Abstract
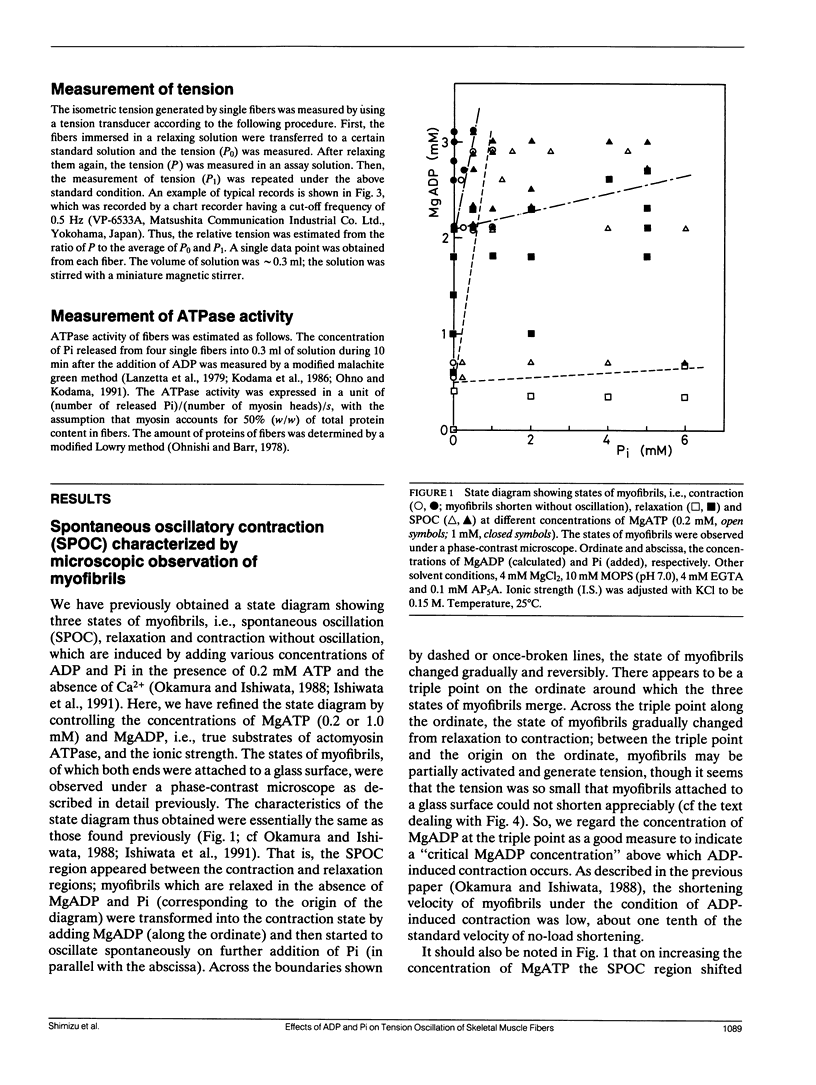
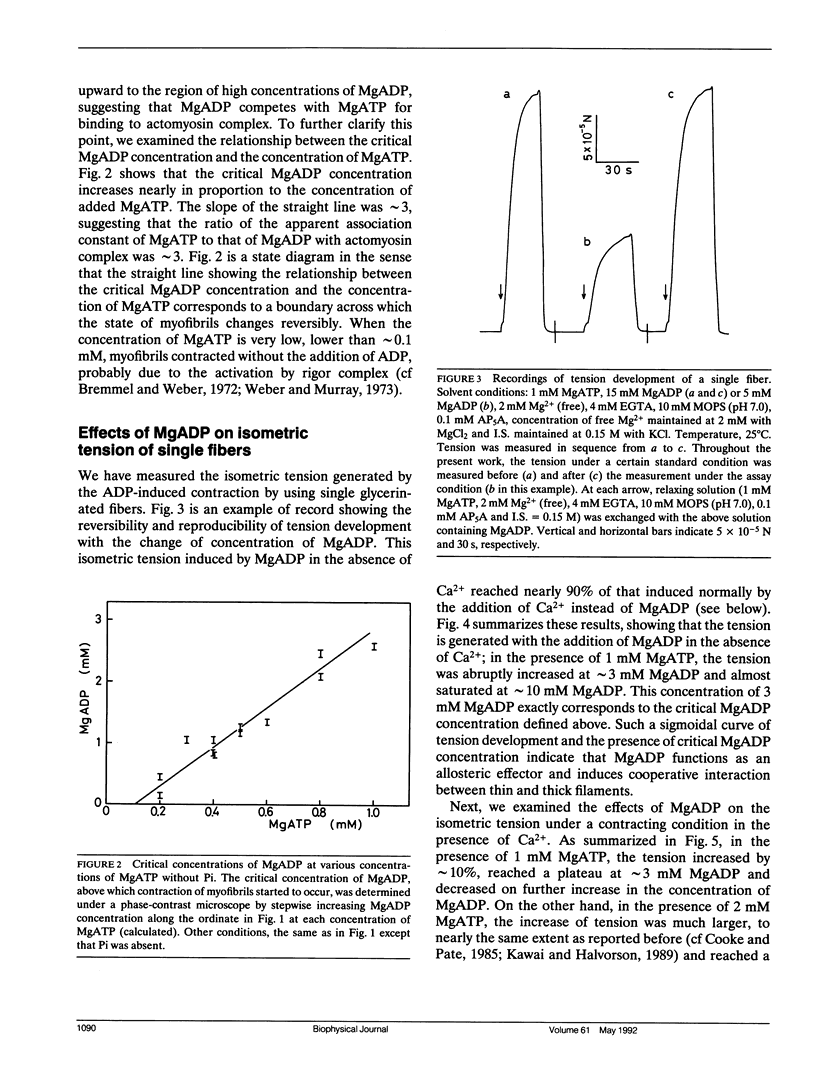
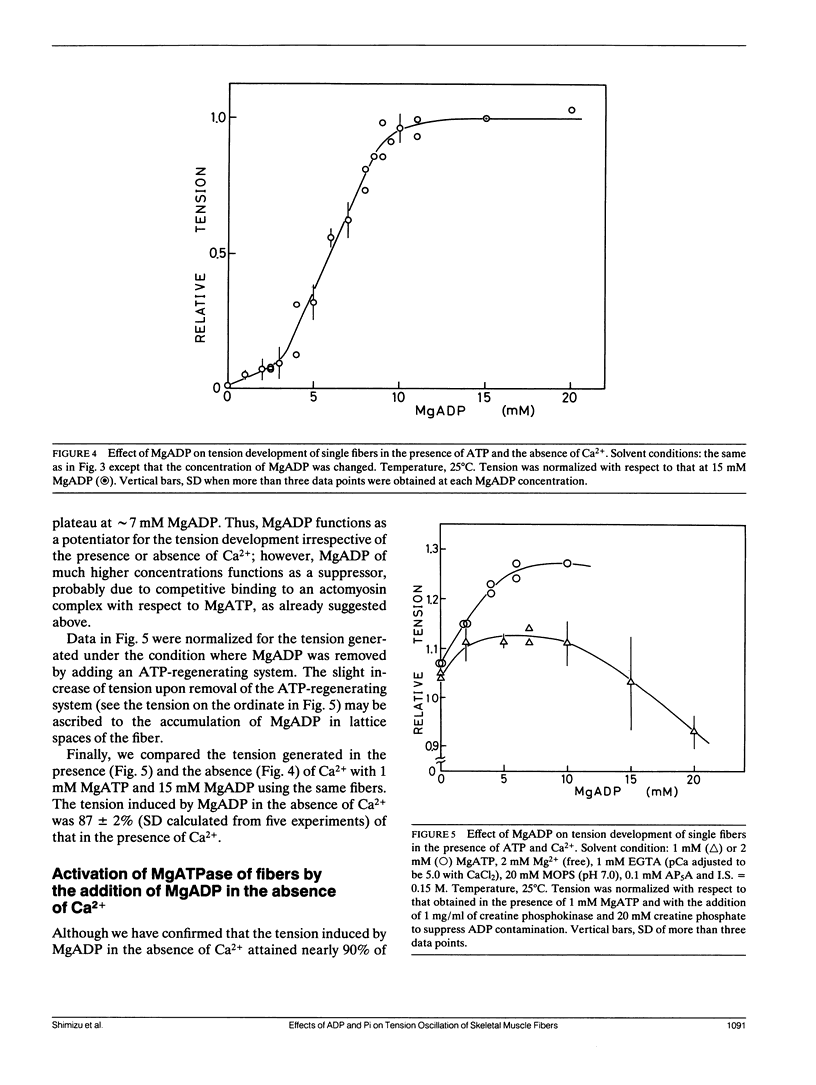
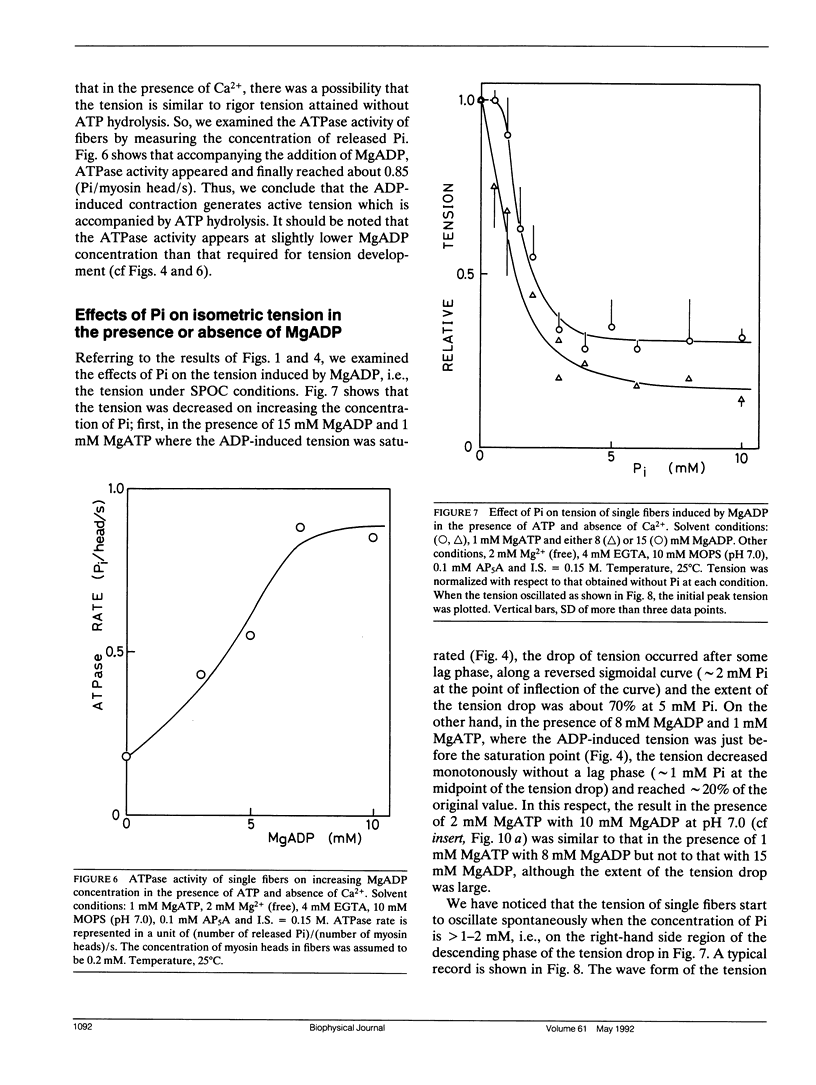
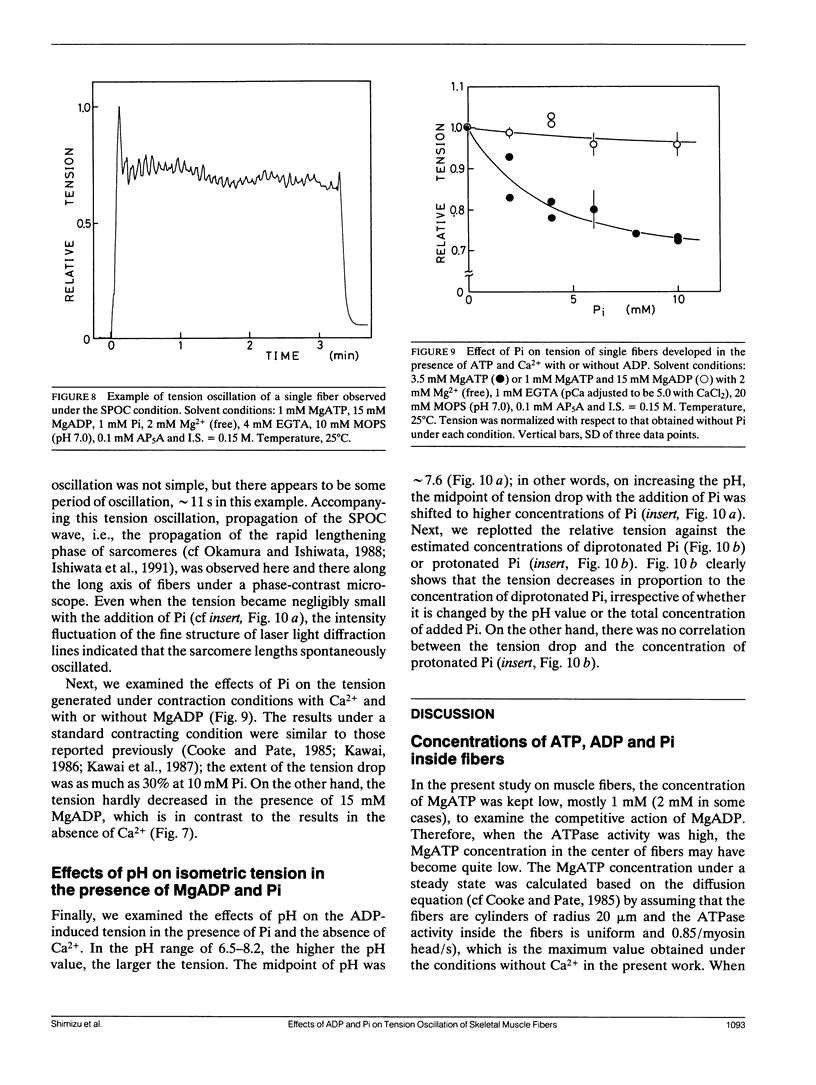
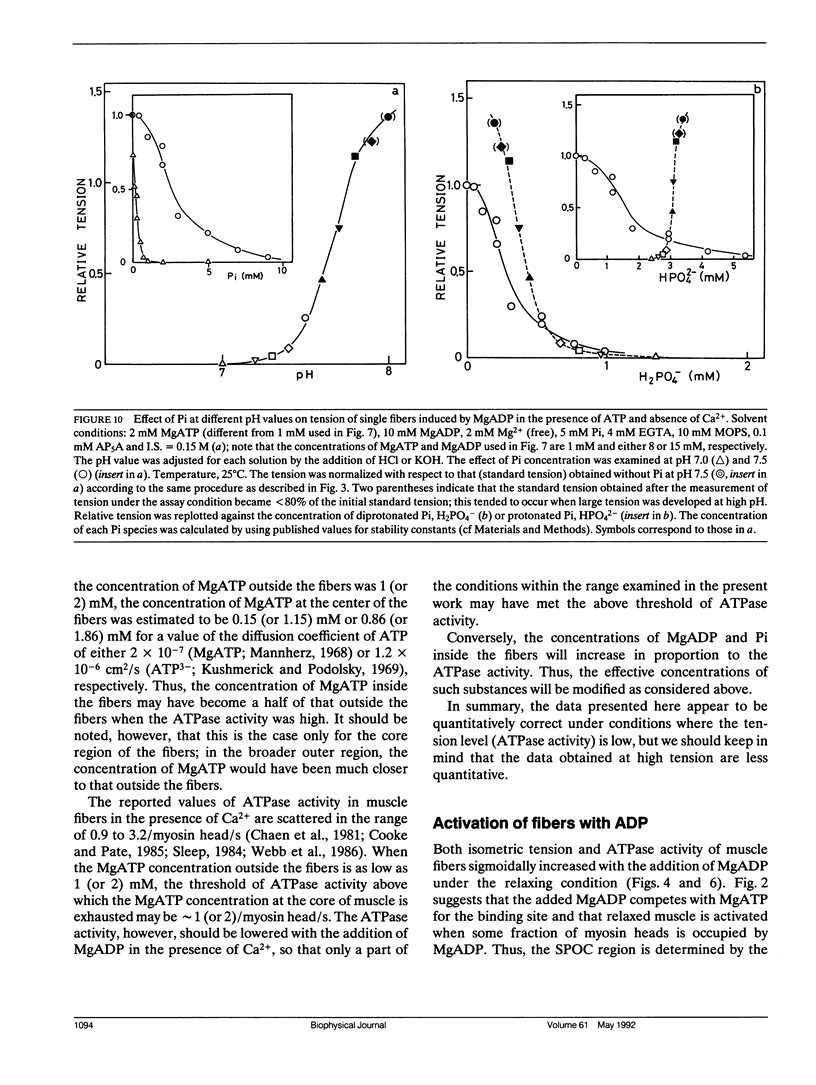
The length of sarcomeres in isolated myofibrils fixed at both ends spontaneously oscillates when MgADP and Pi coexist with MgATP in the absence of Ca2+ (Okamura, N., and S. Ishiwata, 1988. J. Muscle Res. Cell. Motil. 9:111-119). Here, we report that MgADP and Pi function as an activator and an inhibitor, respectively, of tension development of single skeletal muscle fibers in the absence of Ca2+ and the coexistence of MgADP and Pi with MgATP induces spontaneous tension oscillation. First, the isometric tension sharply increased when the concentration of MgADP became higher than approximately 3x that of MgATP and saturated at approximately 90% of the tension obtained under full Ca2+ activation; in parallel with this sigmoidal increase of tension, MgATPase activity appeared. The inhibition of contraction by the regulatory system seems to be desuppressed by the allosteric effect of actomyosin-ADP complex, similarly to so-called rigor complex. The ADP-induced tension was decreased along a reversed sigmoidal curve by the addition of Pi; actomyosin-ADP-Pi complex, which has no desuppression function, may be formed by exogenous Pi; accompanying the decline of tension, spontaneous oscillations of tension and sarcomere length appeared. It is suggested that the length oscillation of each (half) sarcomere would occur through the transition of cross-bridges between force-generating (on) and non-force-generating (off) states, which may be regulated by the mechanical states (strain) of cross-bridges and/or thin filaments.