice (Sellheyer et al., 1993). Therefore, it is possible that TGFβ induces K6 expression, and overexpression of Smad7 is able to block this effect. A role for activin in hair follicle differentiation has also been suggested. For example, overexpression of follistatin, an activin binding protein and antagonist, by a metallothionein-1 promoter in transgenic mice leads to irregular hair formation (Guo et al., 1998). Smad7 transgenic skin consistently exhibits downregulation of BMPR, ActR and TGFβR, and reduced phosphorylation of signaling Smads at the stage when hair follicles differentiate. Thus, perturbation of hair follicle differentiation in K5.Smad7 mice may reflect the antagonistic effect of Smad7 on all these signal transduction pathways.
Smad7 overexpression causes epithelial hyperplasia by increasing proliferation and inhibiting apoptosis
Members of the TGFβ, BMP and activin families play important roles in regulating keratinocyte proliferation. Among them, TGFβ1 is expressed at the highest level in normal epidermis and is a potent growth inhibitor for keratinocytes (Pittelkow et al., 1988; Sellheyer et al., 1993; Wang et al., 1999). Therefore, hyperproliferation in the epidermis and stratified epithelia in K5.Smad7 mice may primarily result from blocking TGFβ1-induced growth arrest of epithelial cells. Supporting this, the c-myc proto-oncogene, which is transcriptionally repressed by TGFβ1 and a key event in abrogation of TGFβ1-induced growth inhibition in keratinocytes (Pietenpol et al., 1990), was upregulated in K5.Smad7 transgenic skin. In contrast, the CDK inhibitor p21, which is elevated by TGFβ1 and mediates TGFβ1-induced growth arrest (Datto et al., 1995), was downregulated in K5.Smad7 transgenic skin.
TGFβ has been shown to induce apoptosis in epithelial cells (Chen et al., 1998). If Smad7 blocks this effect, it might further contribute to epithelial hyperplasia. On the other hand, there is compelling evidence showing that Smad7 induces apoptosis (Landström et al., 2000; Lallemand et al., 2001; Mazars et al., 2001). In the present study, we did not observe increased apoptosis in the epidermis or epithelia of the eye in Smad7 transgenic mice (data not shown), indicating that Smad7 overexpression does not directly induce apoptosis in these tissues. Since normal epidermis and eye epithelia have a very low rate of apoptosis, it would be difficult to evaluate the role of Smad7 if it inhibits apoptosis in these tissues. Relative to this point, we did observe an inhibitory effect of Smad7 on apoptosis in the epithelium of the forestomach, where apoptosis occurs more frequently. Therefore, increased proliferation and decreased apoptosis potentially cooperate, which may explain why a low level of transgene expression was sufficient to induce epithelial hyperplasia in the forestomach before hyperplasia became obvious in the epidermis.
Ocular abnormalities in K5.Smad7 mice suggest an important role of Smad signaling in eye development
TGFβ superfamily members play important roles in ocular development (Vassalli et al., 1994; Sanford et al., 1997; Srinivasan et al., 1998). Since the receptors for TGFβR, ActR and BMPR are mainly expressed in the epithelia in the eye (Obata et al., 1999), epithelial abnormalities in K5.Smad7 eyes are likely to be a direct effect of the Smad7 transgene. For instance, failure of eyelid fusion and corneal epithelial hyperplasia have been observed in activin βB knockout mice (Vassalli et al., 1994); in contrast, transgenic mice overexpressing TGFβ1 in the eye have a thinner corneal epithelium (Srinivasan et al., 1998). Therefore, our current study suggests that Smad7 overexpression is able to block the functions of endogenous activin/TGFβ during eye development. In addition to the targeted tissues in the eye, Smad7 overexpression in the epithelial tissues causes indirect effects on non-targeted tissues. For instance, the corneal stroma was thinner in K5.Smad7 transgenic eyes, which is similar to that observed in TGFβ2 knockout eyes (Sanford et al., 1997), but opposite to that seen in TGFβ1 transgenic eyes. Since Smad7 is not a secreted protein, it is unlikely that Smad7 overexpression in the corneal epithelium directly blocks the paracrine effect of TGFβ1 or TGFβ2 on the stroma. However, it is possible that Smad7 overexpression in the corneal epithelium affects epithelial-mesenchymal cross-talk. The failure of eyelid fusion and the abnormalities of the cornea may further affect lens development, which was exposed to an unusual environment during development. This is evidenced by the fact that the lens epithelium appeared to be less organized, even though the Smad7 transgene was not expressed in the lens epithelium.
