Abstract
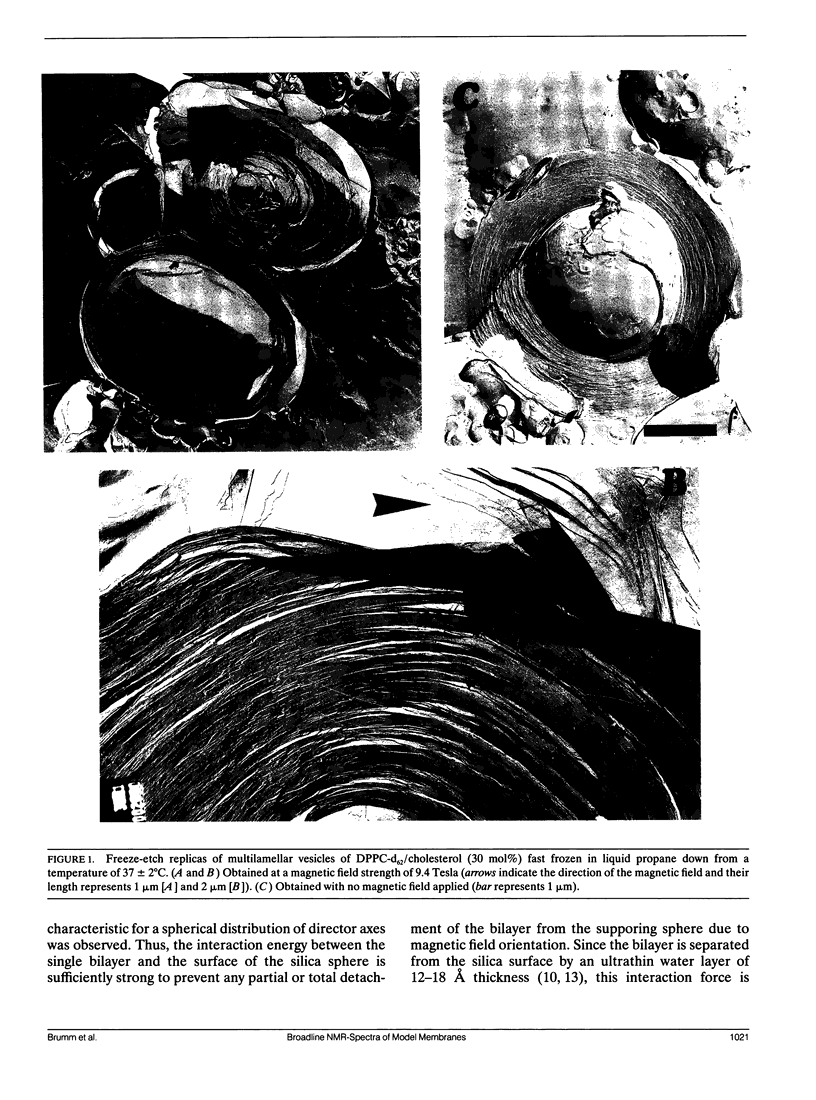
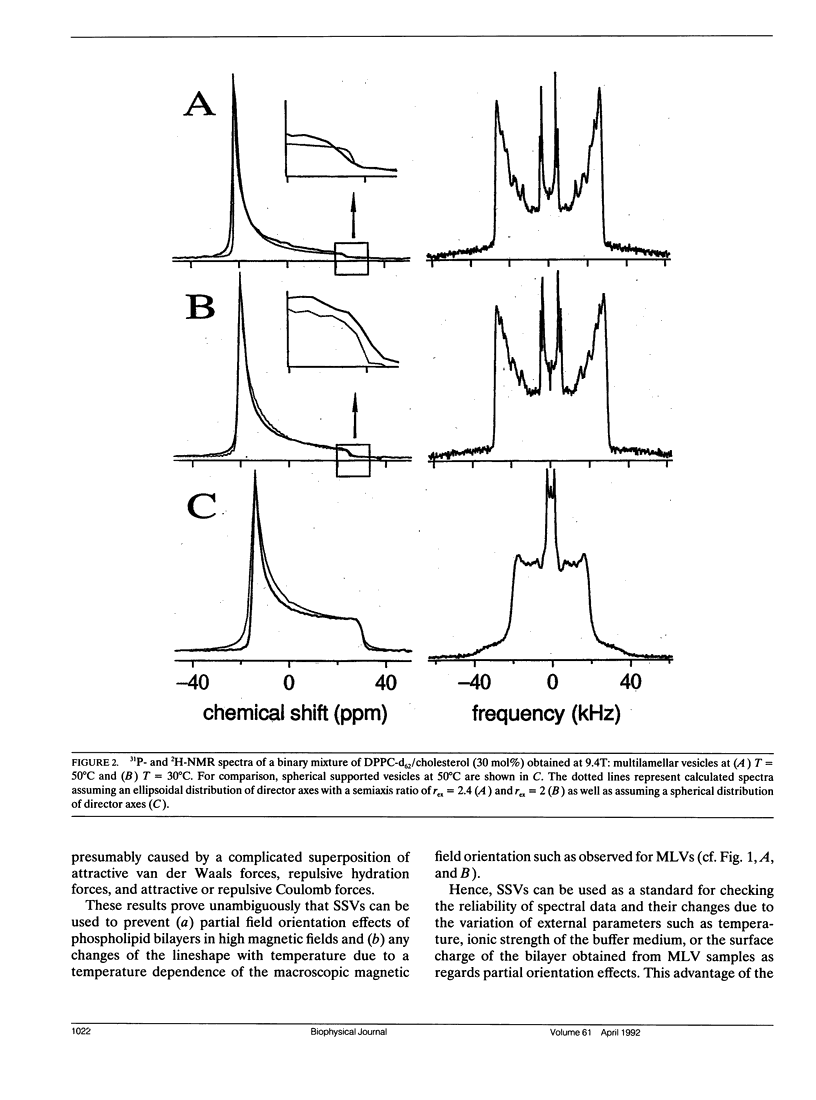
The partial orientation of multilamellar vesicles (MLV) in high magnetic fields has been studied and a method to prevent such effects is herewith proposed. The orientation effect was measured with 2H-, 31P-NMR and electron microscopy on MLVs of dipalmitoyl phosphatidylcholine with 30 mol% cholesterol. We present the first freeze—etch electron microscopy data obtained from MLV samples that were frozen directly in the NMR magnet at a field strength of 9.4 Tesla. These experiments clearly show that the MLVs adopt an ellipsoidal (but not a cylindrical) shape in the magnetic field. Best fit 31P-NMR lineshape calculations assuming an ellipsoidal distribution of molecular director axes to the experimentally obtained spectra provide a quantitative measure of the average semiaxis ratio of the ellipsoidal MLVs and its change with temperature. The application of so-called spherical supported vesicles (SSV) is found to prevent any partial orientation effects so that undistorted NMR powder pattern of the bilayer can be measured independently of magnetic field strength and temperature.
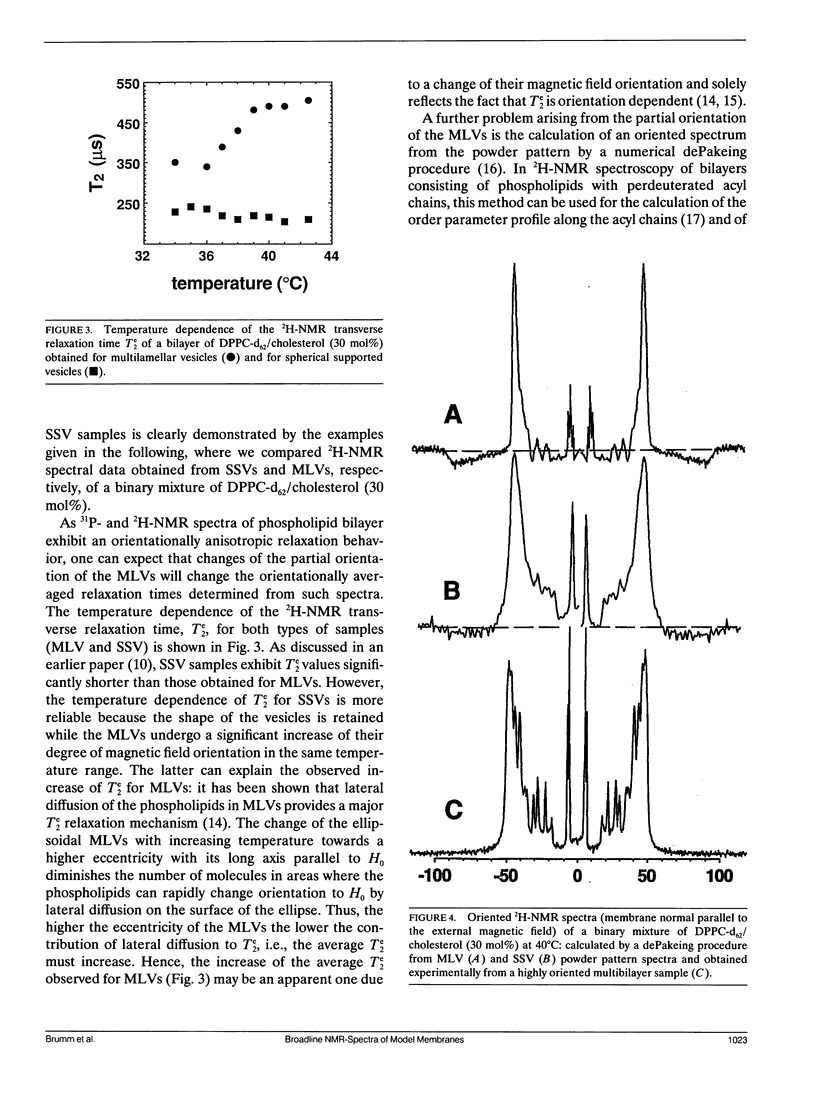
