Abstract
The electrophoretic mobility of liposomes containing a negatively charged derivative of phosphatidylethanolamine with a large headgroup composed of the hydrophilic polymer polyethylene glycol (PEG-PE) was determined by Doppler electrophoretic light scattering. The results show that this method is improved by the use of measurements at multiple angles to eliminate artifacts and that very small mobilities can be measured. The electrophoretic mobility of liposomes with 5 to 10 mol% PEG-PE is approximately -0.5 mu ms-1/Vcm-1 regardless of PEG-PE content compared with approximately -2 mu ms-1/Vcm-1 for similar liposomes but containing 7.5% phosphatidylglycerol (PG) instead of PEG-PE. Measurements of surface potential by distribution of an anionic fluorescent probe show that the PEG-PE imparts a negative charge identical to that by PG, consistent with the expectation of similar locations of the ionized phosphate responsible for the charge. The reduced mobility imparted by the surface bound PEG is attributed to a mechanism similar to that described for colloidal steric stabilization: hydrodynamic drag moves the hydrodynamic plane of shear, or the hydrodynamic radius, away from the charge-bearing plane, that of the phosphate moities. An extended length of approximately 50 A for the 2,000 molecular weight PEG is estimated from the reduction in electrophoretic mobility.
Full text
PDF


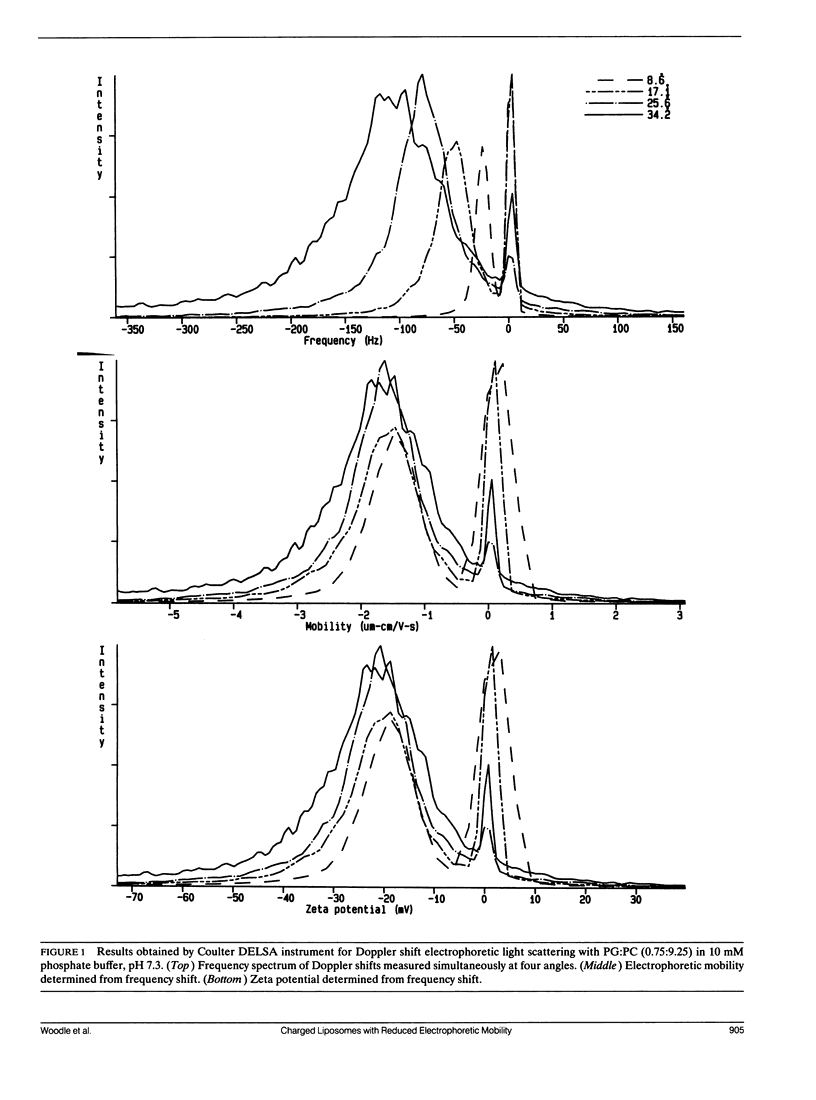
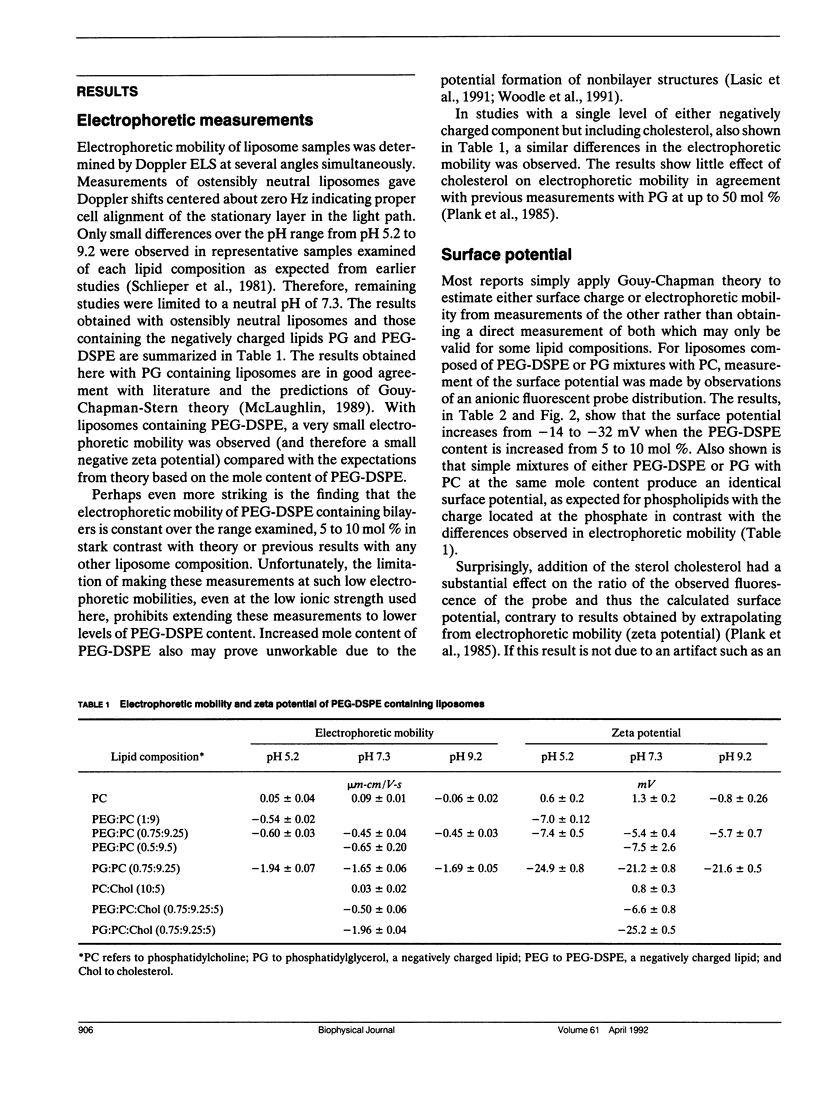
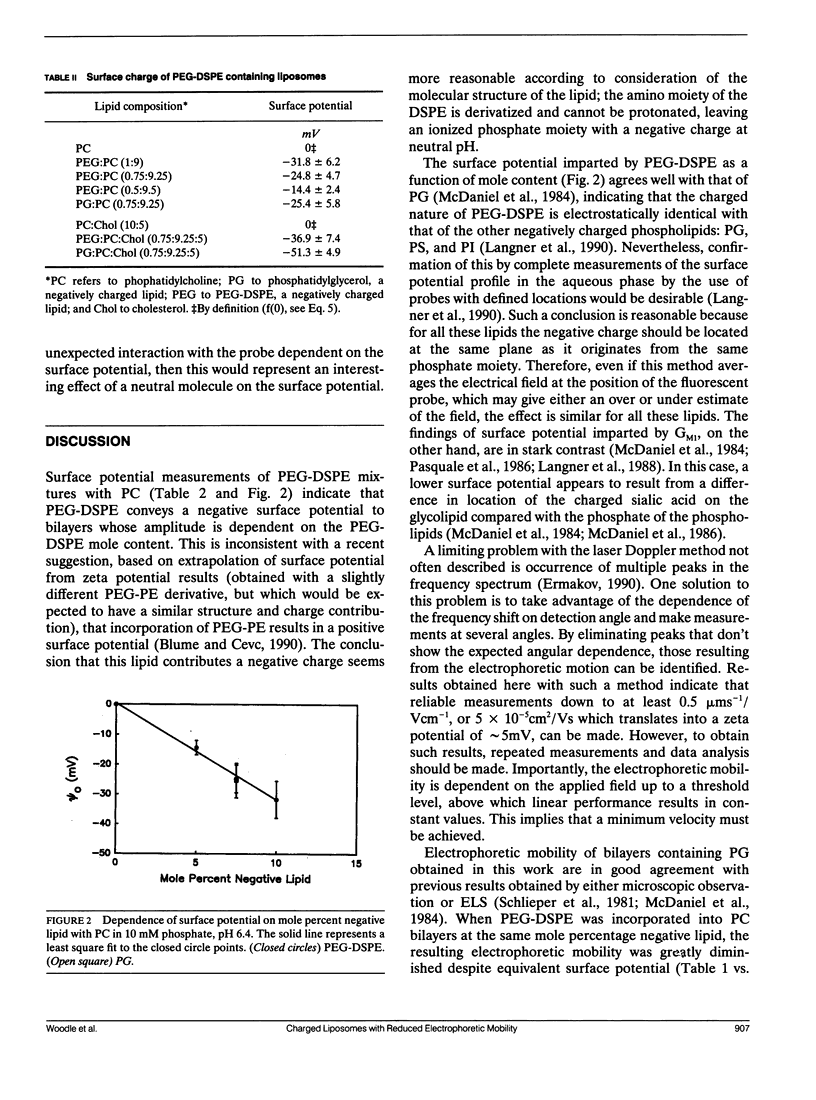



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. M., Chonn A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 1987 Oct 19;223(1):42–46. doi: 10.1016/0014-5793(87)80506-9. [DOI] [PubMed] [Google Scholar]
- Allen T. M., Hansen C., Rutledge J. Liposomes with prolonged circulation times: factors affecting uptake by reticuloendothelial and other tissues. Biochim Biophys Acta. 1989 May 19;981(1):27–35. doi: 10.1016/0005-2736(89)90078-3. [DOI] [PubMed] [Google Scholar]