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altringham J. D., Johnston I. A. Effects of phosphate on the contractile properties of fast and slow muscle fibres from an Antarctic fish. J Physiol. 1985 Nov;368:491–500. doi: 10.1113/jphysiol.1985.sp015871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anazawa T., Yasuda K., Ishiwata S. Spontaneous oscillation of tension and sarcomere length in skeletal myofibrils. Microscopic measurement and analysis. Biophys J. 1992 May;61(5):1099–1108. doi: 10.1016/S0006-3495(92)81919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Brenner B. Indirekter Nachweis einer dehnungsinduzierten Ca++-Freisetzung aus dem sarkoplasmatischen Retikulum glyzerinisierter Skelett- und Herzmuskelpräparate. Basic Res Cardiol. 1979 Mar-Apr;74(2):177–202. doi: 10.1007/BF01907820. [DOI] [PubMed] [Google Scholar]
- Chaen S., Kometani K., Yamada T., Shimizu H. Substrate-concentration of dependences of tension, shortening velocity and ATPase activity of glycerinated single muscle fibers. J Biochem. 1981 Dec;90(6):1611–1621. doi: 10.1093/oxfordjournals.jbchem.a133636. [DOI] [PubMed] [Google Scholar]
- Chalovich J. M., Chock P. B., Eisenberg E. Mechanism of action of troponin . tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem. 1981 Jan 25;256(2):575–578. [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys J. 1979 Nov;28(2):241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988 Jan;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells. J Gen Physiol. 1978 Nov;72(5):667–699. doi: 10.1085/jgp.72.