Abstract
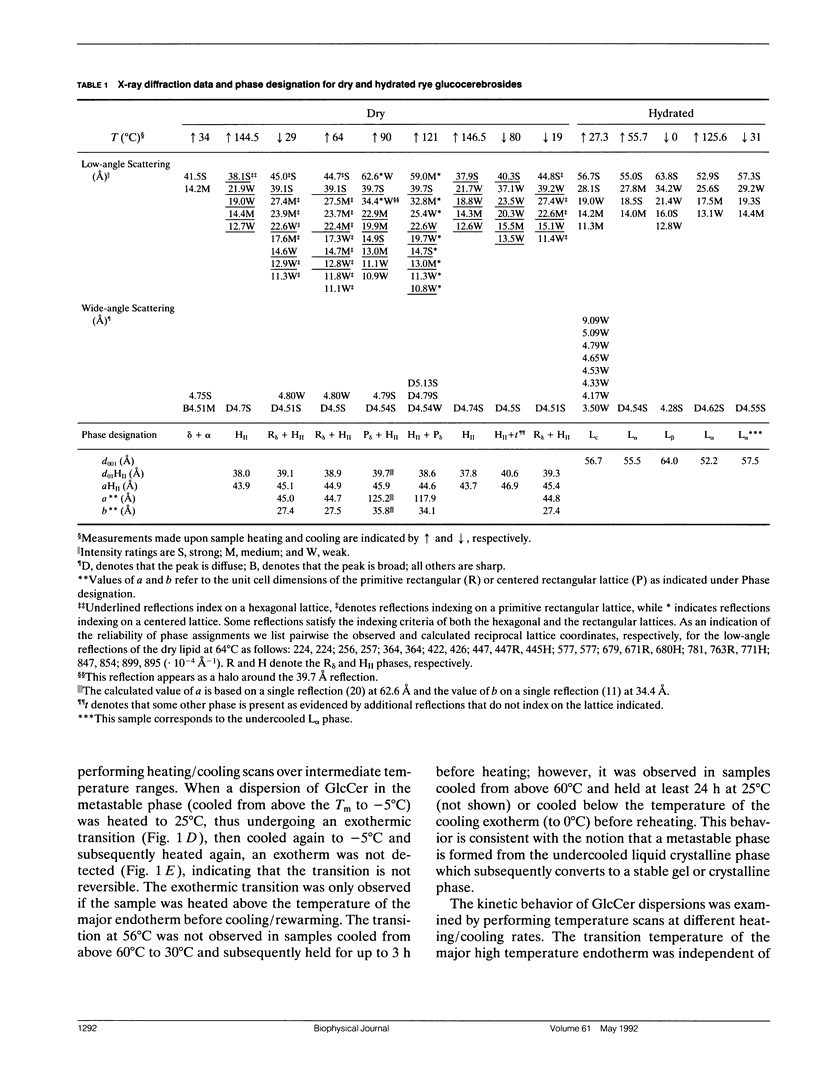
