Abstract
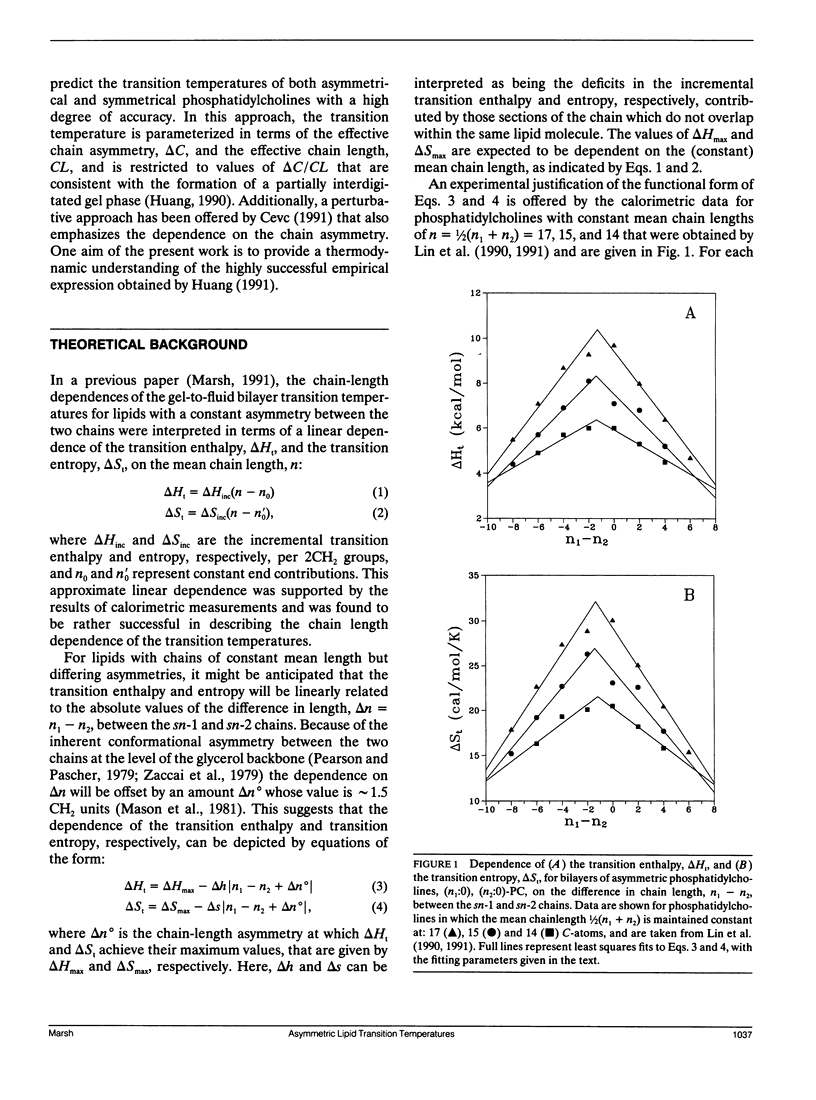
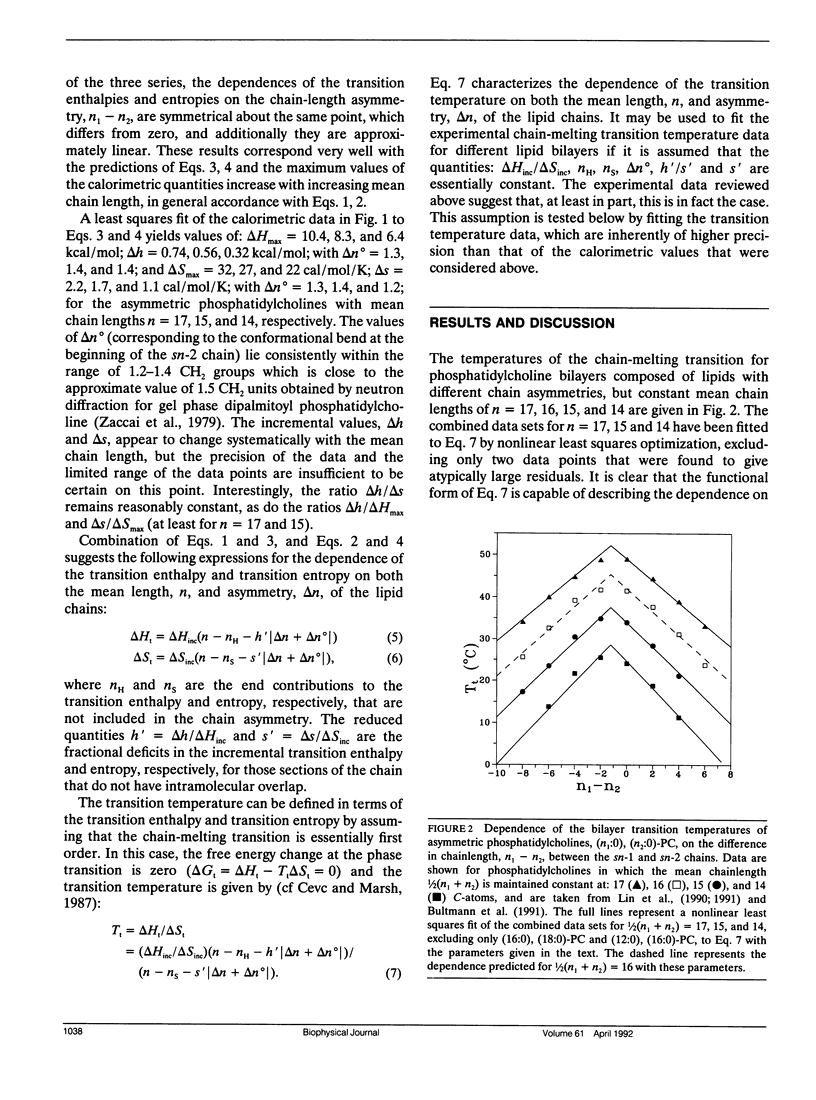
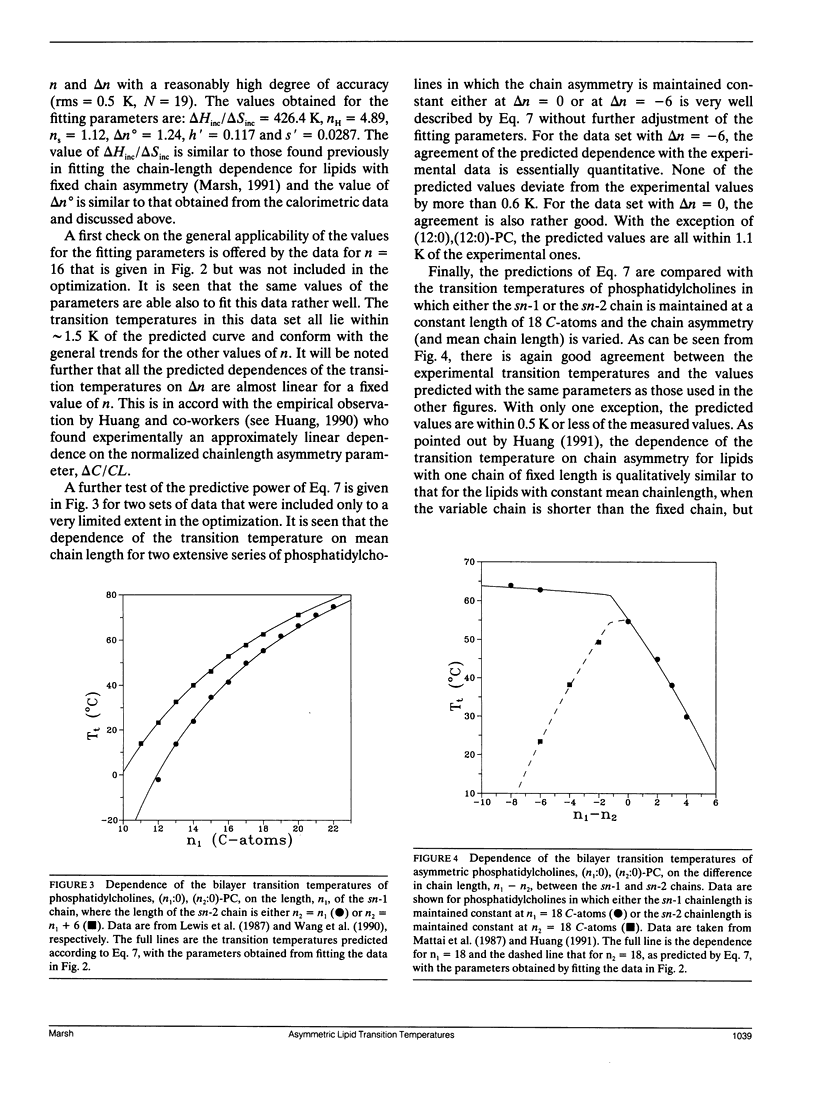
The analysis of the chain-length dependence of the chain-melting transition temperatures of bilayers composed of lipids with identical chains (Marsh, D. 1991. Biochim. Biophys. Acta. 1062: 1-6) is extended to include lipids with chains of unequal length. The bilayer transition temperatures of saturated asymmetrical phosphatidylcholines are interpreted by assuming that the transition enthalpy and transition entropy are linearly related to the absolute value of the difference in chain length between the sn-1 and sn-2 chains, with constant end contributions. Such an assumption is supported by calorimetric data on phosphatidylcholines of constant mean chainlength and varying chain asymmetry. In particular, a symmetrical linear dependence is observed on the chain asymmetry, Δn, which is centered around a value Δn° that corresponds to the conformational inequivalence of the sn-1 and sn-2 chains. The transition temperature then takes the form: Tt = Tt∞(n - nH - h′ ǀ Δn + Δn° ǀ)/(n - ns - s′ ǀ Δn + Δn°) where nH, ns are the end contributions, and h′, s′ are fractional deficits in the incremental transition enthalpy and entropy, respectively, arising from the overlapping regions of the longer chains. Optimization on the transition temperature data for the dependence on chain asymmetry of three series of phosphatidylcholines with constant mean chainlength, n, yields parameters that are capable of predicting the dependence of the transition temperatures on chain asymmetry for other mean chainlengths. The dependence of the transition temperature on mean chainlength for phosphatidylcholines in which the chain asymmetry is maintained constant, as well as the dependence on both mean chain length and chain asymmetry for phosphatidylcholines in which one of the two chains is maintained of constant length, are also described with high accuracy by using the same parameters.