Thymic atrophy in Smad7 transgenic mice suggests an important role for Smad signaling in thymic epithelia
Interestingly, neonatal K5.Smad7 pups have already demonstrated thymic atrophy prior to onset of growth retardation, suggesting that thymic atrophy is most likely induced by Smad7 transgene expression in thymic epithelia rather than as a result of systemic sickness. The severe thymic atrophy appears to be a consequence of massive apoptosis of lymphoid thymocytes. TGFβ1 has been documented to be an anti-apoptotic factor for T cell survival (Cerwenka and Swain, 1999). Since Smad7 transgene expression is restricted to thymic epithelia, it suggests that TGFβ signaling in thymic epithelia may also have an indirect role in thymocyte apoptosis/survival. Both TGFβ ligands and receptors have been detected in cultured thymic epithelial cells, which regulate cytokine expression (Schluns et al., 1997). Thus, it is possible that Smad7 transgene expression perturbs TGFβ-induced cytokine production in thymic epithelia, which affects thymocyte apoptosis and/or survival. In addition to regulation of apoptosis, TGFβ regulates thymocyte differentiation. Once TGFβ1 null mice develop a wasting syndrome (at 2–3 weeks of age), their thymocyte profile reveals an increase in differentiated T cell populations (i.e. a reduction in CD4+CD8+, and an increased percentage of CD4+CD8– and CD4–CD8+) (Christ et al., 1994). Interestingly, neonatal K5.Smad7 thymi already exhibited aberrant thymocyte profiles that are strikingly similar to those in adult TGFβ1 null thymi. These abnormalities may result from either the aberrant development and differentiation of thymocytes, or the massive apoptosis that disproportionally affects subpopulations of thymocytes. It is possible that Smad7 transgene expression also affects thymic epithelial development/differentiation, and thereby perturbs the normal niche for thymocyte survival and differentiation. Since the Smad7 transgene may not only block TGFβ signaling, but also potentially block BMP/activin signaling in thymic epithelia, it is not surprising that thymic atrophy and apoptosis developed in K5.Smad7 mice are more severe than previously reported TGFβ1- or Smad3-knockout mice (Christ et al., 1994; Yang et al., 1999). However, since TGFβ1- or Smad3-knockout mice abolished TGFβ signaling in both thymocytes and thymic epithelial cells, our study suggests that Smad signaling in thymic epithelia plays an essential role in the homeostasis of the thymus.
In summary, elevated Smad7 expression in epithelial cells results in pathological alterations that are lethal in mice. The pathological changes were obvious at birth, indicating that Smad7 overexpression begins to affect these tissues during embryonic development. Addition ally, since K5.Smad7 transgene expression affects both proliferation and apoptosis, it is of interest to examine whether overexpression of Smad7 results in a higher susceptibility to epithelial carcinogenesis. To circumvent the premature lethality of K5.Smad7 mice, future studies will utilize an inducible and tissue-specific transgenic system (Wang et al., 1999), in which Smad7 overexpression can be induced at specific stages of development and carcinogenesis.