The usefulness of SSVs is further demonstrated by a direct comparison of spectral data such as 31P-and 2H-NMR lineshapes and relaxation times as well as 2H-NMR dePaked spectra obtained for both model systems. These experiments show that spectral data obtained from partially oriented MLVs are not unambiguous to interpret, in particular, if an external parameter such as temperature is varied.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayerl T. M., Bloom M. Physical properties of single phospholipid bilayers adsorbed to micro glass beads. A new vesicular model system studied by 2H-nuclear magnetic resonance. Biophys J. 1990 Aug;58(2):357–362. doi: 10.1016/S0006-3495(90)82382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayerl T. M., Köchy T., Brückner S. On the modulation of a high-enthalpy pretransition in binary mixtures of DMPC and DMPG by polar headgroup interaction. Biophys J. 1990 Mar;57(3):675–680. doi: 10.1016/S0006-3495(90)82587-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroske E., Helfrich W. Magnetic anisotropy of egg lecithin membranes. Biophys J. 1978 Dec;24(3):863–868. doi: 10.1016/S0006-3495(78)85425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Johnson S. J., Bayerl T. M., McDermott D. C., Adam G. W., Rennie A. R., Thomas R. K., Sackmann E. Structure of an adsorbed dimyristoylphosphatidylcholine bilayer measured with specular reflection of neutrons. Biophys