Abstract
Diffuse scattering analyses are emerging as a technique to extract additional dynamic information from x-ray diffraction data. In fact, when examined carefully, most protein crystals show significant diffuse scattering in addition to the usual Bragg diffraction. This diffuse scattering contains information about the disorder in the crystal that cannot be obtained from the Bragg diffraction data. Diffraction from tropomyosin crystals shows characteristic diffuse scattering streaks that are directly related to motion of the molecules. The structure of tropomyosin to 15 A resolution shows that the limited molecular contacts between molecules allow large conformational fluctuations of up to 8 A amplitude. Models for the three-dimensional motion of tropomyosin have been tested by comparing their predicted diffuse scattering patterns with the experimental data. From the parameters of the successful simulations, we were able to determine the amplitudes, directions, and distances over which the atomic displacements are correlated.
Full text
PDF

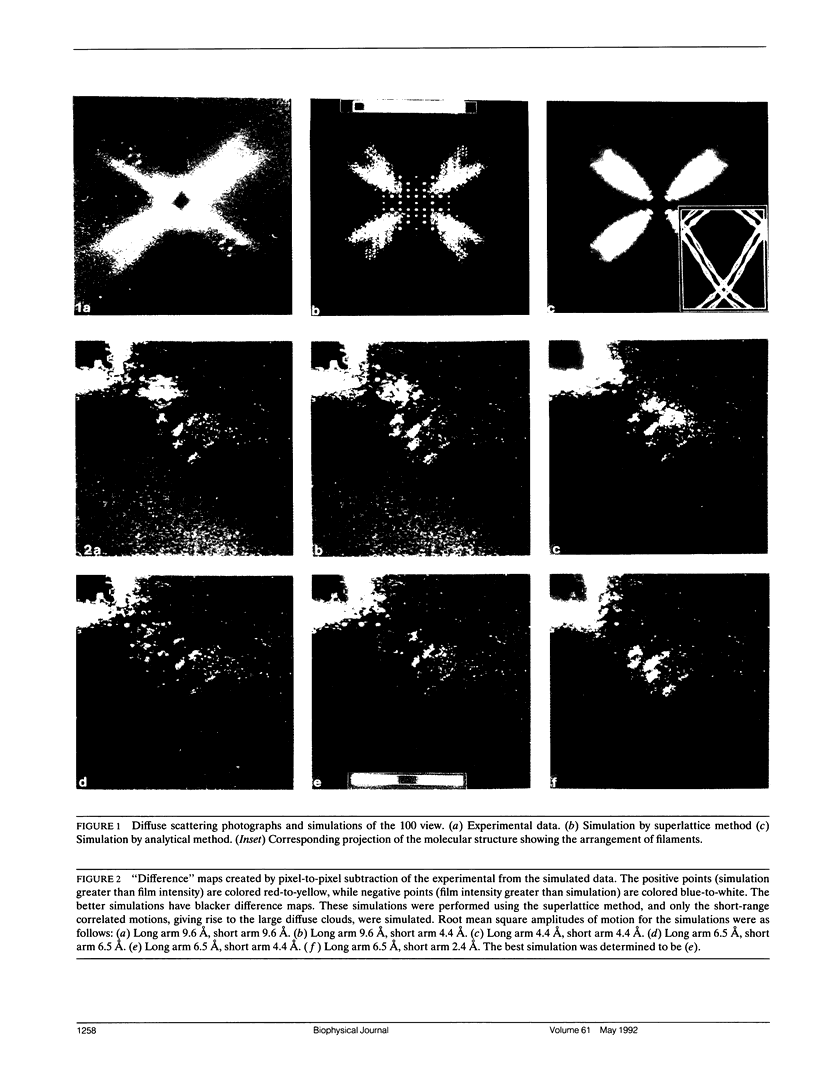


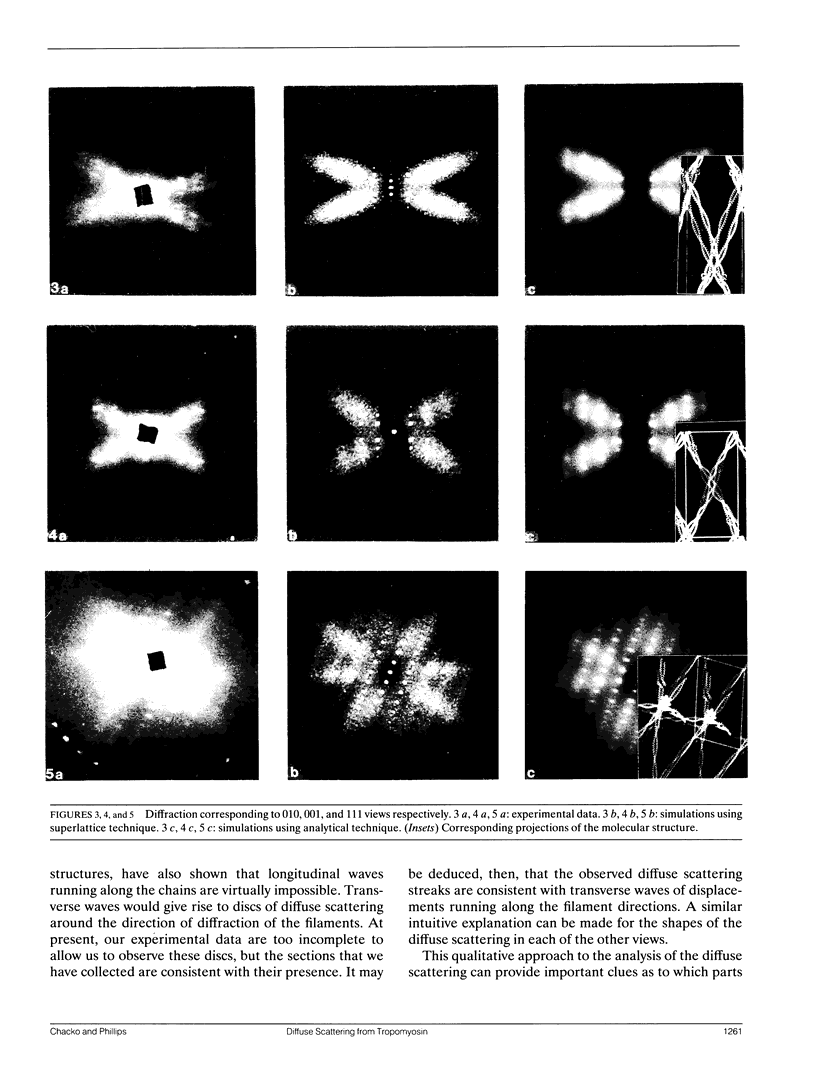
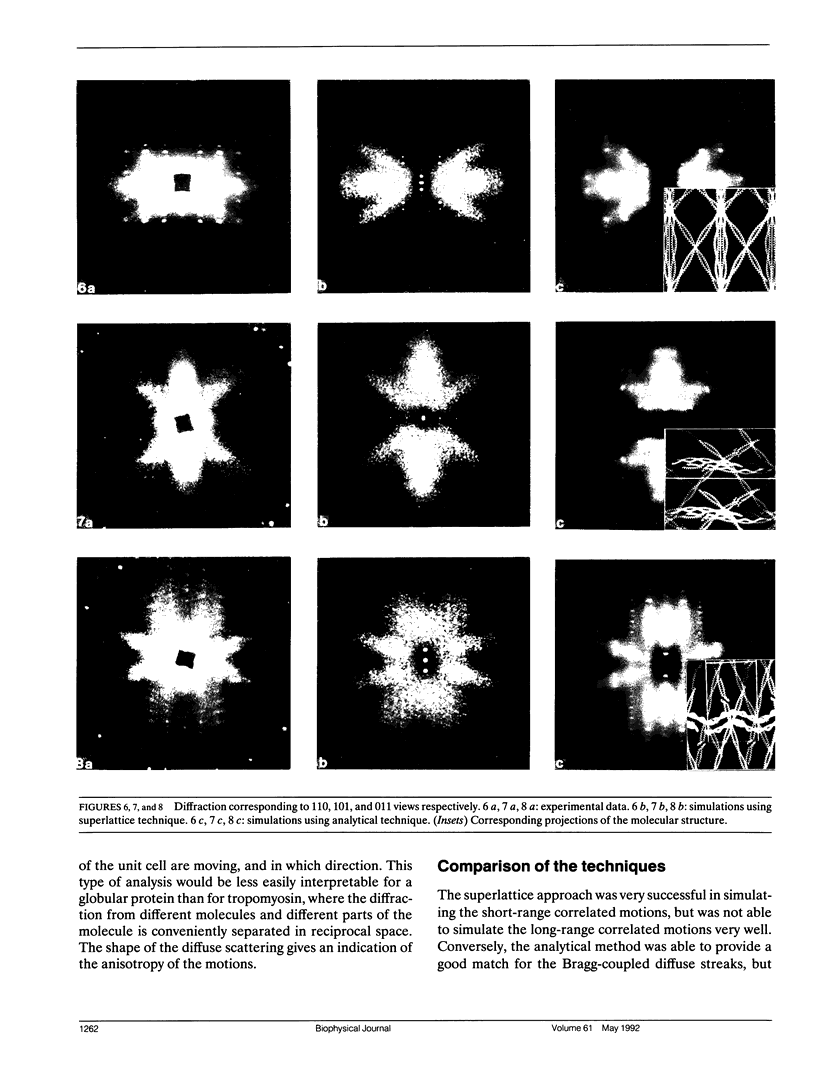




