Abstract
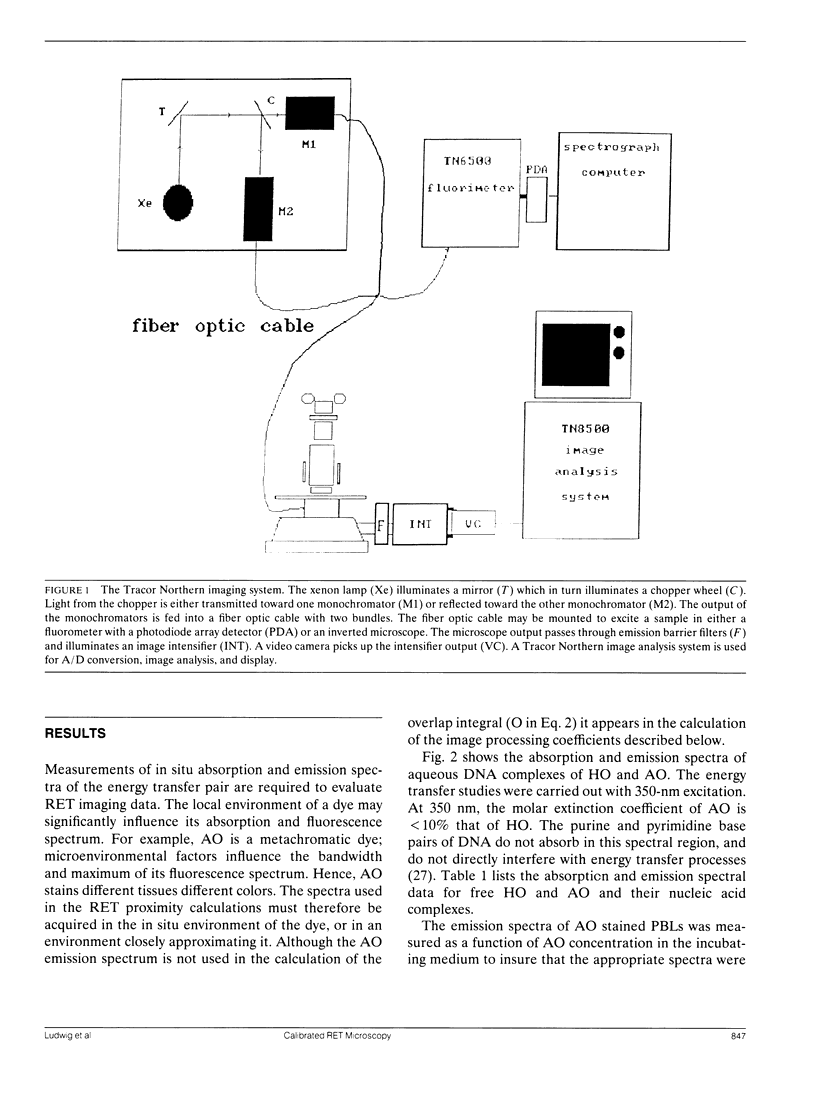
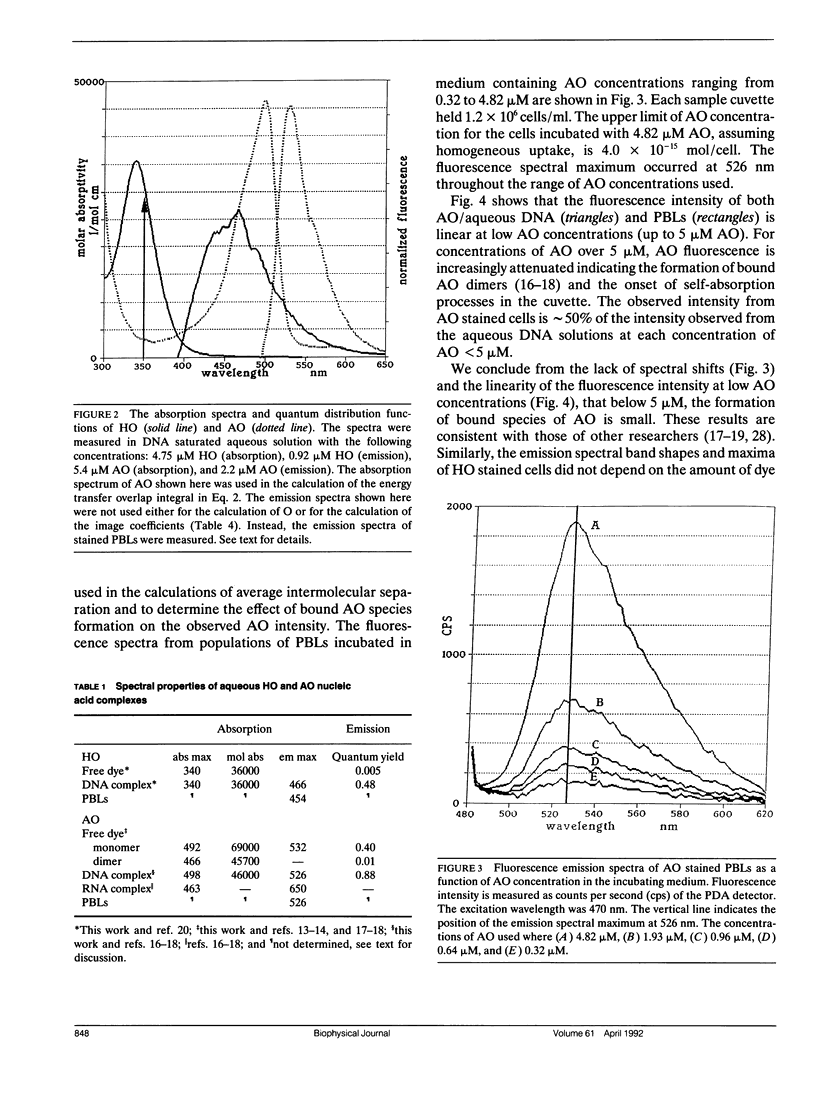
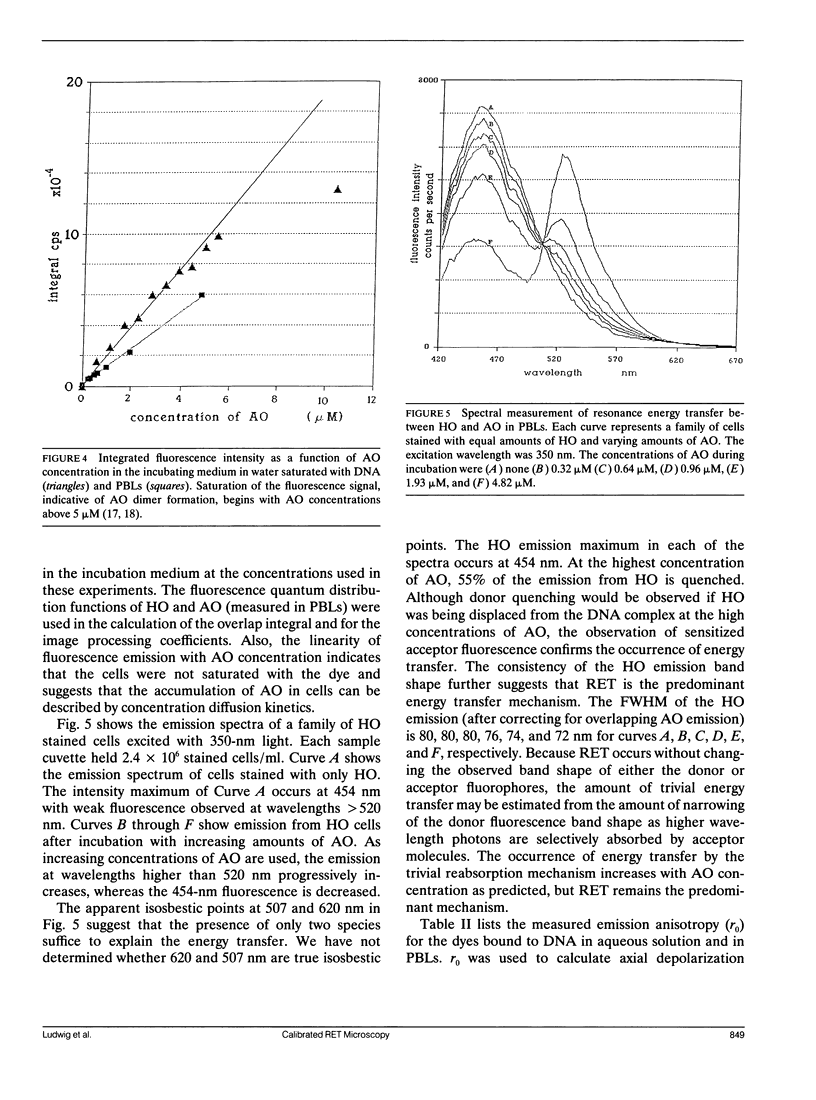
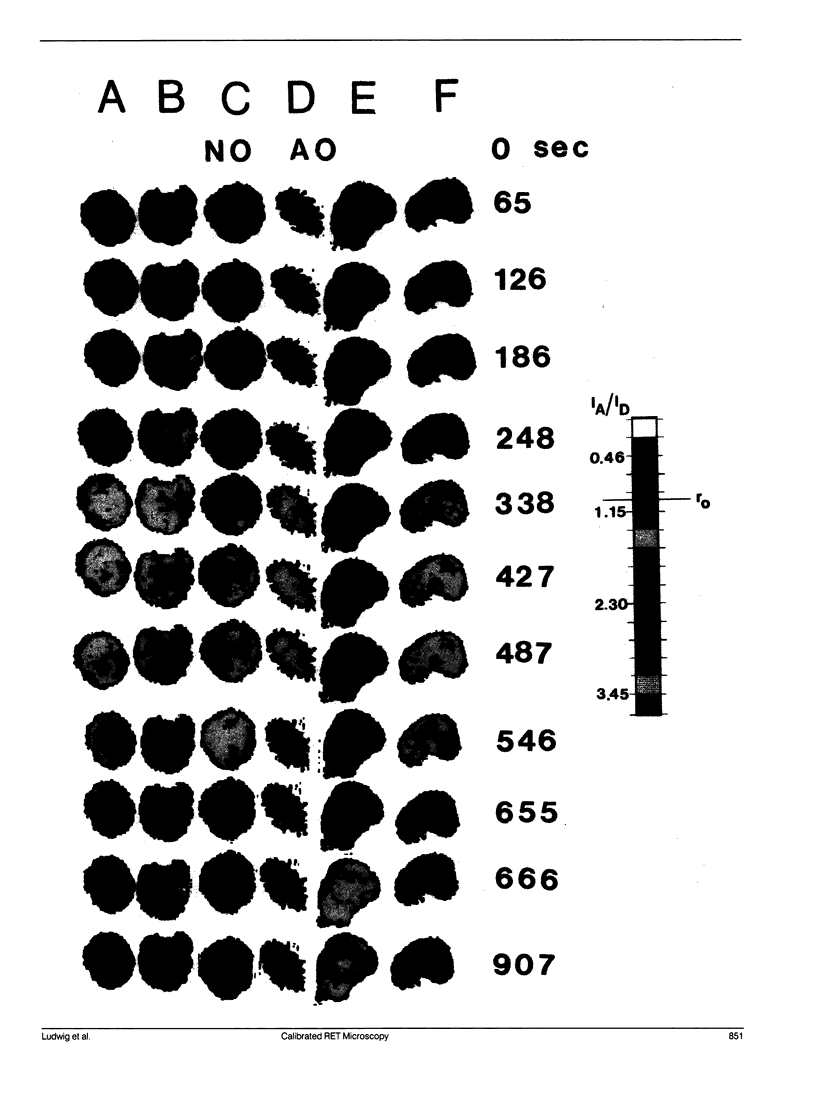
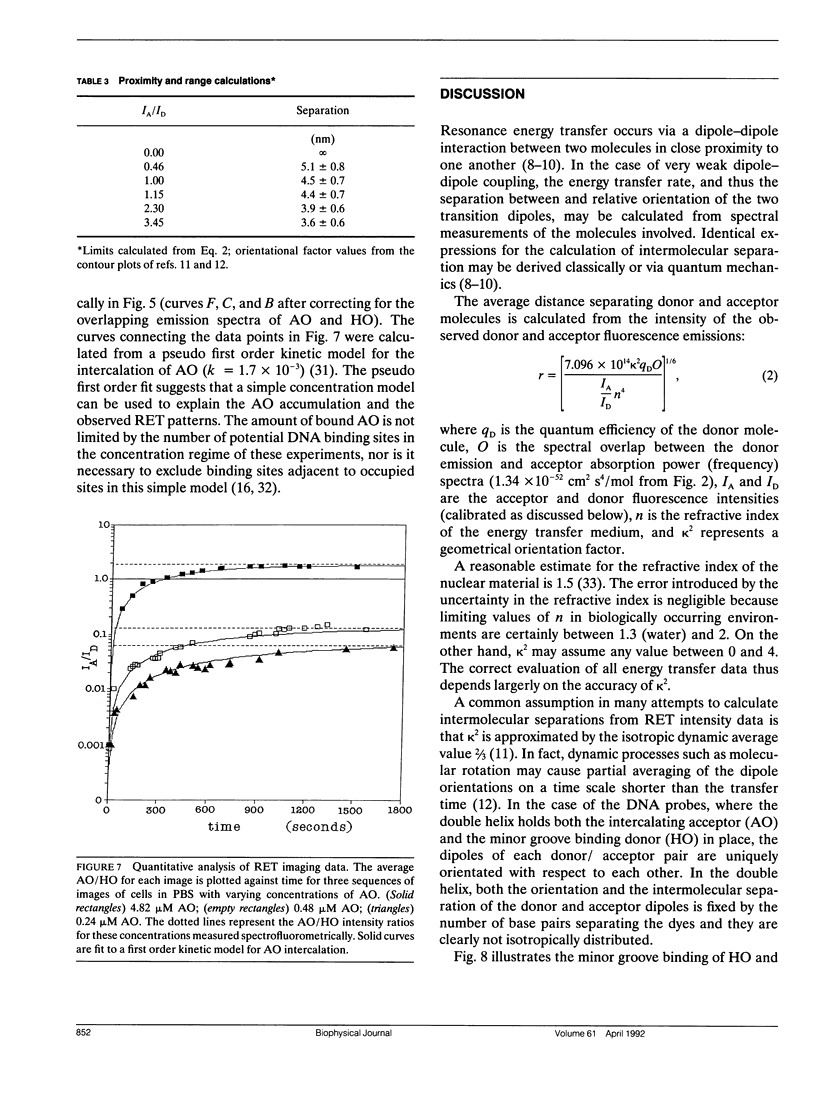
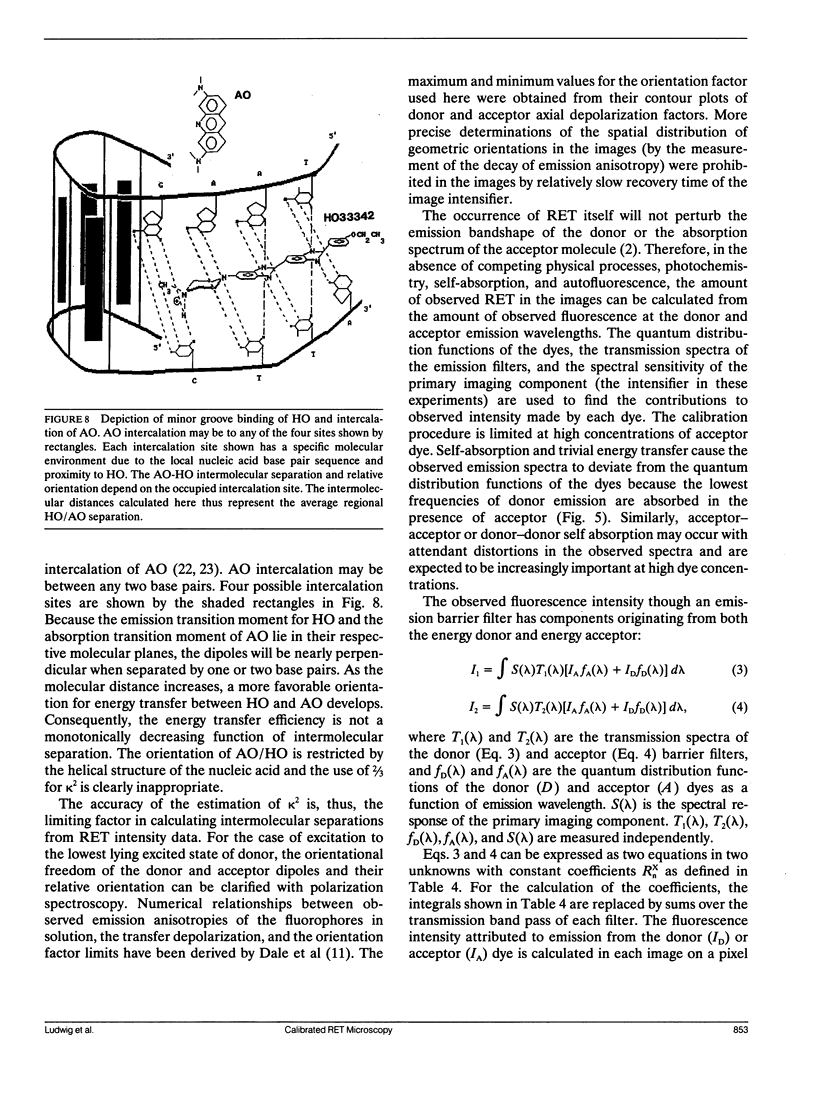
A quantitative technique for the nondestructive visualization of nanometer scale intermolecular separations in a living system is described. A calibration procedure for the acquisition and analysis of resonance energy transfer (RET) image data is outlined. The factors limiting RET imaging of biological samples are discussed. Measurements required for the calibration include: (a) the spectral sensitivity of the image intensifier (or camera); (b) the transmission spectra of the emission filters; and (c) the quantum distribution functions of the energy transfer pair measured in situ. Resonance energy transfer imaging is demonstrated for two DNA specific dyes. The Förster critical distance for energy transfer between Hoechst 33342 (HO) and acridine orange (AO) is 4.5 +/- 0.7 nm. This distance is slightly greater than the distance of a single turn of the DNA helix (3.5 nm or approximately 10 base pairs), and is well below the optical diffraction limit. Timed sequences of intracellular energy transfer reveal nuclear structure, strikingly similar to that observed with confocal and electron microscopy, and may show the spatial distribution of eu- and hetero- chromatin in the interphase nuclei.