Abstract
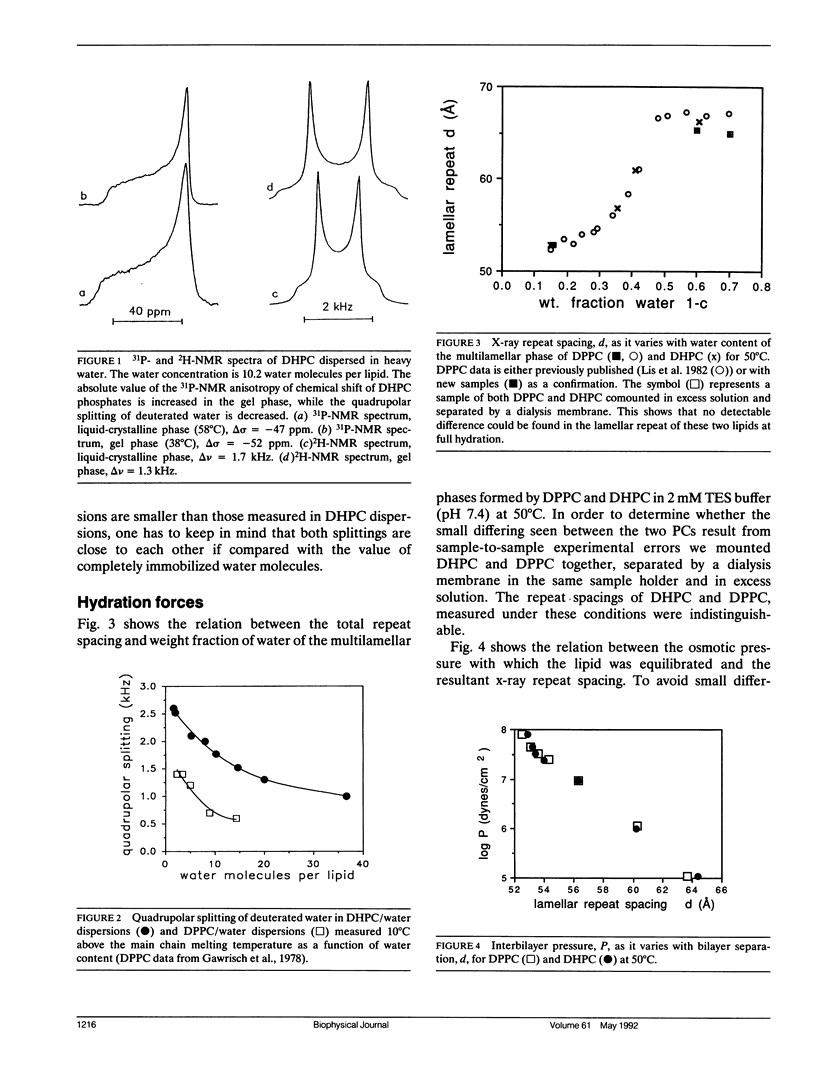
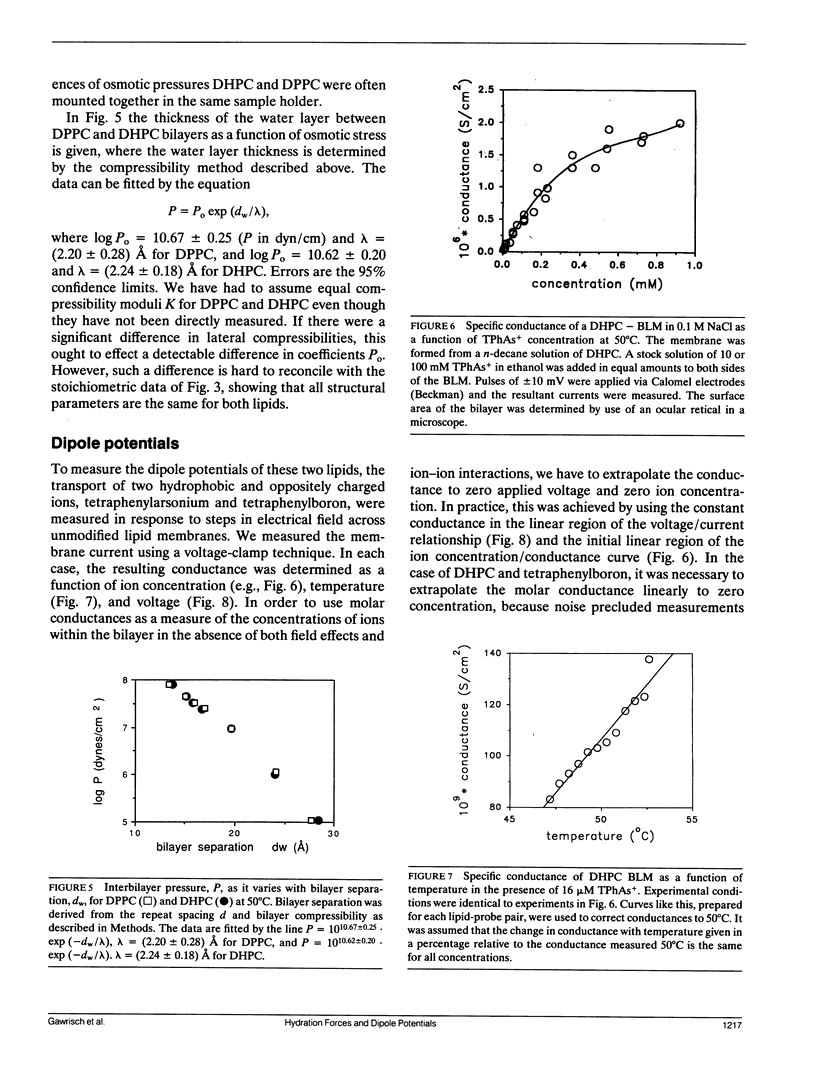
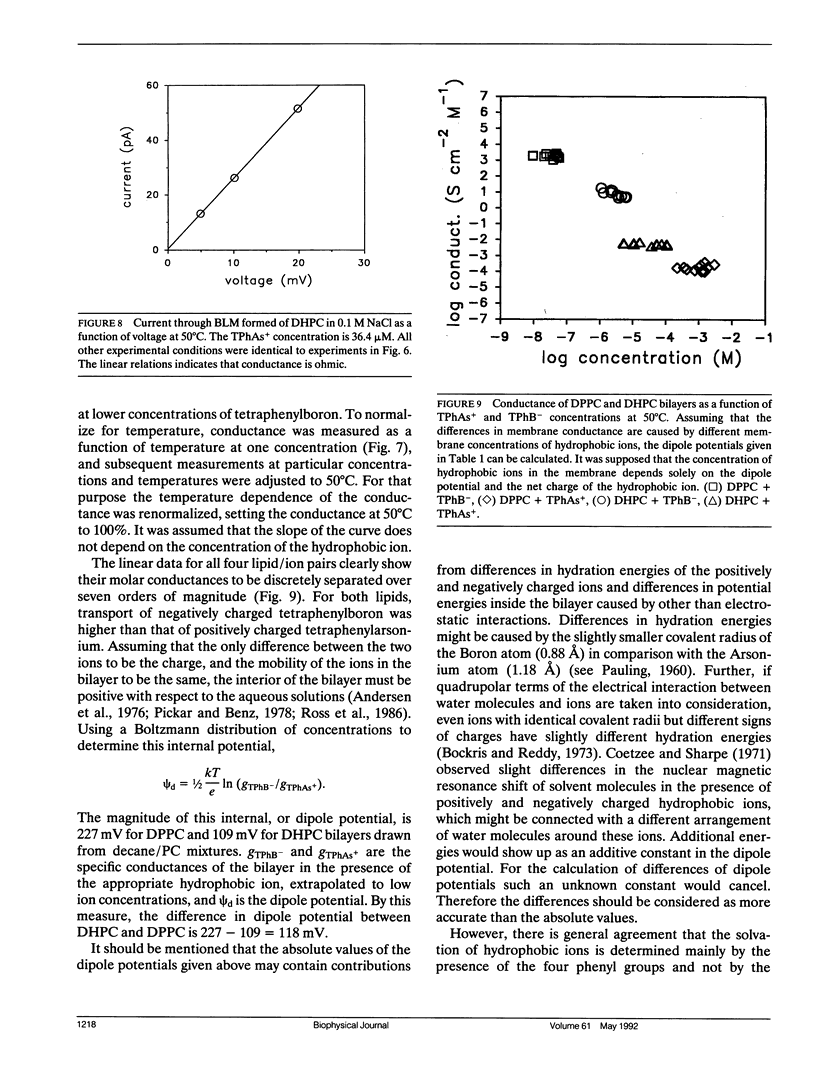
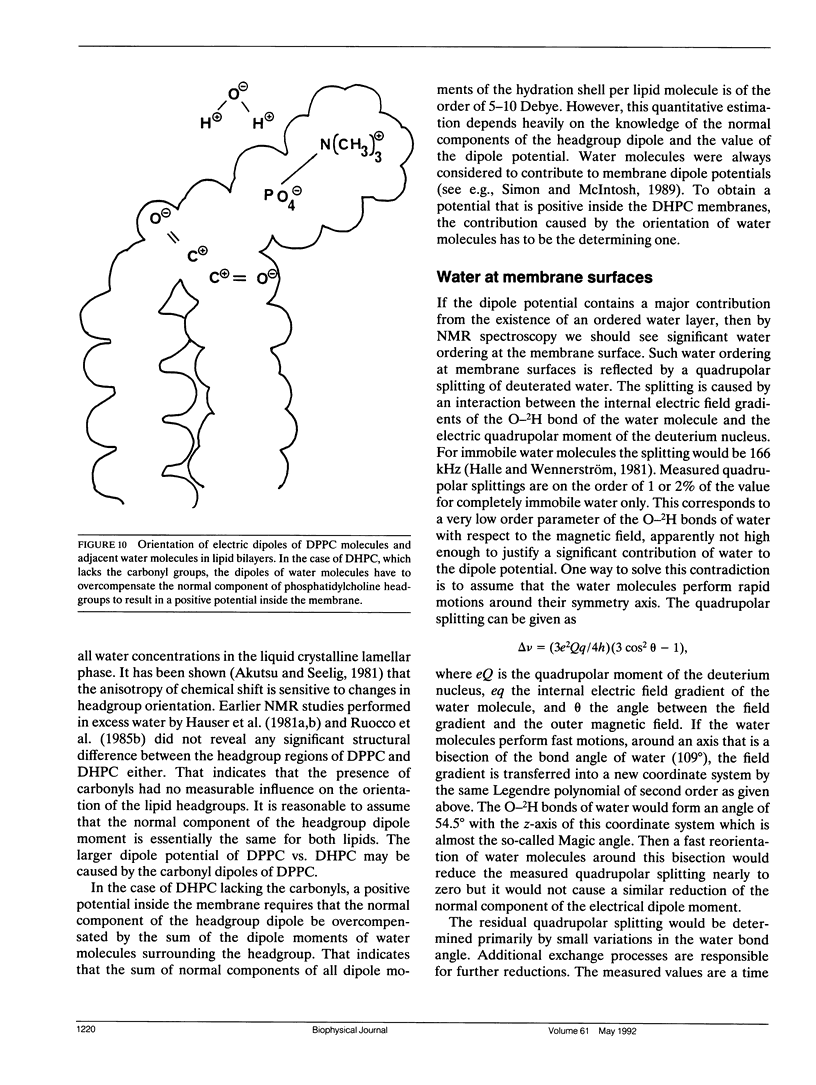
We have compared hydration forces, electrical dipole potentials, and structural parameters of dispersions of dipalmitoylphosphatidylcholine (DPPC) and dihexadecylphosphatidylcholine (DHPC) to evaluate the influence of fatty acid carbonyl groups on phospholipid bilayers. NMR and x-ray investigations performed over a wide range of water concentrations in the samples show, that in the liquid crystalline lamellar phase, the presence of carbonyl groups is not essential for lipid structure and hydration. Within experimental error, the two lipids have identical repulsive hydration forces between their bilayers. The higher transport rate of the negatively charged tetraphenylboron over the positively charged tetraphenylarsonium indicates that the dipole potential is positive inside the membranes of both lipids. However, the lack of fatty acid carbonyl groups in the ether lipid DHPC decreased the potential by (118 +/- 15) mV. By considering the sign of the potential and the orientation of carbonyl groups and headgroups, we conclude that the first layer of water molecules at the lipid water interface makes a major contribution to the dipole potential.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akutsu H., Seelig J. Interaction of metal ions with phosphatidylcholine bilayer membranes. Biochemistry. 1981 Dec 22;20(26):7366–7373. doi: 10.1021/bi00529a007. [DOI] [PubMed] [Google Scholar]
