Abstract
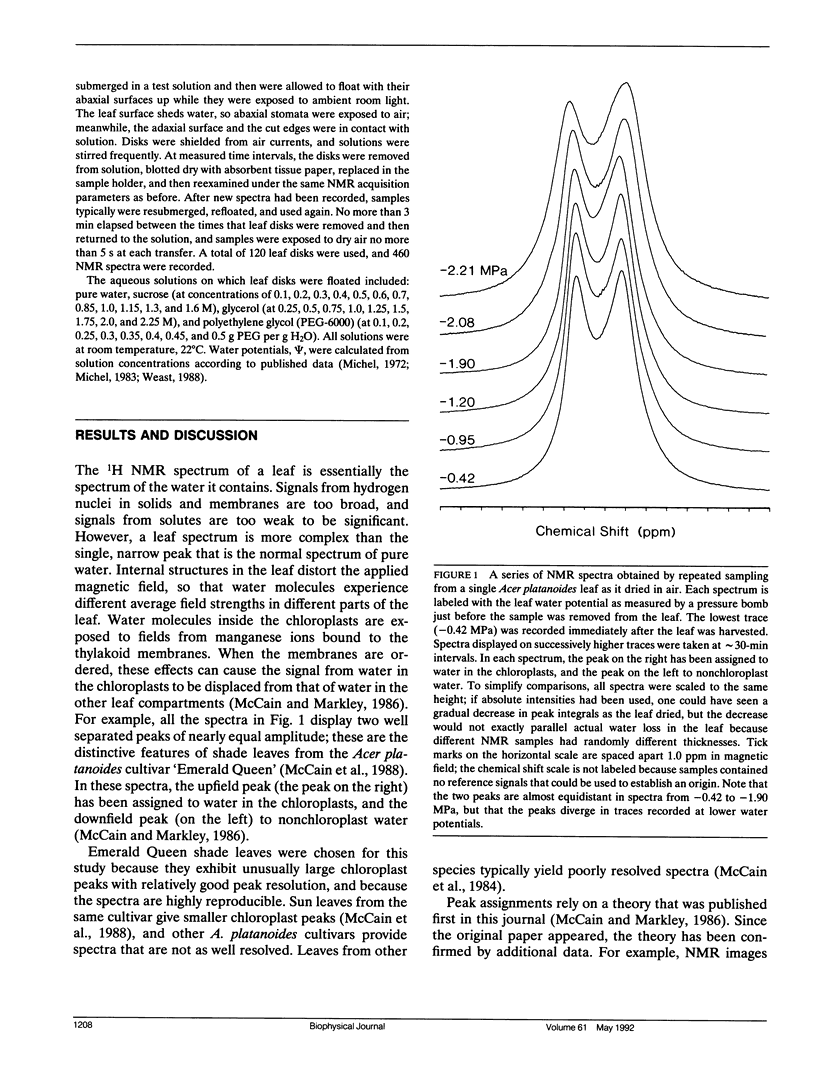
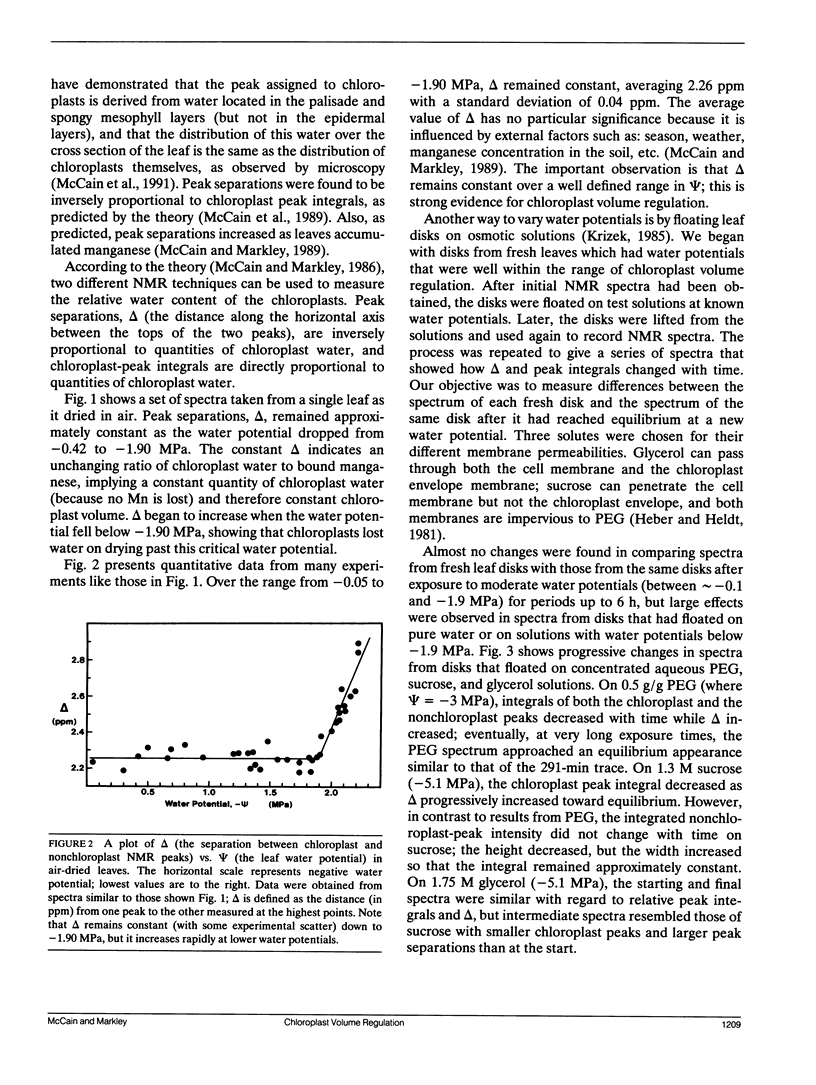
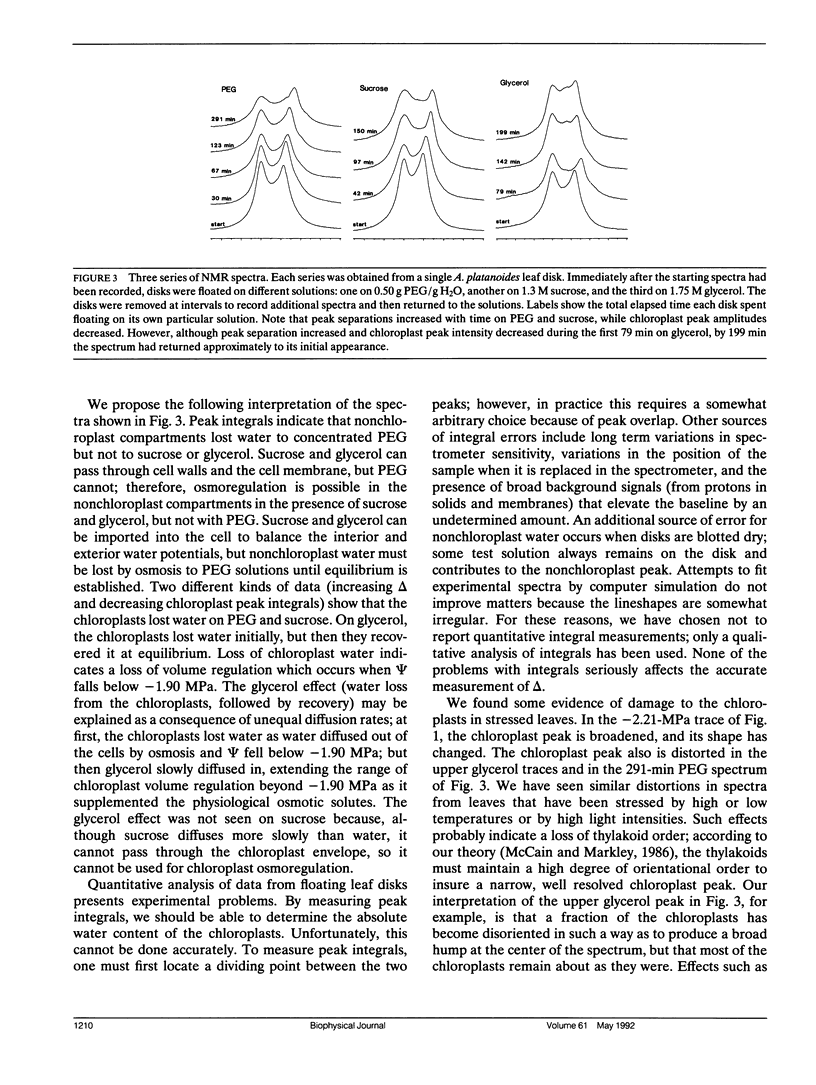
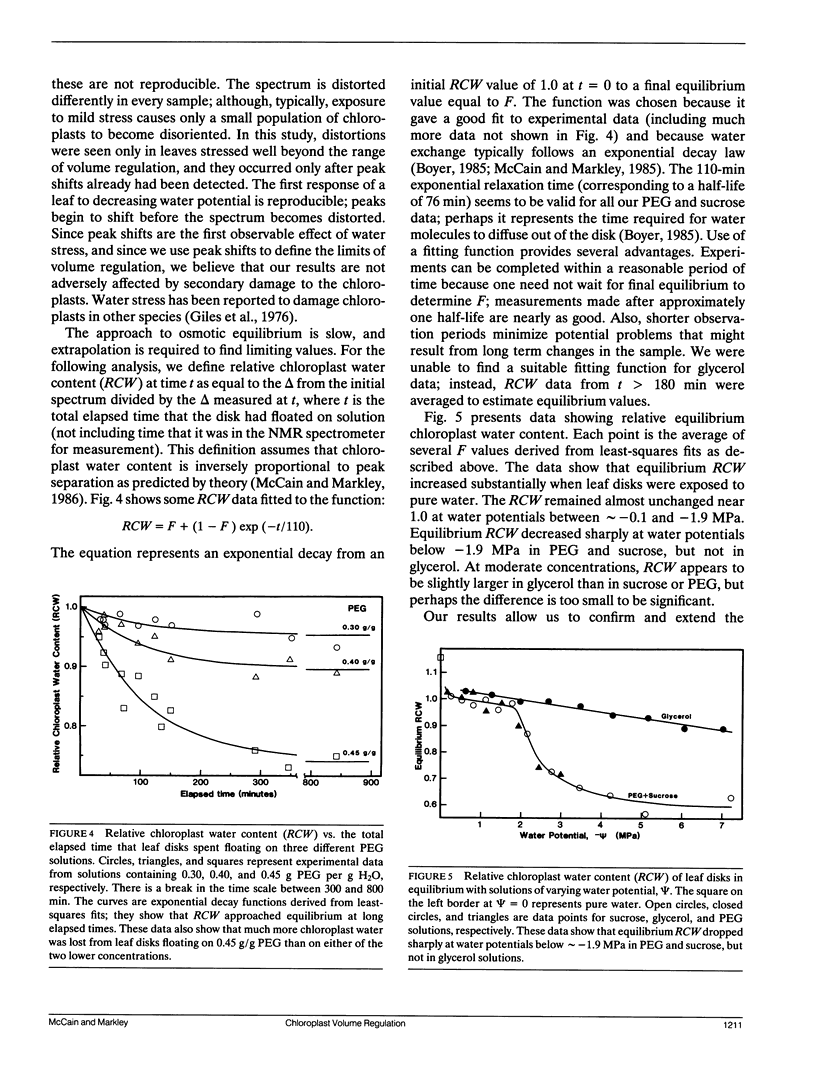
This paper describes a new technique that can be used to study chloroplast volume regulation in vivo. Nuclear magnetic resonance spectroscopy was used to measure relative amounts of chloroplast water in Acer platanoides leaves as they dried in air, and also in leaf disks exposed to aqueous polyethylene glycol, sucrose, or glycerol. The chloroplasts retained a constant quantity of water as leaf water potentials varied between -0.05 and -1.90 MPa, indicating that volume regulation was effective throughout this range. The chloroplasts lost water when the water potential fell below -1.90 MPa, except when leaf disks were exposed to glycerol, suggesting that the lower limit of effective volume regulation is determined by physiological levels of osmotic solutes and that glycerol can be used for chloroplast osmoregulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Giles K. L., Cohen D., Beardsell M. F. Effects of Water Stress on the Ultrastructure of Leaf Cells of Sorghum bicolor. Plant Physiol. 