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Sedat J. W. Three-dimensional architecture of a polytene nucleus. Nature. 1983 Apr 21;302(5910):676–681. doi: 10.1038/302676a0. [DOI] [PubMed] [Google Scholar]
- Armstrong R. W., Kurucsev T., Strauss U. P. The interaction between acridine dyes and deoxyribonucleic acid. J Am Chem Soc. 1970 May 20;92(10):3174–3181. doi: 10.1021/ja00713a041. [DOI] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Jovin T. M. Analysis and sorting of living cells according to deoxyribonucleic acid content. J Histochem Cytochem. 1977 Jul;25(7):585–589. doi: 10.1177/25.7.70450. [DOI] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Robert-Nicoud M., Jovin T. M. Probing DNA structure and function with a multi-wavelength fluorescence confocal laser microscope. J Microsc. 1990 Jan;157(Pt 1):61–72. doi: 10.1111/j.1365-2818.1990.tb02947.x. [DOI] [PubMed] [Google Scholar]
- Aubin J. E. Autofluorescence of viable cultured mammalian cells. J Histochem Cytochem. 1979 Jan;27(1):36–43. doi: 10.1177/27.1.220325. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, HENDLEY D. D., STEINER R. F. Inhibition and activation of polynucleotide phosphorylase through the formation of complexes between acridine orange and polynucleotides. Nature. 1958 Jul 26;182(4630):242–244. doi: 10.1038/182242a0. [DOI] [PubMed] [Google Scholar]