- Andersen O. S., Feldberg S., Nakadomari H., Levy S., McLaughlin S. Electrostatic interactions among hydrophobic ions in lipid bilayer membranes. Biophys J. 1978 Jan;21(1):35–70. doi: 10.1016/S0006-3495(78)85507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Finkelstein A., Katz I., Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976 Jun;67(6):749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Fuchs M. Potential energy barriers to ion transport within lipid bilayers. Studies with tetraphenylborate. Biophys J. 1975 Aug;15(8):795–830. doi: 10.1016/S0006-3495(75)85856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevc G. How membrane chain melting properties are regulated by the polar surface of the lipid bilayer. Biochemistry. 1987 Oct 6;26(20):6305–6310. doi: 10.1021/bi00394a002. [DOI] [PubMed] [Google Scholar]
- Ellena J. F., Dominey R. N., Archer S. J., Xu Z. C., Cafiso D. S. Localization of hydrophobic ions in phospholipid bilayers using 1H nuclear Overhauser effect spectroscopy. Biochemistry. 1987 Jul 14;26(14):4584–4592. doi: 10.1021/bi00388a062. [DOI] [PubMed] [Google Scholar]
- Finer E. G., Darke A. Phospholipid hydration studied by deuteron magnetic resonace spectroscopy. Chem Phys Lipids. 1974 Feb;12(1):1–16. doi: 10.1016/0009-3084(74)90064-4. [DOI] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys J. 1986 Feb;49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys J. 1986 Feb;49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fookson J. E., Wallach D. F. Structural differences among phosphatidylcholine, phosphatidylethanolamine, and mixed phosphatidylcholine/phosphatidylethanolamine multilayers: an infrared absorption study. Arch Biochem Biophys. 1978 Jul;189(1):195–204. doi: 10.1016/0003-9861(78)90132-7. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Tate M. W., Kirk G. L., So P. T., Turner D. C., Keane D. T., Tilcock C. P., Cullis P. R. X-ray diffraction study of the polymorphic behavior of N-methylated dioleoylphosphatidylethanolamine. Biochemistry. 1988 Apr 19;27(8):2853–2866. doi: 10.1021/bi00408a029. [DOI] [PubMed] [Google Scholar]
