Abstract
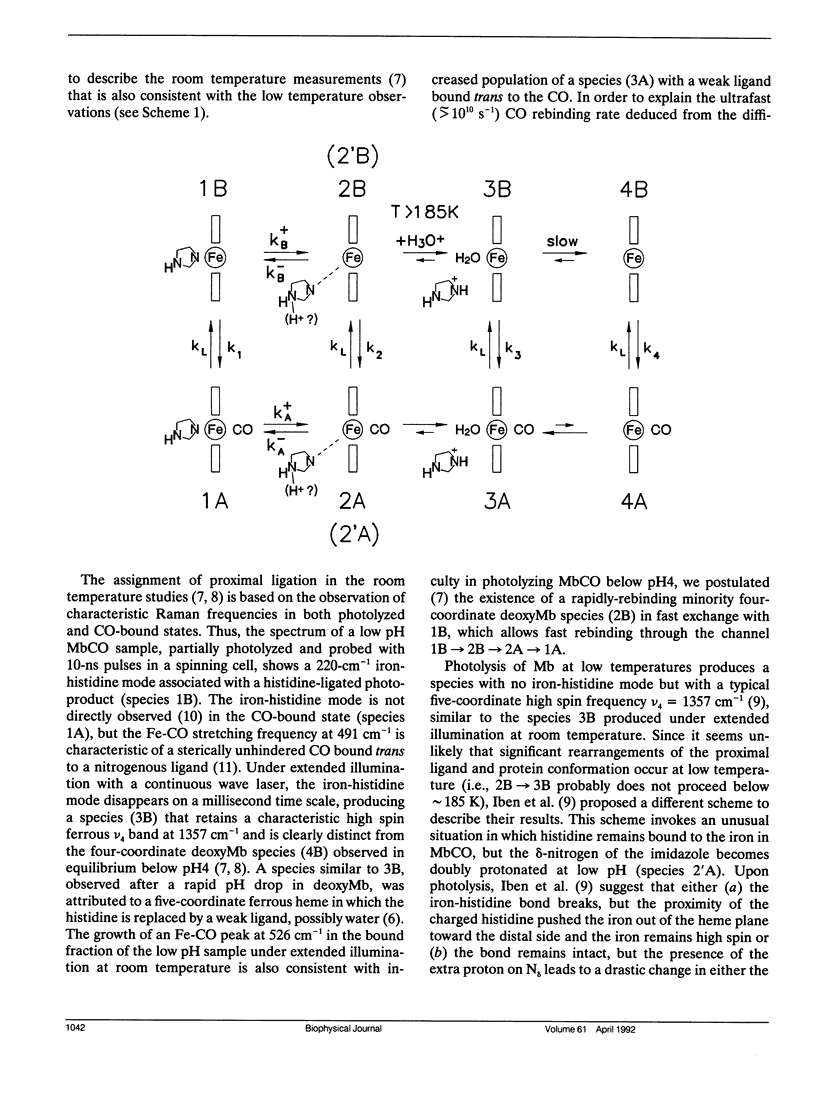
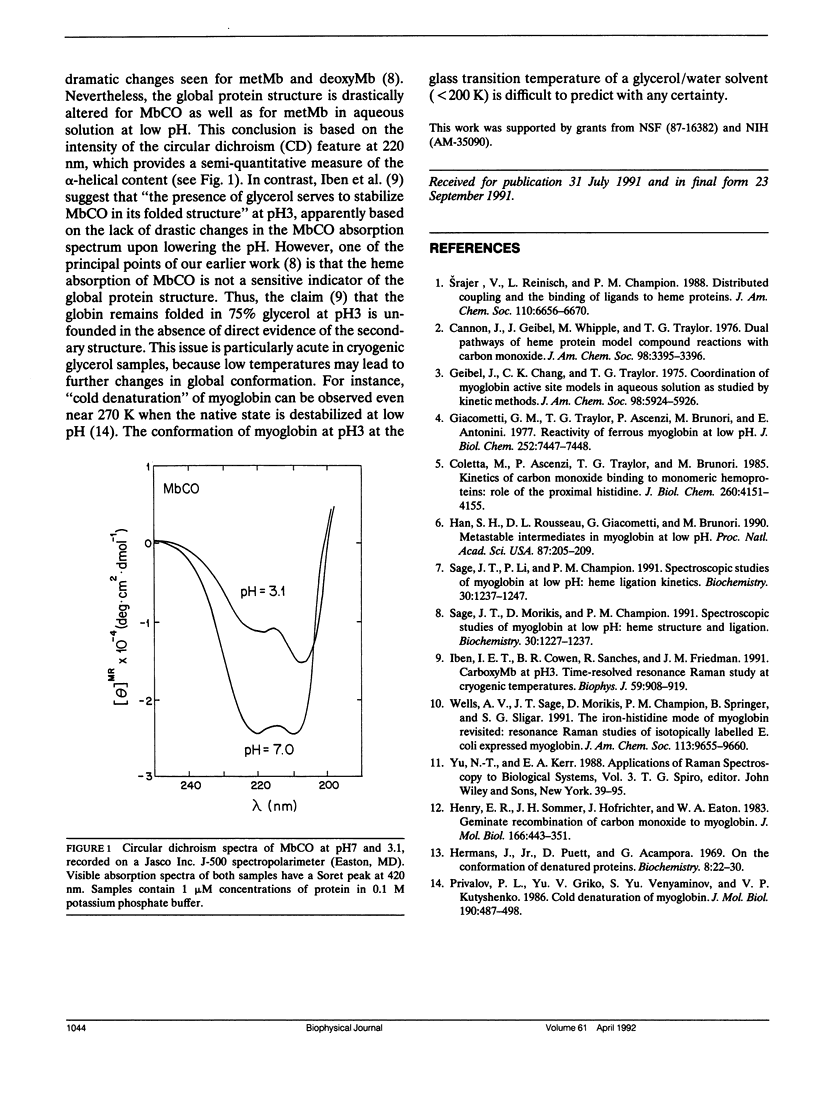
Recently, there has been interest in determining the conditions under which the iron-histidine bond ruptures in myoglobin at low pH, so that the effect of proximal heme ligation can be studied. A 220-cm-1 Raman mode, assigned to iron-histidine stretching, is clearly visible after photolysis of aqueous MbCO samples below pH4 at room temperature (Sage et al. Biochemistry. 30:1237-1247). In contrast, Iben et al. (Biophys. J. 59:908-919) do not observe this mode upon photolysis of a pH3 MbCO sample in a glycerol/water glass at low temperature. In order to account for both the low temperature and the room temperature experiments, Iben et al. suggest a scheme involving an unusual protonation state of the proximal histidine. Here, we discuss some inconsistencies in their explanation of the room temperature results and offer instead a simple modification of an earlier model. In addition, circular dichroism data are presented that indicate partial unfolding of MbCO in aqueous solution below pH4, and raise questions about the claim of Iben et al. that MbCO remains folded in 75% glycerol at pH3.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannon J., Geibel J., Whipple M., Traylor T. G. Letter: Dual pathways of heme protein model compound reactions with carbon monoxide. J Am Chem Soc. 1976 May 26;98(11):3395–3396. doi: 10.1021/ja00427a069. [DOI] [PubMed] [Google Scholar]
- Coletta M., Ascenzi P., Traylor T. G., Brunori M. Kinetics of carbon monoxide binding to monomeric hemoproteins. Role of the proximal histidine. J Biol Chem. 1985 Apr 10;260(7):4151–4155. [PubMed] [Google Scholar]