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu T., Higuchi H., Ishiwata S. Elastic filaments in skeletal muscle revealed by selective removal of thin filaments with plasma gelsolin. J Cell Biol. 1990 Jan;110(1):53–62. doi: 10.1083/jcb.110.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODALL M. C. Auto-oscillations in extracted muscle fibre systems. Nature. 1956 Jun 30;177(4522):1238–1239. doi: 10.1038/1771238b0. [DOI] [PubMed] [Google Scholar]
- Geeves M. A. The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochem J. 1991 Feb 15;274(Pt 1):1–14. doi: 10.1042/bj2740001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Brenner B. Special topic: molecular mechanism of muscle contraction. General introduction. Annu Rev Physiol. 1987;49:629–636. doi: 10.1146/annurev.ph.49.030187.003213. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E. Kinetics of the actomyosin ATPase in muscle fibers. Annu Rev Physiol. 1987;49:637–654. doi: 10.1146/annurev.ph.49.030187.003225. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig J. W., Peterson J. W., Rüegg J. C., Solaro R. J. Vanadate and phosphate ions reduce tension and increase cross-bridge kinetics in chemically skinned heart muscle. Biochim Biophys Acta. 1981 Jan 21;672(2):191–196. doi: 10.1016/0304-4165(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Trentham D. R. Relationships between chemical and mechanical events during muscular contraction. Annu Rev Biophys Biophys Chem. 1986;15:119–161. doi: 10.1146/annurev.bb.15.060186.001003. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Mahoney C. W., Kerrick W. G. MgADP- increases maximum tension and Ca2+ sensitivity in skinned rabbit soleus fibers. Pflugers Arch. 1987 Sep;410(1-2):30–36. doi: 10.1007/BF00581892. [DOI] [PubMed] [Google Scholar]