J. 1991 Feb;59(2):289–294. doi: 10.1016/S0006-3495(91)82222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur M., Fine B., Sternin E., Cullis P. R., Bloom M. Smoothed orientational order profile of lipid bilayers by 2H-nuclear magnetic resonance. Biophys J. 1989 Nov;56(5):1037–1041. doi: 10.1016/S0006-3495(89)82749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Modulation of phospholipid acyl chain order by cholesterol. A solid-state 2H nuclear magnetic resonance study. Biochemistry. 1990 Nov 27;29(47):10676–10684. doi: 10.1021/bi00499a015. [DOI] [PubMed] [Google Scholar]
- Scholz F., Boroske E., Helfrich W. Magnetic anisotropy of lecithin membranes. A new anisotropy susceptometer. Biophys J. 1984 Mar;45(3):589–592. doi: 10.1016/S0006-3495(84)84196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Speyer J. B., Sripada P. K., Das Gupta S. K., Shipley G. G., Griffin R. G. Magnetic orientation of sphingomyelin-lecithin bilayers. Biophys J. 1987 Apr;51(4):687–691. doi: 10.1016/S0006-3495(87)83394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick P. I., Dea P., Chan S. I. Characterization of the transverse relaxation rates in lipid bilayers. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2082–2086. doi: 10.1073/pnas.87.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]