- Geibel J., Chang C. K., Traylor T. G. Letter: Coordination of myoglobin active site models in aqueous solution as studied by kinetic methods. J Am Chem Soc. 1975 Oct 1;97(20):5924–5926. doi: 10.1021/ja00853a053. [DOI] [PubMed] [Google Scholar]
- Giacometti G. M., Traylor T. G., Ascenzi P., Brunori M., Antonini E. Reactivity of ferrous myoglobin at low pH. J Biol Chem. 1977 Nov 10;252(21):7447–7448. [PubMed] [Google Scholar]
- Han S., Rousseau D. L., Giacometti G., Brunori M. Metastable intermediates in myoglobin at low pH. Proc Natl Acad Sci U S A. 1990 Jan;87(1):205–209. doi: 10.1073/pnas.87.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. R., Sommer J. H., Hofrichter J., Eaton W. A. Geminate recombination of carbon monoxide to myoglobin. J Mol Biol. 1983 May 25;166(3):443–451. doi: 10.1016/s0022-2836(83)80094-1. [DOI] [PubMed] [Google Scholar]
- Hermans J., Jr, Acampora G. On the conformation of denatured proteins. Biochemistry. 1969 Jan;8(1):22–30. doi: 10.1021/bi00829a005. [DOI] [PubMed] [Google Scholar]
- Iben I. E., Cowen B. R., Sanches R., Friedman J. M. Carboxy Mb at pH 3. Time-resolved resonance Raman study at cryogenic temperatures. Biophys J. 1991 Apr;59(4):908–919. doi: 10.1016/S0006-3495(91)82304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalov P. L., Griko YuV, Venyaminov SYu, Kutyshenko V. P. Cold denaturation of myoglobin. J Mol Biol. 1986 Aug 5;190(3):487–498. doi: 10.1016/0022-2836(86)90017-3. [DOI] [PubMed] [Google Scholar]
- Sage J. T., Li P. S., Champion P. M. Spectroscopic studies of myoglobin at low pH: heme ligation kinetics. Biochemistry. 1991 Feb 5;30(5):1237–1247. doi: 10.1021/bi00219a011. [DOI] [PubMed] [Google Scholar]
- Sage J. T., Morikis D., Champion P. M. Spectroscopic studies of myoglobin at low pH: heme structure and ligation. Biochemistry. 1991 Feb 5;30(5):1227–1237. doi: 10.1021/bi00219a010. [DOI] [PubMed] [Google Scholar]