Materials and methods
Generation and identification of K5.Smad7 mice
The full-length mouse Smad7 cDNA with a 5′ flag epitope was inserted into the K5 vector (Ramirez et al., 1994; Figure 1A). The K5.Smad7 transgene was microinjected into mouse embryos obtained from matings between ICR females and B6D2 males. After birth, transgenic mice were identified by PCR analysis of tail DNA. The K5.Smad7 transgene was identified by PCR using forward primer 1 (Figure 1A), specific for the 5′ K5 vector (5′-TACAGCTCCTGGGCAACGTG-3′), and reverse primer 2 (Figure 1A), specific for mouse Smad7 (5′-GCCGCTCCTTGAGTTTCTTG-3′). The PCR product positive for the Smad7 transgene was ∼320 bp.
Tissue histology
Tissue samples were fixed in 10% neutral-buffered formalin at 4°C overnight, embedded in paraffin, sectioned to 6 µm thickness, and stained with hematoxylin and eosin.
Immunofluorescence and BrdU staining
Immunofluorescence analyses for keratin K6 and BrdU labeling were performed on frozen sections as described previously (Wang et al., 1997). Each tissue section was measured using a micrometer, and the average number of BrdU positive cells/mm epithelium ± S.D. represents the labeling index for the epidermis and other stratified epithelia. Double stain immunofluorescence for the flag epitope of the Smad7 transgene and K5 in thymic epithelia was performed on formalin-fixed thymus sections, utilizing a biotinylated flag antibody (1:100, Sigma) and rabbit anti-K5 (1:500), followed by incubation with FITC-conjugated anti-rabbit IgG (Dako) and streptavidin–Texas Red (Gibco).
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue sections, except for TGFβRI, which was performed on acetone-fixed frozen sections. The flag epitope of the Smad7 transgene was detected utilizing a biotinylated Flag antibody (1:100, Sigma). Staining for activin receptors (ActR-IA, ActR-IIA, ActR-IIB) and BMP receptors (BMPR-IA, BMPR-IB, BMPR-II) was performed utilizing their specific antibodies (1:1000), produced as described previously (Ishidou et al., 1995; Nagamine et al., 1998; Onishi et al., 1998). The antibodies for ActR-IB (T-17, 2 µg/ml), TGFβRI (V-22, 2 µg/ml) and TGFβRII (L21, 2 µg/ml) and their blocking peptides were purchased from Santa Cruz. Controls included sections incubated with rabbit or goat IgG with the same concentration of the primary antibody, and sections incubated with the primary antibody were pre-incubated (4°C, overnight) with 20 µg/ml of its blocking peptide. Immune complexes were detected by the avidin–biotin–peroxidase complex using Vectastain kits (Vector) and visualized with diaminobenzidine.
Apoptosis assays
Apoptosis was evaluated using a deoxynucleotidetransferase uridine end-labeling kit (Promega), on formalin fixed tissue sections.
Immunofluorescence staining and flow cytometry for T cells
Single cell suspensions were prepared and red blood cells were depleted by incubation in 0.83% NH4Cl. Cells were then washed with phosphate-buffered saline (pH 7.4) containing 2% fetal calf serum at 4°C. Cells were stained with FITC-labeled anti-CD4 and PE-conjugated anti-CD8 (PharMingen). Events were collected on an EPICS Profile I or XL of the flow cytometer (Coulter Corp., Hialeah, FL). The live lymphocytes, which were selected by forward-side scatter pattern, were used to calculate the percentage of CD4+ and /or CD8+ cells, using the Flow Jo software (Tree Star Inc., San Carlos, CA).
Western blot analysis
Total skin protein extraction and western blot analyses were performed as described (Go et al., 2000). To detect TGFβR, ActR and BMPR, the antibodies and their concentrations were used in the same way as those in the immunohistochemical staining (above). To determine levels of phosphorylated and total Smads, we used rabbit antisera produced as described previously (Persson et al., 1998; Korchynskyi et al., 1999; Brodin et al., 2000). Western blot analysis for Smad7 was also performed utilizing the rabbit antiserum produced as described previously (Brodin et al., 1999). Controls included applying the primary antibodies after pre-incubation (4°C, overnight) with 20 µg/ml of the corresponding blocking peptides.