- Homsher E., Millar N. C. Caged compounds and striated muscle contraction. Annu Rev Physiol. 1990;52:875–896. doi: 10.1146/annurev.ph.52.030190.004303. [DOI] [PubMed] [Google Scholar]
- Horiuti K. Some properties of the contractile system and sarcoplasmic reticulum of skinned slow fibres from Xenopus muscle. J Physiol. 1986 Apr;373:1–23. doi: 10.1113/jphysiol.1986.sp016032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata S., Fujime S. Effect of calcium ions on the flexibility of reconstituted thin filaments of muscle studied by quasielastic scattering of laser light. J Mol Biol. 1972 Jul 28;68(3):511–522. doi: 10.1016/0022-2836(72)90103-9. [DOI] [PubMed] [Google Scholar]
- Ishiwata S., Funatsu T. Does actin bind to the ends of thin filaments in skeletal muscle? J Cell Biol. 1985 Jan;100(1):282–291. doi: 10.1083/jcb.100.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata S., Muramatsu K., Higuchi H. Disassembly from both ends of thick filaments in rabbit skeletal muscle fibers. An optical diffraction study. Biophys J. 1985 Mar;47(3):257–266. doi: 10.1016/S0006-3495(85)83915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata S., Okamura N., Shimizu H., Anazawa T., Yasuda K. Spontaneous oscillatory contraction (SPOC) of sarcomeres in skeletal muscle. Adv Biophys. 1991;27:227–235. doi: 10.1016/0065-227x(91)90022-6. [DOI] [PubMed] [Google Scholar]
- Iwazumi T., Pollack G. H. The effect of sarcomere non-uniformity on the sarcomere length-tension relationship of skinned fibers. J Cell Physiol. 1981 Mar;106(3):321–337. doi: 10.1002/jcp.1041060302. [DOI] [PubMed] [Google Scholar]