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan D., Phillips G. N. Motions of tropomyosin: characterization of anisotropic motions and coupled displacements in crystals. Biophys J. 1986 Jan;49(1):76–78. doi: 10.1016/s0006-3495(86)83599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar D. L., Clarage J., Salunke D. M., Clarage M. Liquid-like movements in crystalline insulin. Nature. 1988 Apr 14;332(6165):659–662. doi: 10.1038/332659a0. [DOI] [PubMed] [Google Scholar]
- Clarage J. B., Clarage M. S., Phillips W. C., Sweet R. M., Caspar D. L. Correlations of atomic movements in lysozyme crystals. Proteins. 1992 Feb;12(2):145–157. doi: 10.1002/prot.340120208. [DOI] [PubMed] [Google Scholar]
- Doucet J., Benoit J. P., Cruse W. B., Prange T., Kennard O. Coexistence of A- and B-form DNA in a single crystal lattice. Nature. 1989 Jan 12;337(6203):190–192. doi: 10.1038/337190a0. [DOI] [PubMed] [Google Scholar]
- Doucet J., Benoit J. P. Molecular dynamics studied by analysis of the X-ray diffuse scattering from lysozyme crystals. Nature. 1987 Feb 12;325(6105):643–646. doi: 10.1038/325643a0. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Hartmann H., Parak F., Steigemann W., Petsko G. A., Ponzi D. R., Frauenfelder H. Conformational substates in a protein: structure and dynamics of metmyoglobin at 80 K. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4967–4971. doi: 10.1073/pnas.79.16.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsko G. A., Ringe D. Fluctuations in protein structure from X-ray diffraction. Annu Rev Biophys Bioeng. 1984;13:331–371. doi: 10.1146/annurev.bb.13.060184.001555. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr Diffraction methods for biological macromolecules. Crystallization in capillary tubes. Methods Enzymol. 1985;114:128–131. doi: 10.1016/0076-6879(85)14010-3. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Fillers J. P., Cohen C. Motions of tropomyosin. Crystal as metaphor. Biophys J. 1980 Oct;32(1):485–502. doi: 10.1016/S0006-3495(80)84985-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Fillers J. P., Cohen C. Tropomyosin crystal structure and muscle regulation. J Mol Biol. 1986 Nov 5;192(1):111–131. doi: 10.1016/0022-2836(86)90468-7. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Lattman E. E., Cummins P., Lee K. Y., Cohen C. Crystal structure and molecular interactions of tropomyosin. Nature. 1979 Mar 29;278(5703):413–417. doi: 10.1038/278413a0. [DOI] [PubMed] [Google Scholar]









