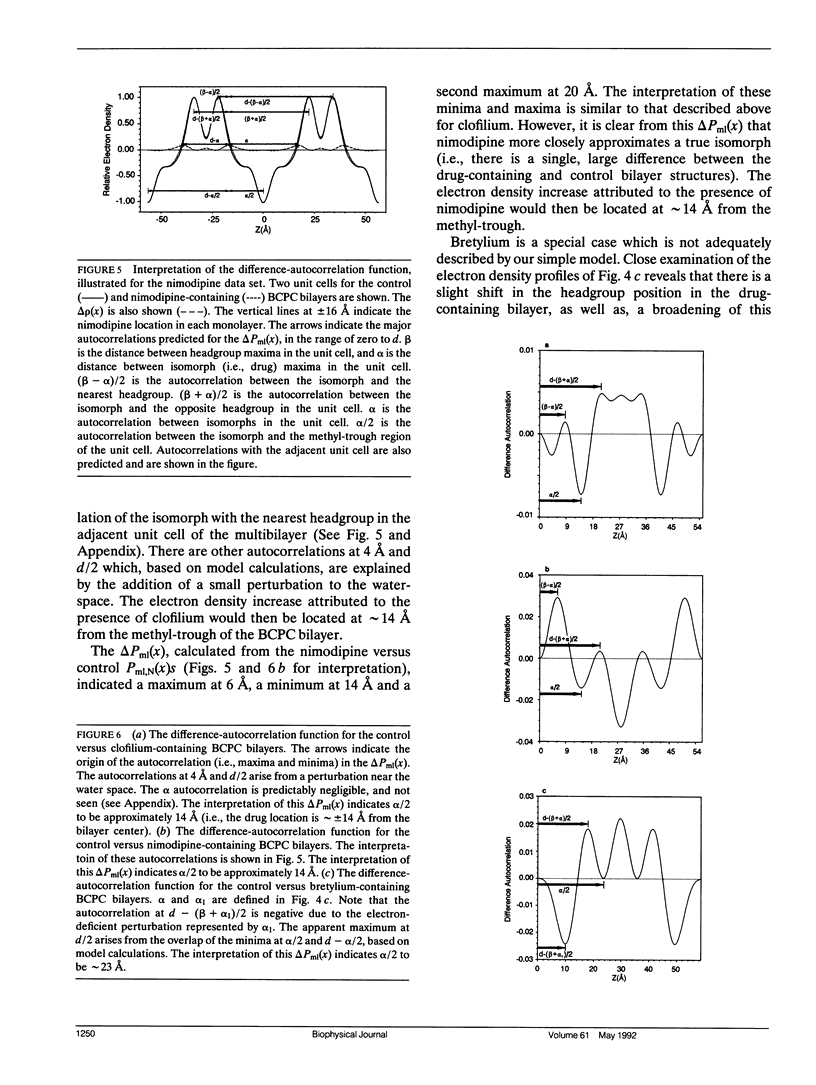
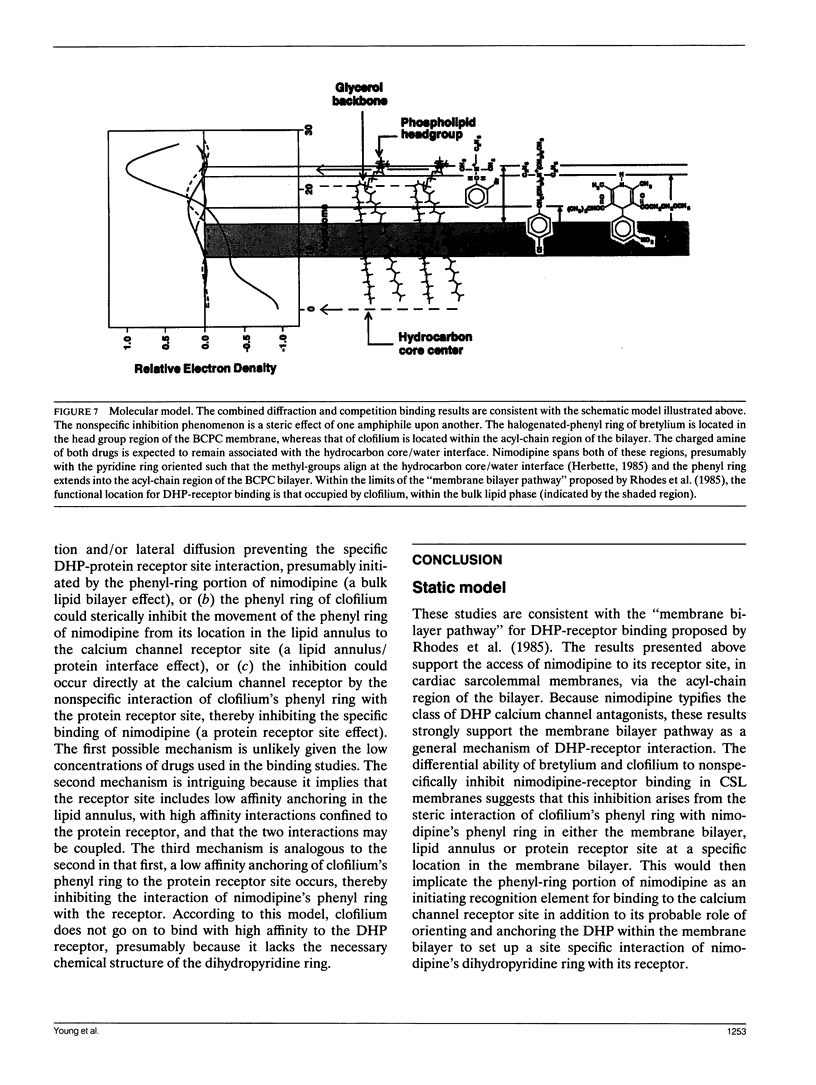
Abstract
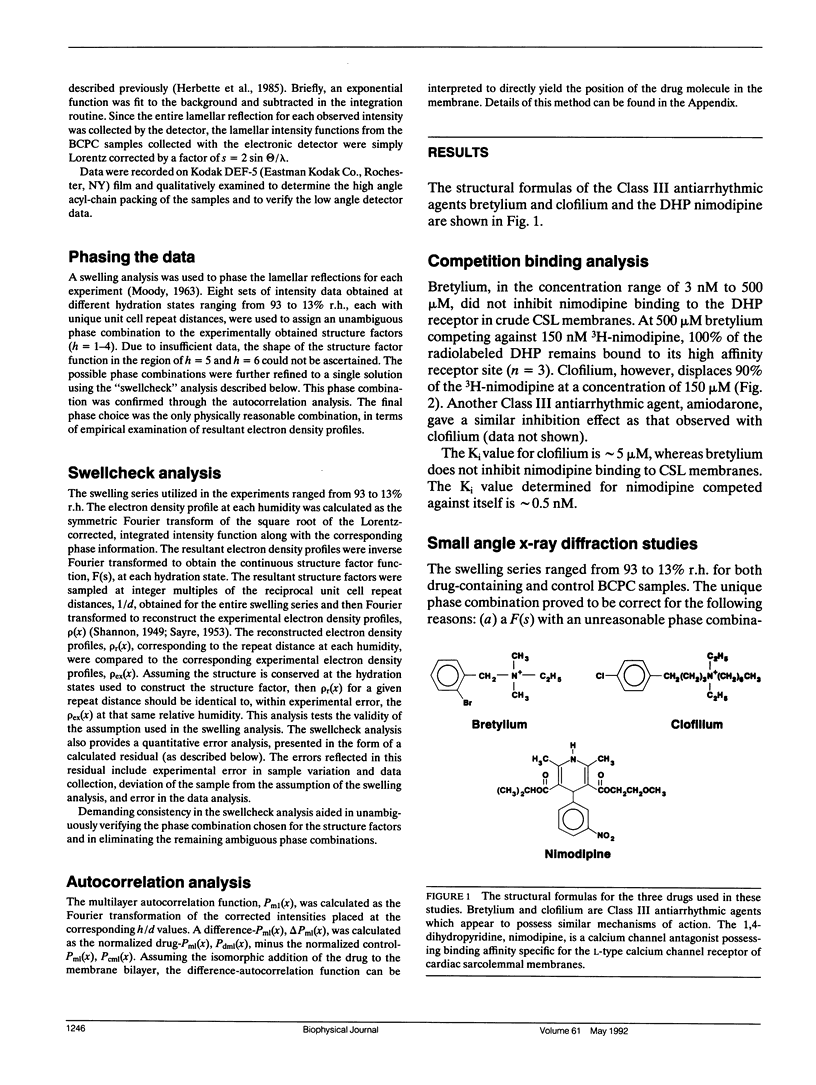
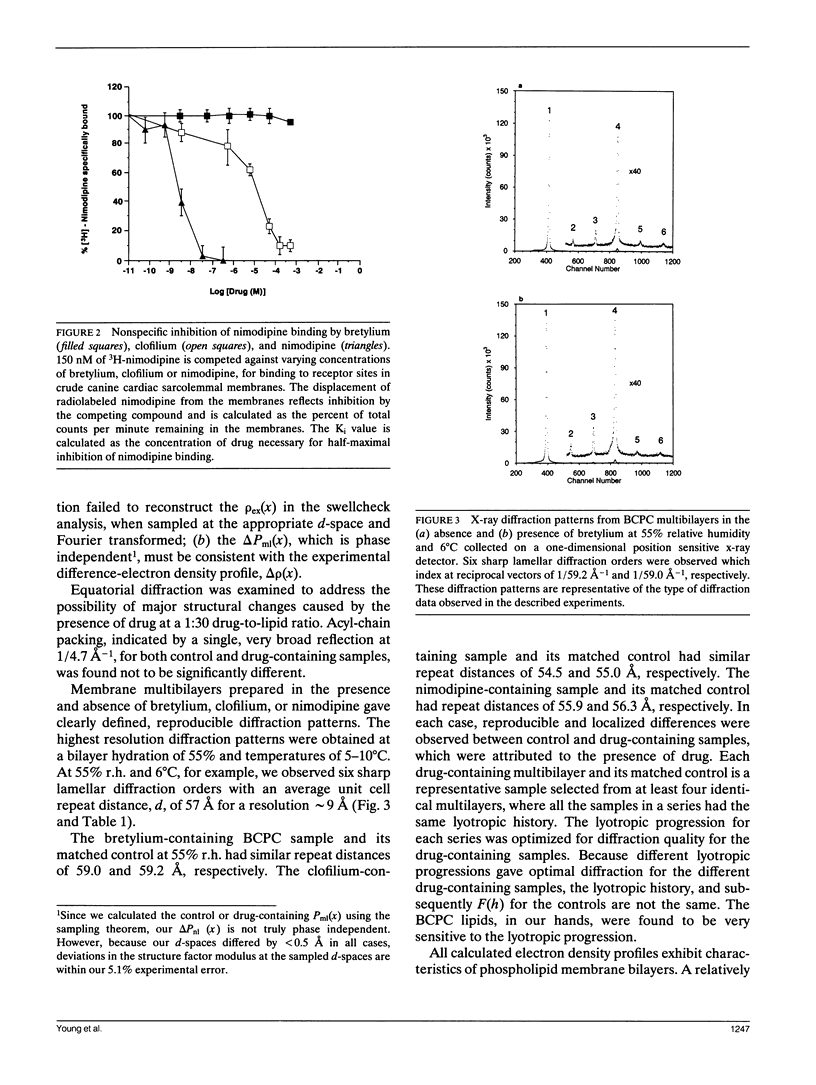
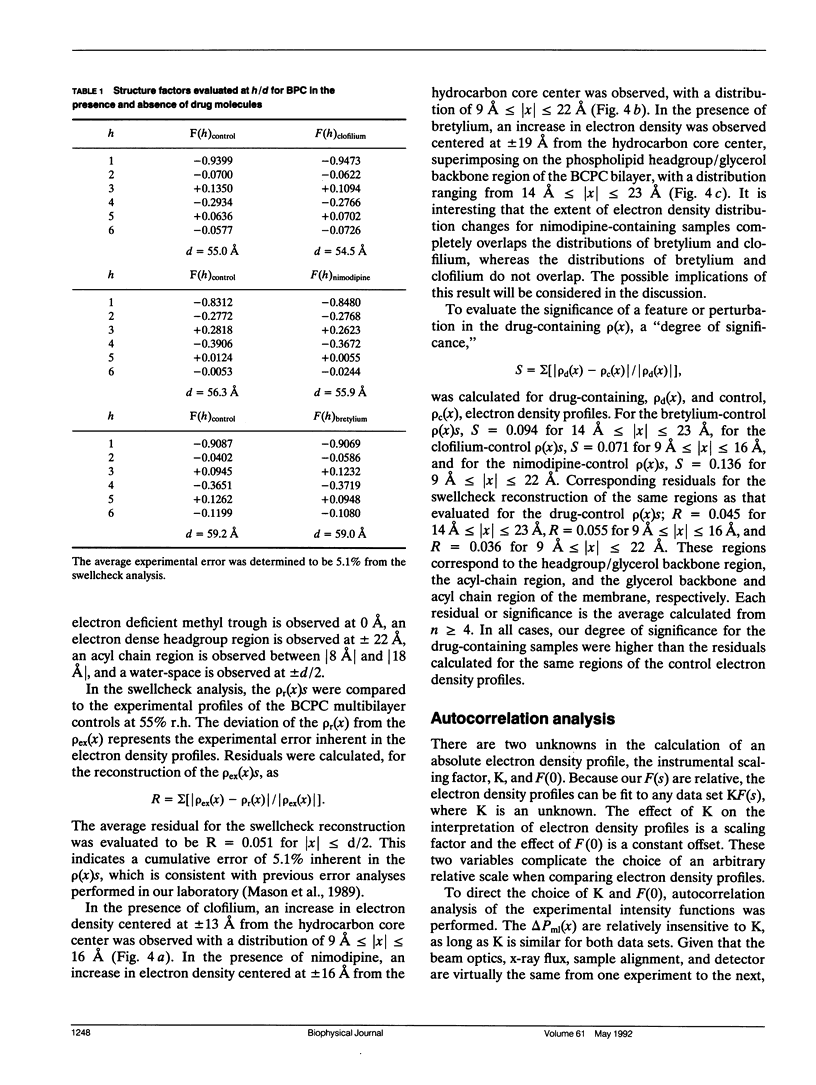
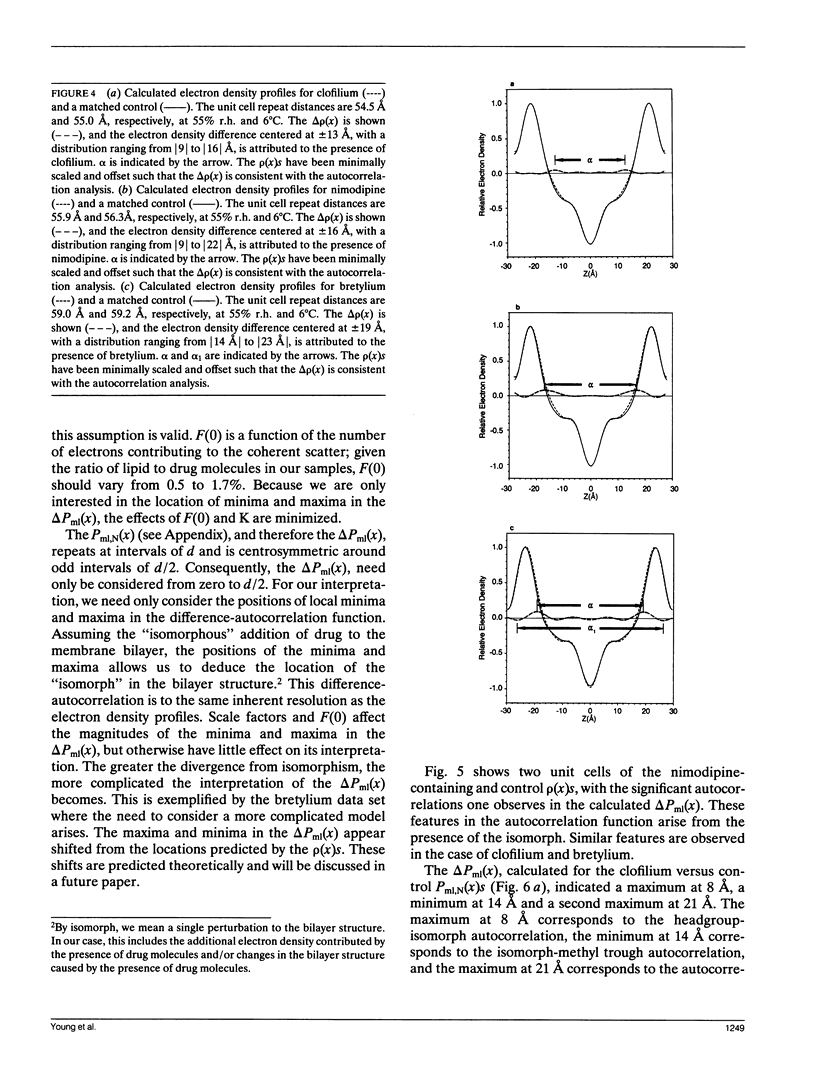
The "membrane bilayer" pathway (Rhodes, D. G., J. G. Sarmiento, and L. G. Herbette. 1985. Mol. Pharmacol. 27:612-623.) for 1,4-dihydropyridine calcium channel drug (DHP) binding to receptor sites in cardiac sarcolemmal membranes has been extended to include the interaction of amphiphiles within the lipid bilayer. These studies focused on the ability of the Class III antiarrhythmic agents bretylium and clofilium to nonspecifically inhibit DHP-receptor binding in canine cardiac sarcolemma. Clofilium was found to inhibit nimodipine binding with an inhibition constant of approximately 5 microM, whereas bretylium had no effect on nimodipine binding. Small angle x-ray diffraction was then used to examine the differential ability of these two Class III agents to inhibit DHP-receptor binding. The