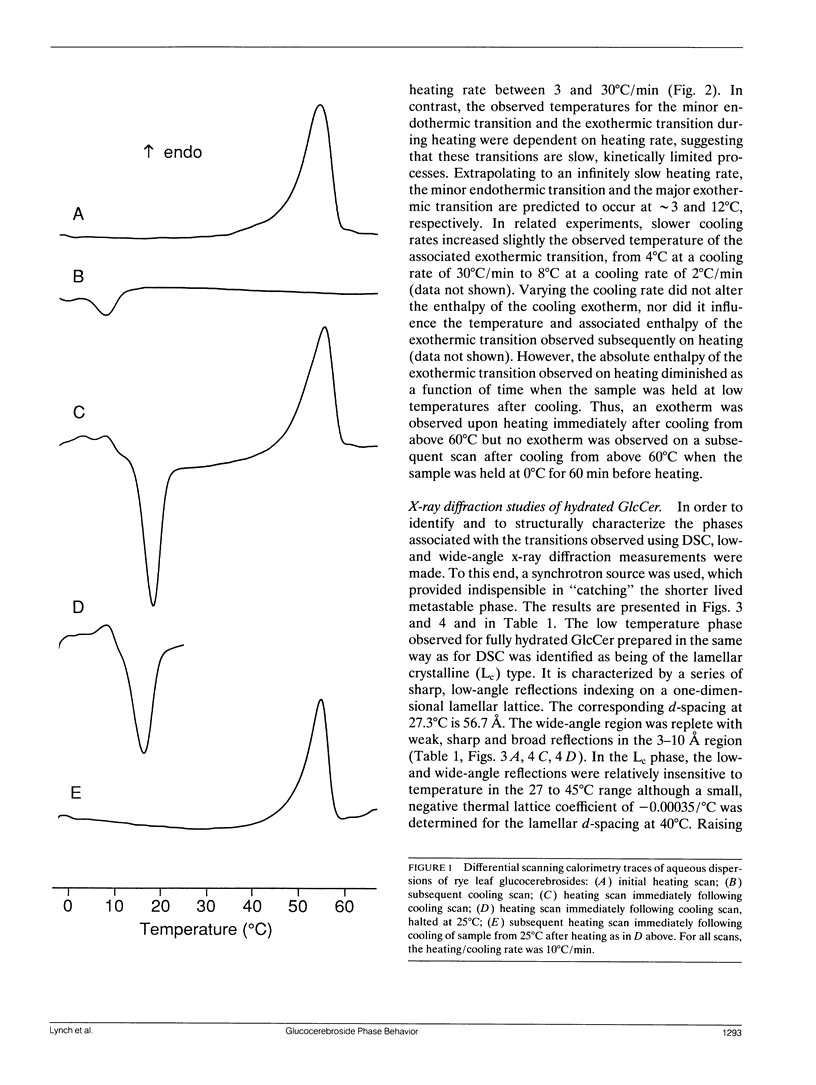
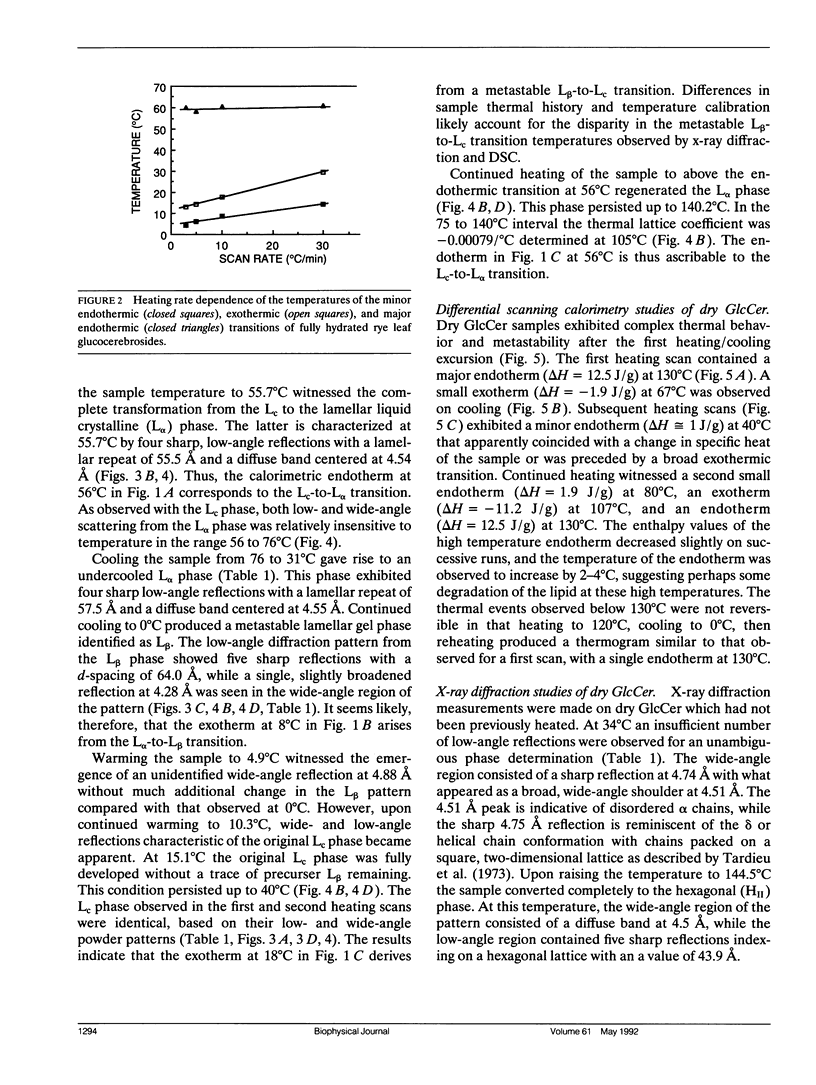