- Haas N. S., Sripada P. K., Shipley G. G. Effect of chain-linkage on the structure of phosphatidyl choline bilayers. Hydration studies of 1-hexadecyl 2-palmitoyl-sn-glycero-3-phosphocholine. Biophys J. 1990 Jan;57(1):117–124. doi: 10.1016/S0006-3495(90)82512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. T., Mattai J., Shipley G. G. Bilayer interactions of ether- and ester-linked phospholipids: dihexadecyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1987 Oct 20;26(21):6599–6603. doi: 10.1021/bi00395a006. [DOI] [PubMed] [Google Scholar]
- LUZZATI V., HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962 Feb;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Interactions between neutral phospholipid bilayer membranes. Biophys J. 1982 Mar;37(3):657–665. [PMC free article] [PubMed] [Google Scholar]
- Lohner K., Schuster A., Degovics G., Müller K., Laggner P. Thermal phase behaviour and structure of hydrated mixtures between dipalmitoyl- and dihexadecylphosphatidylcholine. Chem Phys Lipids. 1987 Jun;44(1):61–70. doi: 10.1016/0009-3084(87)90005-3. [DOI] [PubMed] [Google Scholar]
- Paltauf F., Hauser H., Phillips M. C. Monolayer characteristics of some 1,2-diacyl, I-alkyl-2-acyl and 1,2-dialkyl phospholipids at the air-water interface. Biochim Biophys Acta. 1971 Dec 3;249(2):539–547. doi: 10.1016/0005-2736(71)90129-5. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Fuller N. L., Rau D. C. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 1986;127:400–416. doi: 10.1016/0076-6879(86)27032-9. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N., Parsegian V. A., Rau D. C. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988 Oct 4;27(20):7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Makriyannis A., Siminovitch D. J., Griffin R. G. Deuterium NMR investigation of ether- and ester-linked phosphatidylcholine bilayers. Biochemistry. 