RPA
Total skin RNA was isolated as described previously (Wang et al., 1995). RPA was performed using the RPA II kit (Ambion) and 32P-labeled riboprobes. Probes specific for the Smad7 transgene and p21 were generated as described previously (Go et al., 2000; He et al., 2001). The template for the c-myc oncogene was provided by Dr Yu-Lee (Baylor College of Medicine). To generate riboprobe templates specific for mouse TGFβRs, ActRs and BMPRs, 200–380 bp of PCR fragments corresponding to the mRNA sequences of individual receptors were produced from cDNAs synthesized from normal mouse epidermis (Table I), and cloned into the pGem-T vector (Promega). To normalize each RNA sample for differences in loading, a [32P]cyclophilin riboprobe was used. The intensity of protected bands was determined by densitometeric scanning of X-ray films.
Table I. PCR products as riboprobe templates of the receptors for TGFβ, activin and BMP.
| Genes | DDBJ/EMBL/GenBank accession No. | PCR encompassing region |
|---|---|---|
| TGFβRI | D25540 | bp 230–480 |
| TGFβRII | NM_009371 | bp 860–1120 |
| Act-RIA | NM_007394 | bp 2034–2414 |
| Act-RIB | NM_007395 | bp 123–456 |
| Act-RIIA | NM_007396 | bp 1647–1876 |
| Act-RIIB | NM_007397 | bp 346–586 |
| BMPR-1A | Z23154 | bp 85–459 |
| BMPR-1B | Z23143 | bp 221–530 |
| BMPRII | NM_007561 | bp 678–931 |
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
The authors would like to thank Dr Jose Jorcano for providing the K5 vector, Dr Li-Yuan Yu-Lee for providing mouse c-myc cDNA, and Drs Dennis R.Roop and Paul A.Overbeek for their critical comments on the manuscript. This work was supported by NIH grants CA87849 and AR47898 to X.-J.W. and the Dutch Organization for Scientific Research (MW 902–16–295) to P.t.D. W.H. is a recipient of the postdoctoral fellowship from the Dermatology Foundation.
References
- Böttinger E.P., Letterio,J.J. and Roberts,A.B. (1997) Biology of TGF–β in knockout and transgenic mouse models. Kidney Int., 51, 1355–1360. [DOI] [PubMed] [Google Scholar]
- Boulay J.L. et al. (2001) Combined copy status of 18q21 genes in colorectal cancer shows frequent retention of SMAD7. Genes Chromosomes Cancer, 31, 240–247. [DOI] [PubMed] [Google Scholar]
- Brodin G., ten Dijke,P., Funa,K., Heldin,C.-H. and Landström,M. (1999) Increased smad expression and activation are associated with apoptosis in normal and malignant prostate after castration. Cancer Res., 59, 2731–2738. [PubMed] [Google Scholar]
- Brodin G., Ahgren,A., ten Dijke,P., Heldin,C.-H. and Heuchel,R. (2000) Efficient TGF-β induction of the Smad7 gene requires cooperation between AP-1, Sp1, and Smad proteins on the mouse Smad7 promoter. J. Biol. Chem., 275, 29023–29030. [DOI] [PubMed] [Google Scholar]
- Casellas R. and Brivanlou,A.H. (1998) Xenopus Smad7 inhibits both the activin and BMP pathways and acts as a neural inducer. Dev. Biol., 198, 1–12. [DOI] [PubMed] [Google Scholar]
- Cerwenka A. and Swain,S.L. (1999) TGF–β1: immunosuppressant and viability factor for T lymphocytes. Microbes Infect., 1, 1291–1296. [DOI] [PubMed] [Google Scholar]
- Chen R.H., Su,Y.H., Chuang,R.L. and Chang,T.Y. (1998) Suppression of transforming growth factor-β-induced apoptosis through a phosphatidylinositol 3-kinase/Akt-dependent pathway. Oncogene, 17, 1959–1968. [DOI] [PubMed] [Google Scholar]