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bultmann T., Lin H. N., Wang Z. Q., Huang C. H. Thermotropic and mixing behavior of mixed-chain phosphatidylcholines with molecular weights identical with that of L-alpha-dipalmitoylphosphatidylcholine. Biochemistry. 1991 Jul 23;30(29):7194–7202. doi: 10.1021/bi00243a022. [DOI] [PubMed] [Google Scholar]
- Cevc G. How membrane chain-melting phase-transition temperature is affected by the lipid chain asymmetry and degree of unsaturation: an effective chain-length model. Biochemistry. 1991 Jul 23;30(29):7186–7193. doi: 10.1021/bi00243a021. [DOI] [PubMed] [Google Scholar]
- Huang C. Empirical estimation of the gel to liquid-crystalline phase transition temperatures for fully hydrated saturated phosphatidylcholines. Biochemistry. 1991 Jan 8;30(1):26–30. doi: 10.1021/bi00215a004. [DOI] [PubMed] [Google Scholar]
- Huang C. Mixed-chain phospholipids and interdigitated bilayer systems. Klin Wochenschr. 1990 Feb 1;68(3):149–165. doi: 10.1007/BF01649079. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Mason J. T., Huang C. Acyl chain interdigitation in saturated mixed-chain phosphatidylcholine bilayer dispersions. Biochemistry. 1984 Nov 6;23(23):5570–5577. doi: 10.1021/bi00318a029. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Mak N., McElhaney R. N. A differential scanning calorimetric study of the thermotropic phase behavior of model membranes composed of phosphatidylcholines containing linear saturated fatty acyl chains. Biochemistry. 1987 Sep 22;26(19):6118–6126. doi: 10.1021/bi00393a026. [DOI] [PubMed] [Google Scholar]
- Lin H. N., Wang Z. Q., Huang C. H. Differential scanning calorimetry study of mixed-chain phosphatidylcholines with a common molecular weight identical with diheptadecanoylphosphatidylcholine. Biochemistry. 1990 Jul 31;29(30):7063–7072. doi: 10.1021/bi00482a017. [DOI] [PubMed] [Google Scholar]