time-averaged locations of bretylium, clofilium, and nimodipine in bovine cardiac phosphatidylcholine (BCPC) bilayers (supplemented with 13 mol% cholesterol) were determined to a resolution of 9 A. The location of bretylium as dominated by its phenyl ring in BCPC bilayers was found to be at the hydrocarbon core/water interface, similar to that of the dihydropyridine ring of nimodipine. The location of clofilium as dominated by its phenyl ring was found to be below the hydrocarbon/core water interface within the hydrocarbon chain region of the bilayer, similar to that of the phenyl ring of nimodipine. The location of the dihydropyridine ring portion of nimodipine has previously been shown by neutron diffraction to be located at the hydrocarbon core/water interface of native sarcoplasmic reticulum, consistent with the small angle x-ray data from model membranes in this paper. Therefore, we speculate that the nonspecific inhibition arises from the interaction of clofilium's phenyl ring with the site on the calcium channel receptor where the phenyl ring portion of nimodipine must interact. The DHP-receptor binding pathway would then involve both nonspecific (membrane) and specific (protein) binding components, both of which are necessary for receptor binding.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter H., Coronado R. Agonists Bay-K8644 and CGP-28392 open calcium channels reconstituted from skeletal muscle transverse tubules. Biophys J. 1985 Aug;48(2):341–347. doi: 10.1016/S0006-3495(85)83789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham A. D., Standish M. M., Watkins J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965 Aug;13(1):238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- Bellemann P., Schade A., Towart R. Dihydropyridine receptor in rat brain labeled with [3H]nimodipine. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2356–2360. doi: 10.1073/pnas.80.8.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester D. W., Herbette L. G., Mason R. P., Joslyn A. F., Triggle D. J., Koppel D. E. Diffusion of dihydropyridine calcium channel