- Beisker W., Eisert W. G. Denaturation and condensation of intracellular nucleic acids monitored by fluorescence depolarization of intercalating dyes in individual cells. J Histochem Cytochem. 1989 Nov;37(11):1699–1704. doi: 10.1177/37.11.2478614. [DOI] [PubMed] [Google Scholar]
- Benson D. M., Bryan J., Plant A. L., Gotto A. M., Jr, Smith L. C. Digital imaging fluorescence microscopy: spatial heterogeneity of photobleaching rate constants in individual cells. J Cell Biol. 1985 Apr;100(4):1309–1323. doi: 10.1083/jcb.100.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum A. Separation of lymphocytes, lymphocyte subgroups and monocytes: a review. Lymphology. 1977 Jun;10(2):71–76. [PubMed] [Google Scholar]
- Cohen G., Eisenberg H. Deoxyribonucleate solutions: sedimentation in a density gradient, partial specific volumes, density and refractive index increments, and preferential interactions. Biopolymers. 1968;6(8):1077–1100. doi: 10.1002/bip.1968.360060805. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Eisinger J., Blumberg W. E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J. 1979 May;26(2):161–193. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Kapuscinski J., Staiano-Coico L., Melamed M. R. Accessibility of DNA in situ to various fluorochromes: relationship to chromatin changes during erythroid differentiation of Friend leukemia cells. Cytometry. 1984 Jul;5(4):355–363. doi: 10.1002/cyto.990050411. [DOI] [PubMed] [Google Scholar]