- Choy L., Skillington,J. and Derynck,R. (2000) Roles of autocrine TGF-β receptor and Smad signaling in adipocyte differentiation. J. Cell Biol., 149, 667–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ M. et al. (1994) Immune dysregulation in TGF-β 1-deficient mice. J. Immunol., 153, 1936–1946. [PubMed] [Google Scholar]
- Datto M.B., Yu,Y. and Wang,X.F. (1995) Functional analysis of the transforming growth factor β responsive elements in the WAF1/Cip1/p21 promoter. J. Biol. Chem., 270, 28623–28628. [DOI] [PubMed] [Google Scholar]
- Ebisawa T., Fukuchi,M., Murakami,G., Chiba,T., Tanaka,K., Imamura,T. and Miyazono,K. (2001) Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. J. Biol. Chem., 276, 12477–12480. [DOI] [PubMed] [Google Scholar]
- Foitzik K., Paus,R., Doetschman,T. and Dotto,G.P. (1999) The TGF-β2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev. Biol., 212, 278–289. [DOI] [PubMed] [Google Scholar]
- Go C., He,W., Zhong,L., Li,P., Huang,J., Brinkley,B.R. and Wang,X.J. (2000) Aberrant cell cycle progression contributes to the early-stage accelerated carcinogenesis in transgenic epidermis expressing the dominant negative TGFβRII. Oncogene, 19, 3623–3631. [DOI] [PubMed] [Google Scholar]
- Guo Q., Kumar,T.R., Woodruff,T., Hadsell,L.A., DeMayo,F.J. and Matzuk,M.M. (1998) Overexpression of mouse follistatin causes reproductive defects in transgenic mice. Mol. Endocrinol., 12, 96–106. [DOI] [PubMed] [Google Scholar]
- Hata A., Lagna,G., Massague,J. and Hemmati-Brivanlou,A. (1998) Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev., 12, 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H. et al. (1997) The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell, 89, 1165–1173. [DOI] [PubMed] [Google Scholar]
- He W., Cao,T., Smith,D.A., Myers,T.E. and Wang,X.J. (2001) Smads mediate signaling of the TGFβ superfamily in normal keratinocytes but are lost during skin chemical carcinogenesis. Oncogene, 20, 471–483. [DOI] [PubMed] [Google Scholar]
- Imamura T., Takase,M., Nishihara,A., Oeda,E., Hanai,J.-I., Kawabata,M. and Miyazono,K. (1997) Smad6 inhibits signalling by the TGF-β superfamily. Nature, 389, 622–626. [DOI] [PubMed] [Google Scholar]
- Ishidou Y. et al. (1995) Enhanced expression of type I receptors for bone morphogenetic proteins during bone formation. J. Bone Miner. Res., 10, 1651–1659. [DOI] [PubMed] [Google Scholar]
- Kavsak P., Rasmussen,R.K., Causing,C.G., Bonni,S., Zhu,H., Thomsen,G.H. and Wrana,J.L. (2000) Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol. Cell, 6, 1365–1375. [DOI] [PubMed] [Google Scholar]
- Kleeff J., Ishiwata,T., Maruyama,H., Friess,H., Truong,P., Buchler,M.W., Falb,D. and Korc,M. (1999) The TGF-β signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene, 18, 5363–5372. [DOI] [PubMed] [Google Scholar]
- Korchynskyi O., Landström,M., Stoika,R., Funa,K., Heldin,C.-H., ten Dijke,P. and Souchelnytskyi,S. (1999) Expression of Smad proteins in human colorectal cancer. Int. J. Cancer, 82, 197–202. [DOI] [PubMed] [Google Scholar]
- Kulessa H., Turk,G. and Hogan,B.L. (2000) Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J., 19, 6664–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand F., Mazars,A., Prunier,C., Bertrand,F., Kornprost,M., Gallea,S., Roman-Roman,S., Cherqui,G. and Atfi,A. (2001) Smad7 inhibits the survival nuclear factor κB and potentiates apoptosis in epithelial cells. Oncogene, 20, 879–884. [DOI] [PubMed] [Google Scholar]