1976 Jan;57(1):11–14. doi: 10.1104/pp.57.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. S., Berkowitz G. A. Chloroplast osmotic adjustment and water stress effects on photosynthesis. Plant Physiol. 1988 Sep;88(1):200–206. doi: 10.1104/pp.88.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain D. C., Croxdale J., Markley J. L. Thermal damage to chloroplast envelope membranes. Plant Physiol. 1989 Jun;90(2):606–609. doi: 10.1104/pp.90.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain D. C., Croxdale J., Markley J. L. Water is allocated differently to chloroplasts in sun and shade leaves. Plant Physiol. 1988 Jan;86(1):16–18. doi: 10.1104/pp.86.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain D. C., Markley J. L. A theory and a model for interpreting the proton nuclear magnetic resonance spectra of water in plant leaves. Biophys J. 1985 Nov;48(5):687–694. doi: 10.1016/S0006-3495(85)83826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain D. C., Markley J. L. More manganese accumulates in maple sun leaves than in shade leaves. Plant Physiol. 1989 Aug;90(4):1417–1421. doi: 10.1104/pp.90.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain D. C., Markley J. L. Water permeability of chloroplast envelope membranes. In vivo measurement by saturation-transfer NMR. FEBS Lett. 1985 Apr 22;183(2):353–358. doi: 10.1016/0014-5793(85)80809-7. [DOI] [PubMed] [Google Scholar]
- McCain D. C., Selig T. C., Govindjee, Markley J. L. Some plant leaves have orientation-dependent EPR and NMR spectra. Proc Natl Acad Sci U S A. 1984 Feb;81(3):748–752. doi: 10.1073/pnas.81.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B. E. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 1983 May;72(1):66–70. doi: 10.1104/pp.72.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B. E. Solute potentials of sucrose solutions. Plant Physiol. 1972 Jul;50(1):196–198. doi: 10.1104/pp.50.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P. Osmotic adjustment by intact isolated chloroplasts in response to osmotic stress and its effect on photosynthesis and chloroplast volume. Plant Physiol. 1985 Dec;79(4):996–1002. doi: 10.1104/pp.79.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]