- Davies H. G., Murray A. B., Walmsley M. E. Electron-microscope observations on the organization of the nucleus in chicken erythrocytes and a superunit thread hypothesis for chromosome structure. J Cell Sci. 1974 Nov;16(2):261–299. doi: 10.1242/jcs.16.2.261. [DOI] [PubMed] [Google Scholar]
- Dix J. A., Verkman A. S. Mapping of fluorescence anisotropy in living cells by ratio imaging. Application to cytoplasmic viscosity. Biophys J. 1990 Feb;57(2):231–240. doi: 10.1016/S0006-3495(90)82526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ E., OTH A., FONTAINE F. The ultraviolet spectrum of deoxyribonucleic acids and their constituents. J Mol Biol. 1961 Feb;3:11–17. doi: 10.1016/s0022-2836(61)80003-x. [DOI] [PubMed] [Google Scholar]
- Gerace L., Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Herman B. Resonance energy transfer microscopy. Methods Cell Biol. 1989;30:219–243. doi: 10.1016/s0091-679x(08)60981-4. [DOI] [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Interactions of acridine orange with double stranded nucleic acids. Spectral and affinity studies. J Biomol Struct Dyn. 1987 Aug;5(1):127–143. doi: 10.1080/07391102.1987.10506381. [DOI] [PubMed] [Google Scholar]