- Arnold K., Zschoernig O., Barthel D., Herold W. Exclusion of poly(ethylene glycol) from liposome surfaces. Biochim Biophys Acta. 1990 Mar;1022(3):303–310. doi: 10.1016/0005-2736(90)90278-v. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., HEARD D. H., FLEMANS R., SEAMAN G. V. An apparatus for microelectrophoresis of small particles. Nature. 1958 Sep 6;182(4636):642–644. doi: 10.1038/182642a0. [DOI] [PubMed] [Google Scholar]
- Bangham A. D., Standish M. M., Miller N. Cation permeability of phospholipid model membranes: effect of narcotics. Nature. 1965 Dec 25;208(5017):1295–1297. doi: 10.1038/2081295a0. [DOI] [PubMed] [Google Scholar]
- Bazzi M. D., Nelsestuen G. L. Association of protein kinase C with phospholipid vesicles. Biochemistry. 1987 Jan 13;26(1):115–122. doi: 10.1021/bi00375a017. [DOI] [PubMed] [Google Scholar]
- Beitinger H., Vogel V., Möbius D., Rahmann H. Surface potentials and electric dipole moments of ganglioside and phospholipid bilayers: contribution of the polar headgroup at the water/lipid interface. Biochim Biophys Acta. 1989 Sep 18;984(3):293–300. doi: 10.1016/0005-2736(89)90296-4. [DOI] [PubMed] [Google Scholar]
- Blume G., Cevc G. Liposomes for the sustained drug release in vivo. Biochim Biophys Acta. 1990 Nov 2;1029(1):91–97. doi: 10.1016/0005-2736(90)90440-y. [DOI] [PubMed] [Google Scholar]
- Cafiso D., McLaughlin A., McLaughlin S., Winiski A. Measuring electrostatic potentials adjacent to membranes. Methods Enzymol. 1989;171:342–364. doi: 10.1016/s0076-6879(89)71019-3. [DOI] [PubMed] [Google Scholar]
- Cevc G. Membrane electrostatics. Biochim Biophys Acta. 1990 Oct 8;1031(3):311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- Ermakov Y. A. The determination of binding site density and association constants for monovalent cation adsorption onto liposomes made from mixtures of zwitterionic and charged lipids. Biochim Biophys Acta. 1990 Mar 30;1023(1):91–97. doi: 10.1016/0005-2736(90)90013-e. [DOI] [PubMed] [Google Scholar]