- Lin H. N., Wang Z. Q., Huang C. H. The influence of acyl chain-length asymmetry on the phase transition parameters of phosphatidylcholine dispersions. Biochim Biophys Acta. 1991 Aug 5;1067(1):17–28. doi: 10.1016/0005-2736(91)90021-y. [DOI] [PubMed] [Google Scholar]
- Marsh D. Analysis of the chainlength dependence of lipid phase transition temperatures: main and pretransitions of phosphatidylcholines; main and non-lamellar transitions of phosphatidylethanolamines. Biochim Biophys Acta. 1991 Feb 11;1062(1):1–6. doi: 10.1016/0005-2736(91)90326-4. [DOI] [PubMed] [Google Scholar]
- Mason J. T., Huang C., Biltonen R. L. Calorimetric investigations of saturated mixed-chain phosphatidylcholine bilayer dispersions. Biochemistry. 1981 Oct 13;20(21):6086–6092. doi: 10.1021/bi00524a026. [DOI] [PubMed] [Google Scholar]
- Mattai J., Sripada P. K., Shipley G. G. Mixed-chain phosphatidylcholine bilayers: structure and properties. Biochemistry. 1987 Jun 16;26(12):3287–3297. doi: 10.1021/bi00386a007. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., Ellington J. C., Jr, Porter N. A. New structural model for mixed-chain phosphatidylcholine bilayers. Biochemistry. 1984 Aug 28;23(18):4038–4044. doi: 10.1021/bi00313a005. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Wang Z. Q., Lin H. N., Huang C. H. Differential scanning calorimetric study of a homologous series of fully hydrated saturated mixed-chain C(X):C(X + 6) phosphatidylcholines. Biochemistry. 1990 Jul 31;29(30):7072–7076. doi: 10.1021/bi00482a018. [DOI] [PubMed] [Google Scholar]
- Zaccai G., Büldt G., Seelig A., Seelig J. Neutron diffraction studies on phosphatidylcholine model membranes. II. Chain conformation and segmental disorder. J Mol Biol. 1979 Nov 15;134(4):693–706. doi: 10.1016/0022-2836(79)90480-7. [DOI] [PubMed] [Google Scholar]