antagonists in cardiac sarcolemmal lipid multibilayers. Biophys J. 1987 Dec;52(6):1021–1030. doi: 10.1016/S0006-3495(87)83295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbette L. G., Chester D. W., Rhodes D. G. Structural analysis of drug molecules in biological membranes. Biophys J. 1986 Jan;49(1):91–94. doi: 10.1016/S0006-3495(86)83605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbette L. G., MacAlister T., Ashavaid T. F., Colvin R. A. Structure-function studies of canine cardiac sarcolemmal membranes. II. Structural organization of the sarcolemmal membrane as determined by electron microscopy and lamellar X-ray diffraction. Biochim Biophys Acta. 1985 Feb 14;812(3):609–623. doi: 10.1016/0005-2736(85)90254-8. [DOI] [PubMed] [Google Scholar]
- Herbette L. G., Rhodes D. G., Mason R. P. New approaches to drug design and delivery based on drug-membrane interactions. Drug Des Deliv. 1991 Apr;7(2):75–118. [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. R., Maddock S. W., Besch H. R., Jr Unmasking effect of alamethicin on the (Na+,K+)-ATPase, beta-adrenergic receptor-coupled adenylate cyclase, and cAMP-dependent protein kinase activities of cardiac sarcolemmal vesicles. J Biol Chem. 1980 Oct 25;255(20):9971–9980. [PubMed] [Google Scholar]
- Kokubun S., Reuter H. Dihydropyridine derivatives prolong the open state of Ca channels in cultured cardiac cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOODY M. F. X-RAY DIFFRACTION PATTERN OF NERVE MYELIN: A METHOD FOR DETERMINING THE PHASES. Science. 1963 Nov 29;142(3596):1173–1174. doi: 10.1126/science.142.3596.1173. [DOI] [PubMed] [Google Scholar]
- Martin B. R. Cellular effects of cannabinoids. Pharmacol Rev. 1986 Mar;38(1):45–74. [PubMed] [Google Scholar]
- Mason R. P., Gonye G. E., Chester D. W., Herbette L. G. Partitioning and location of Bay K 8644, 1,4-dihydropyridine calcium channel agonist, in model and biological membranes. Biophys J. 1989 Apr;55(4):769–778. doi: 10.1016/S0006-3495(89)82875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. P., Moring J., Herbette L. G. A molecular model involving the membrane bilayer in the binding of lipid soluble drugs to their receptors in heart and brain. Int J Rad Appl Instrum B. 1990;17(1):13–33. doi: 10.1016/0883-2897(90)90004-k. [DOI] [PubMed] [Google Scholar]
- McCloskey M. A., Poo M. M. Rates of membrane-associated reactions: reduction of dimensionality revisited. J Cell Biol. 1986 Jan;102(1):88–96. doi: 10.1083/jcb.102.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. G., Sarmiento J. G., Herbette L. G. Kinetics of binding of membrane-active drugs to receptor sites. Diffusion-limited rates for a membrane bilayer approach of 1,4-dihydropyridine calcium channel antagonists to their active site. Mol Pharmacol. 1985 Jun;27(6):612–623. [PubMed] [Google Scholar]
- Trumbore M., Chester D. W., Moring J., Rhodes D., Herbette L. G. Structure and location of amiodarone in a membrane bilayer as determined by molecular mechanics and quantitative x-ray diffraction. Biophys J. 1988 Sep;54(3):535–543. doi: 10.1016/S0006-3495(88)82986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]