- Gabizon A., Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6949–6953. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanov A. L., Maruyama K., Beckerleg A. M., Torchilin V. P., Huang L. Activity of amphipathic poly(ethylene glycol) 5000 to prolong the circulation time of liposomes depends on the liposome size and is unfavorable for immunoliposome binding to target. Biochim Biophys Acta. 1991 Feb 25;1062(2):142–148. doi: 10.1016/0005-2736(91)90385-l. [DOI] [PubMed] [Google Scholar]
- Klibanov A. L., Maruyama K., Torchilin V. P., Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990 Jul 30;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- Kossovsky N., Gelman A., Sponsler E., Millett D. Nanocrystalline Epstein-Barr virus decoys. J Appl Biomater. 1991 Winter;2(4):251–259. doi: 10.1002/jab.770020406. [DOI] [PubMed] [Google Scholar]
- Lang J., Vigo-Pelfrey C., Martin F. Liposomes composed of partially hydrogenated egg phosphatidylcholines: fatty acid composition, thermal phase behavior and oxidative stability. Chem Phys Lipids. 1990 Mar;53(1):91–101. doi: 10.1016/0009-3084(90)90137-g. [DOI] [PubMed] [Google Scholar]
- Langner M., Cafiso D., Marcelja S., McLaughlin S. Electrostatics of phosphoinositide bilayer membranes. Theoretical and experimental results. Biophys J. 1990 Feb;57(2):335–349. doi: 10.1016/S0006-3495(90)82535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Levine M., Sharp K. A., Brooks D. E. Theory of the electrokinetic behavior of human erythrocytes. Biophys J. 1983 May;42(2):127–135. doi: 10.1016/S0006-3495(83)84378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H., Furusawa K. Electrical phenomena at the surface of phospholipid membranes relevant to the sorption of ionic compounds. Adv Colloid Interface Sci. 1989 Jul;30(1-2):71–109. doi: 10.1016/0001-8686(89)80004-1. [DOI] [PubMed] [Google Scholar]