- Landström M., Heldin,N.-E., Bu,S., Hermansson,A., Itoh,S., ten Dijke,P. and Heldin,C.-H. (2000) Smad7 mediates apoptosis induced by transforming growth factor β in prostatic carcinoma cells. Curr. Biol., 10, 535–538. [DOI] [PubMed] [Google Scholar]
- Letterio J.J. and Roberts,A.B. (1997) TGF–β: a critical modulator of immune cell function. Clin. Immunol. Immunopathol., 84, 244–250. [DOI] [PubMed] [Google Scholar]
- Matzuk M.M., Kumar,T.R., Vassalli,A., Bickenbach,J.R., Roop,D.R., Jaenisch,R. and Bradley,A. (1995) Functional analysis of activins during mammalian development. Nature, 374, 354–356. [DOI] [PubMed] [Google Scholar]
- Mazars A., Lallemand,F., Prunier,C., Marais,J., Ferrand,N., Pessah,M., Cherqui,G. and Atfi,A. (2001) Evidence for a role of the JNK cascade in Smad7-mediated apoptosis. J. Biol. Chem., 276, 36797–36803. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Kusanagi,K. and Inoue,H. (2001) Divergence and convergence of TGF-β/BMP signaling. J. Cell Physiol., 187, 265–276. [DOI] [PubMed] [Google Scholar]
- Monteleone G., Kumberova,A., Croft,N.M., McKenzie,C., Steer,H.W. and MacDonald,T.T. (2001) Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J. Clin. Invest., 108, 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine T., Imamura,T., Ishidou,Y., Kato,M., Murata,F., ten Dijke,P. and Sakou,T. (1998) Immunohistochemical detection of activin A, follistatin, and activin receptors during fracture healing in the rat. J. Orthop. Res., 16, 314–321. [DOI] [PubMed] [Google Scholar]
- Nakao A. et al. (1997) Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature, 389, 631–635. [DOI] [PubMed] [Google Scholar]
- Obata H., Kaji,Y., Yamada,H., Kato,M., Tsuru,T. and Yamashita,H. (1999) Expression of transforming growth factor-β superfamily receptors in rat eyes. Acta Ophthalmol. Scand., 77, 151–156. [DOI] [PubMed] [Google Scholar]
- Onishi T., Ishidou,Y., Nagamine,T., Yone,K., Imamura,T., Kato,M., Sampath,T.K., ten Dijke,P. and Sakou,T. (1998) Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone, 22, 605–612. [DOI] [PubMed] [Google Scholar]
- Paus R., Foitzik,K., Welker,P., Bulfone-Paus,S. and Eichmuller,S. (1997) Transforming growth factor-β receptor type I and type II expression during murine hair follicle development and cycling. J. Invest. Dermatol., 109, 518–526. [DOI] [PubMed] [Google Scholar]
- Persson U., Izumi,H., Souchelnytskyi,S., Itoh,S., Grimsby,S., Engström,U., Heldin,C.-H., Funa,K. and ten Dijke,P. (1998) The L45 loop in type I receptors for TGF-β family members is a critical determinant in specifying Smad isoform activation. FEBS Lett., 434, 83–87. [DOI] [PubMed] [Google Scholar]
- Pietenpol J.A., Holt,J.T., Stein,R.W. and Moses,H.L. (1990) Transforming growth factor β1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc. Natl Acad. Sci. USA, 87, 3758–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittelkow M.R., Coffey,R.J.J. and Moses,H.J. (1988) Keratinocytes produce and are regulated by transforming growth factors. Ann. N. Y. Acad. Sci., 548, 211–224. [DOI] [PubMed] [Google Scholar]
- Ramirez A., Bravo,A., Jorcano,J.L. and Vidal,M. (1994) Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation, 58, 53–64. [DOI] [PubMed] [Google Scholar]