- Lalande M. E., Ling V., Miller R. G. Hoechst 33342 dye uptake as a probe of membrane permeability changes in mammalian cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):363–367. doi: 10.1073/pnas.78.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Stetten G. Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J Histochem Cytochem. 1976 Jan;24(1):24–33. doi: 10.1177/24.1.943439. [DOI] [PubMed] [Google Scholar]
- Markovits J., Garbay-Jaureguiberry C., Roques B. P., Le Pecq J. B. Acridine dimers: influence of the intercalating ring and of the linking-chain nature on the equilibrium and kinetic DNA-binding parameters. Eur J Biochem. 1989 Mar 15;180(2):359–366. doi: 10.1111/j.1432-1033.1989.tb14656.x. [DOI] [PubMed] [Google Scholar]
- Müller W., Gautier F. Interactions of heteroaromatic compounds with nucleic acids. A - T-specific non-intercalating DNA ligands. Eur J Biochem. 1975 Jun;54(2):385–394. doi: 10.1111/j.1432-1033.1975.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Nicolini C., Belmont A., Parodi S., Lessin S., Abraham S. Mass action and acridine orange staining: static and flow cytofluorometry. J Histochem Cytochem. 1979 Jan;27(1):102–113. doi: 10.1177/27.1.86559. [DOI] [PubMed] [Google Scholar]