- McDaniel R. V., McLaughlin A., Winiski A. P., Eisenberg M., McLaughlin S. Bilayer membranes containing the ganglioside GM1: models for electrostatic potentials adjacent to biological membranes. Biochemistry. 1984 Sep 25;23(20):4618–4624. doi: 10.1021/bi00315a016. [DOI] [PubMed] [Google Scholar]
- McDaniel R. V., Sharp K., Brooks D., McLaughlin A. C., Winiski A. P., Cafiso D., McLaughlin S. Electrokinetic and electrostatic properties of bilayers containing gangliosides GM1, GD1a, or GT1. Comparison with a nonlinear theory. Biophys J. 1986 Mar;49(3):741–752. doi: 10.1016/S0006-3495(86)83700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Whitaker M. Cations that alter surface potentials of lipid bilayers increase the calcium requirement for exocytosis in sea urchin eggs. J Physiol. 1988 Feb;396:189–204. doi: 10.1113/jphysiol.1988.sp016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser P., Marchand-Arvier M., Labrude P., Handjani-Vila R. M., Vigneron C. Niosomes d'hémoglobine. I. Préparation, propriétés physicochimiques et oxyphoriques, stabilité. Pharm Acta Helv. 1989;64(7):192–202. [PubMed] [Google Scholar]
- Namba Y., Sakakibara T., Masada M., Ito F., Oku N. Glucuronate-modified liposomes with prolonged circulation time. Chem Pharm Bull (Tokyo) 1990 Jun;38(6):1663–1666. doi: 10.1248/cpb.38.1663. [DOI] [PubMed] [Google Scholar]
- Ohki S. Adsorption of local anesthetics on phospholipid membranes. Biochim Biophys Acta. 1984 Oct 17;777(1):56–66. doi: 10.1016/0005-2736(84)90496-6. [DOI] [PubMed] [Google Scholar]
- Olson F., Hunt C. A., Szoka F. C., Vail W. J., Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophys Acta. 1979 Oct 19;557(1):9–23. doi: 10.1016/0005-2736(79)90085-3. [DOI] [PubMed] [Google Scholar]