- Robles A.I., Larcher,F., Whalin,R.B., Murillas,R., Richie,E., Gimenez-Conti,I.B., Jorcano,J.L. and Conti,C.J. (1996) Expression of cyclin D1 in epithelial tissues of transgenic mice results in epidermal hyperproliferation and severe thymic hyperplasia. Proc. Natl Acad. Sci. USA, 93, 7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford L.P., Ormsby,I., Gittenberger-de Groot,A.C., Sariola,H., Friedman,R., Boivin,G.P., Cardell,E.L. and Doetschman,T. (1997) TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development, 124, 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Bitzer,M., Roberts,I.S., Kopp,J.B., ten Dijke,P., Mundel,P. and Böttinger,E.P. (2001) Apoptosis in podocytes induced by TGF-β and Smad7. J. Clin. Invest., 108, 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns K.S., Cook,J.E. and Le,P.T. (1997) TGF-β differentially modulates epidermal growth factor-mediated increases in leukemia-inhibitory factor, IL-6, IL-1α, and IL-1β in human thymic epithelial cells. J. Immunol., 158, 2704–2712. [PubMed] [Google Scholar]
- Sellheyer K., Bickenbach,J.R., Rothnagel,J.A., Bundman,D., Longley,M.A., Krieg,T., Roche,N.S., Roberts,A.B. and Roop,D.R. (1993) Inhibition of skin development by overexpression of transforming growth factor β1 in the epidermis of transgenic mice. Proc. Natl Acad. Sci. USA, 90, 5237–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan Y., Lovicu,F.J. and Overbeek,P.A. (1998) Lens-specific expression of transforming growth factor β1 in transgenic mice causes anterior subcapsular cataracts. J. Clin. Invest., 101, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hogerlinden M., Rozell,B.L., Ahrlund-Richter,L. and Toftgard,R. (1999) Squamous cell carcinomas and increased apoptosis in skin with inhibited Rel/nuclear factor-κB signaling. Cancer Res., 59, 3299–3303. [PubMed] [Google Scholar]
- Vassalli A., Matzuk,M.M., Gardner,H.A., Lee,K.F. and Jaenisch,R. (1994) Activin/inhibin βB subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev., 8, 414–427. [DOI] [PubMed] [Google Scholar]
- Wang X.-J., Greenhalgh,D.A., Lu,X.R., Bickenbach,J.R. and Roop,D.R. (1995) TGFα and v-fos cooperation in transgenic mouse epidermis induces aberrant keratinocyte differentiation and stable, autonomous papillomas. Oncogene, 10, 279–289. [PubMed] [Google Scholar]
- Wang X.-J., Greenhalgh,D.A., Bickenbach,J.R., Jiang,A., Bundman,D.S., Krieg,T., Derynck,R. and Roop,D.R. (1997) Expression of a dominant-negative type II transforming growth factor β (TGF-β) receptor in the epidermis of transgenic mice blocks TGF-β-mediated growth inhibition. Proc. Natl Acad. Sci. USA, 94, 2386–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-J., Liefer,K.M., Tsai,S.Y., O’Malley,B.W. and Roop,D.R. (1999) Development of gene-switch transgenic mice that inducibly express transforming growth factor β1 in the epidermis. Proc. Natl Acad. Sci. USA, 96, 8483–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H., ten Dijke,P., Heldin,C.H. and Miyazono,K. (1996) Bone morphogenetic protein receptors. Bone, 19, 569–574. [DOI] [PubMed] [Google Scholar]
- Yang X., Letterio,J.J., Lechleider,R.J., Chen,L., Hayman,R., Gu,H., Roberts,A.B. and Deng,C. (1999) Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J., 18, 1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]