- Johnson R. E., Adams P. H. ADP binds similarly to rigor muscle myofibrils and to actomyosin-subfragment one. FEBS Lett. 1984 Aug 20;174(1):11–14. doi: 10.1016/0014-5793(84)81067-4. [DOI] [PubMed] [Google Scholar]
- Kawai M., Güth K., Winnikes K., Haist C., Rüegg J. C. The effect of inorganic phosphate on the ATP hydrolysis rate and the tension transients in chemically skinned rabbit psoas fibers. Pflugers Arch. 1987 Jan;408(1):1–9. doi: 10.1007/BF00581833. [DOI] [PubMed] [Google Scholar]
- Kawai M., Halvorson H. R. Role of MgATP and MgADP in the cross-bridge kinetics in chemically skinned rabbit psoas fibers. Study of a fast exponential process (C) Biophys J. 1989 Apr;55(4):595–603. doi: 10.1016/S0006-3495(89)82857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Halvorson H. R. Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas muscle. Biophys J. 1991 Feb;59(2):329–342. doi: 10.1016/S0006-3495(91)82227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M. The role of orthophosphate in crossbridge kinetics in chemically skinned rabbit psoas fibres as detected with sinusoidal and step length alterations. J Muscle Res Cell Motil. 1986 Oct;7(5):421–434. doi: 10.1007/BF01753585. [DOI] [PubMed] [Google Scholar]
- Kodama T., Fukui K., Kometani K. The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J Biochem. 1986 May;99(5):1465–1472. doi: 10.1093/oxfordjournals.jbchem.a135616. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- LORAND L., MOOS C. Auto-oscillations in extracted muscle fibre systems. Nature. 1956 Jun 30;177(4522):1239–1239. doi: 10.1038/1771239a0. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I. P 1 ,P 5 -Di(adenosine-5')pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. J Biol Chem. 1973 Feb 10;248(3):1121–1123. [PubMed] [Google Scholar]