1985 Aug 27;24(18):4844–4851. doi: 10.1021/bi00339a018. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Siminovitch D. J., Griffin R. G. Comparative study of the gel phases of ether- and ester-linked phosphatidylcholines. Biochemistry. 1985 May 7;24(10):2406–2411. doi: 10.1021/bi00331a003. [DOI] [PubMed] [Google Scholar]
- Salsbury N. J., Darke A., Chapman D. Deuteron magnetic resonance studies of water associated with phospholipids. Chem Phys Lipids. 1972 Mar;8(2):142–151. doi: 10.1016/0009-3084(72)90026-6. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Simon S. A., Fink C. A., Kenworthy A. K., McIntosh T. J. The hydration pressure between lipid bilayers. Comparison of measurements using x-ray diffraction and calorimetry. Biophys J. 1991 Mar;59(3):538–546. doi: 10.1016/S0006-3495(91)82270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Magnitude of the solvation pressure depends on dipole potential. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9263–9267. doi: 10.1073/pnas.86.23.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel gating currents. Origin of the rising phase. J Gen Physiol. 1987 Apr;89(4):521–540. doi: 10.1085/jgp.89.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H., Jacobs R. E., King G. I. Partial specific volumes of lipid and water in mixtures of egg lecithin and water. Biophys J. 1987 Oct;52(4):663–665. doi: 10.1016/S0006-3495(87)83259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. A., Pohorille A., Pratt L. R. Surface potential of the water liquid-vapor interface. J Chem Phys. 1988 Mar 1;88(5):3281–3285. doi: 10.1063/1.453923. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Parsegian V. A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986 Sep 4;323(6083):36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]