- Paoletti J., Le Pecq J. B. Resonance energy transfer between ethidium bromide molecules bound to nucleic acids. Does intercalation wind or unwind the DNA helix? J Mol Biol. 1971 Jul 14;59(1):43–62. doi: 10.1016/0022-2836(71)90412-8. [DOI] [PubMed] [Google Scholar]
- Pjura P. E., Grzeskowiak K., Dickerson R. E. Binding of Hoechst 33258 to the minor groove of B-DNA. J Mol Biol. 1987 Sep 20;197(2):257–271. doi: 10.1016/0022-2836(87)90123-9. [DOI] [PubMed] [Google Scholar]
- Rigler R., Jr Microfluorometric characterization of intracellular nucleic acids and nucleoproteins by acridine orange. Acta Physiol Scand Suppl. 1966;267:1–122. [PubMed] [Google Scholar]
- Stryer L., Haugland R. P. Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci U S A. 1967 Aug;58(2):719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Sharpless T., Melamed M. R. Simultaneous staining of ribonucleic and deoxyribonucleic acids in unfixed cells using acridine orange in a flow cytofluorometric system. J Histochem Cytochem. 1977 Jan;25(1):46–56. doi: 10.1177/25.1.64567. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Cereghini S. Structure of transcriptionally active chromatin. CRC Crit Rev Biochem. 1986;21(1):1–26. doi: 10.3109/10409238609113607. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Wähnert U. Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material. Prog Biophys Mol Biol. 1986;47(1):31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]