- Pasquale L., Winiski A., Oliva C., Vaio G., McLaughlin S. An experimental test of new theoretical models for the electrokinetic properties of biological membranes. The effect of UO2++ and tetracaine on the electrophoretic mobility of bilayer membranes and human erythrocytes. J Gen Physiol. 1986 Dec;88(6):697–718. doi: 10.1085/jgp.88.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank L., Dahl C. E., Ware B. R. Effect of sterol incorporation on head group separation in liposomes. Chem Phys Lipids. 1985 Mar;36(4):319–328. doi: 10.1016/0009-3084(85)90039-8. [DOI] [PubMed] [Google Scholar]
- Schlieper P., Medda P. K., Kaufmann R. Drug-induced zeta potential changes in liposomes studied by laser Doppler spectroscopy. Biochim Biophys Acta. 1981 Jun 22;644(2):273–283. doi: 10.1016/0005-2736(81)90385-0. [DOI] [PubMed] [Google Scholar]
- Senior J., Delgado C., Fisher D., Tilcock C., Gregoriadis G. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: studies with poly(ethylene glycol)-coated vesicles. Biochim Biophys Acta. 1991 Feb 11;1062(1):77–82. doi: 10.1016/0005-2736(91)90337-8. [DOI] [PubMed] [Google Scholar]
- Smejtek P., Wang S. R. Adsorption to dipalmitoylphosphatidylcholine membranes in gel and fluid state: pentachlorophenolate, dipicrylamine, and tetraphenylborate. Biophys J. 1990 Nov;58(5):1285–1294. doi: 10.1016/S0006-3495(90)82468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T., Nir S., Wilschut J. Membrane fusion activity of influenza virus. Effects of gangliosides and negatively charged phospholipids in target liposomes. Biochemistry. 1989 Feb 21;28(4):1698–1704. doi: 10.1021/bi00430a041. [DOI] [PubMed] [Google Scholar]
- Tatulian S. A. Binding of alkaline-earth metal cations and some anions to phosphatidylcholine liposomes. Eur J Biochem. 1987 Dec 30;170(1-2):413–420. doi: 10.1111/j.1432-1033.1987.tb13715.x. [DOI] [PubMed] [Google Scholar]
- Uzgiris E. E. Probing immune reactions by laser light scattering spectroscopy. Methods Enzymol. 1981;74(Pt 100):177–198. doi: 10.1016/0076-6879(81)74013-8. [DOI] [PubMed] [Google Scholar]
- Woodle M. C., Papahadjopoulos D. Liposome preparation and size characterization. Methods Enzymol. 1989;171:193–217. doi: 10.1016/s0076-6879(89)71012-0. [DOI] [PubMed] [Google Scholar]