- Mannherz H. G. ATP-Spaltung und ATP-Diffusion in oscillierenden extrahiertenMuskelfasern. Pflugers Arch. 1968;303(3):230–248. doi: 10.1007/BF01890903. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Connectin, an elastic filamentous protein of striated muscle. Int Rev Cytol. 1986;104:81–114. doi: 10.1016/s0074-7696(08)61924-5. [DOI] [PubMed] [Google Scholar]
- Millar N. C., Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J Biol Chem. 1990 Nov 25;265(33):20234–20240. [PubMed] [Google Scholar]
- Nosek T. M., Fender K. Y., Godt R. E. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science. 1987 Apr 10;236(4798):191–193. doi: 10.1126/science.3563496. [DOI] [PubMed] [Google Scholar]
- Ohnishi S. T., Barr J. K. A simplified method of quantitating protein using the biuret and phenol reagents. Anal Biochem. 1978 May;86(1):193–200. doi: 10.1016/0003-2697(78)90334-2. [DOI] [PubMed] [Google Scholar]
- Ohno T., Kodama T. Kinetics of adenosine triphosphate hydrolysis by shortening myofibrils from rabbit psoas muscle. J Physiol. 1991 Sep;441:685–702. doi: 10.1113/jphysiol.1991.sp018773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N., Ishiwata S. Spontaneous oscillatory contraction of sarcomeres in skeletal myofibrils. J Muscle Res Cell Motil. 1988 Apr;9(2):111–119. doi: 10.1007/BF01773733. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. A model of crossbridge action: the effects of ATP, ADP and Pi. J Muscle Res Cell Motil. 1989 Jun;10(3):181–196. doi: 10.1007/BF01739809. [DOI] [PubMed] [Google Scholar]
- Pringle J. W. The Croonian Lecture, 1977. Stretch activation of muscle: function and mechanism. Proc R Soc Lond B Biol Sci. 1978 May 5;201(1143):107–130. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- Pringle J. W. The contractile mechanism of insect fibrillar muscle. Prog Biophys Mol Biol. 1967;17:1–60. doi: 10.1016/0079-6107(67)90003-x. [DOI] [PubMed] [Google Scholar]
- Rüegg J. C., Schädler M., Steiger G. J., Müller G. Effects of inorganic phosphate on the contractile mechanism. Pflugers Arch. 1971;325(4):359–364. doi: 10.1007/BF00592176. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Eisenberg E. ADP binding to myosin cross-bridges and its effect on the cross-bridge detachment rate constants. J Gen Physiol. 1987 Jun;89(6):905–920. doi: 10.1085/jgp.89.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol. 1982 Dec;333:637–653. doi: 10.1113/jphysiol.1982.sp014473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer N. K., Moss R. L. The effect of altered temperature on Ca2(+)-sensitive force in permeabilized myocardium and skeletal muscle. Evidence for force dependence of thin filament activation. J Gen Physiol. 1990 Dec;96(6):1221–1245. doi: 10.1085/jgp.96.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Sarcomere-associated cytoskeletal lattices in striated muscle. Review and hypothesis. Cell Muscle Motil. 1985;6:315–369. doi: 10.1007/978-1-4757-4723-2_10. [DOI] [PubMed] [Google Scholar]
- Webb M. R., Hibberd M. G., Goldman Y. E., Trentham D. R. Oxygen exchange between Pi in the medium and water during ATP hydrolysis mediated by skinned fibers from rabbit skeletal muscle. Evidence for Pi binding to a force-generating state. J Biol Chem. 1986 Nov 25;261(33):15557–15564. [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]