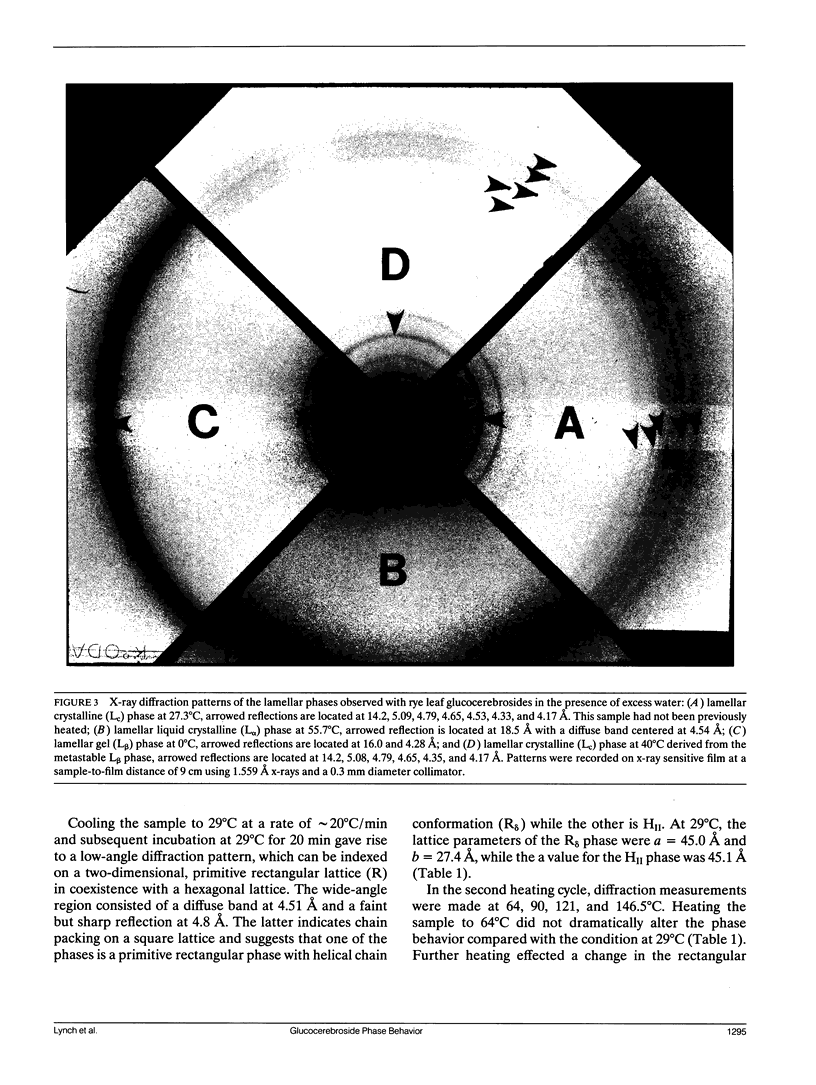
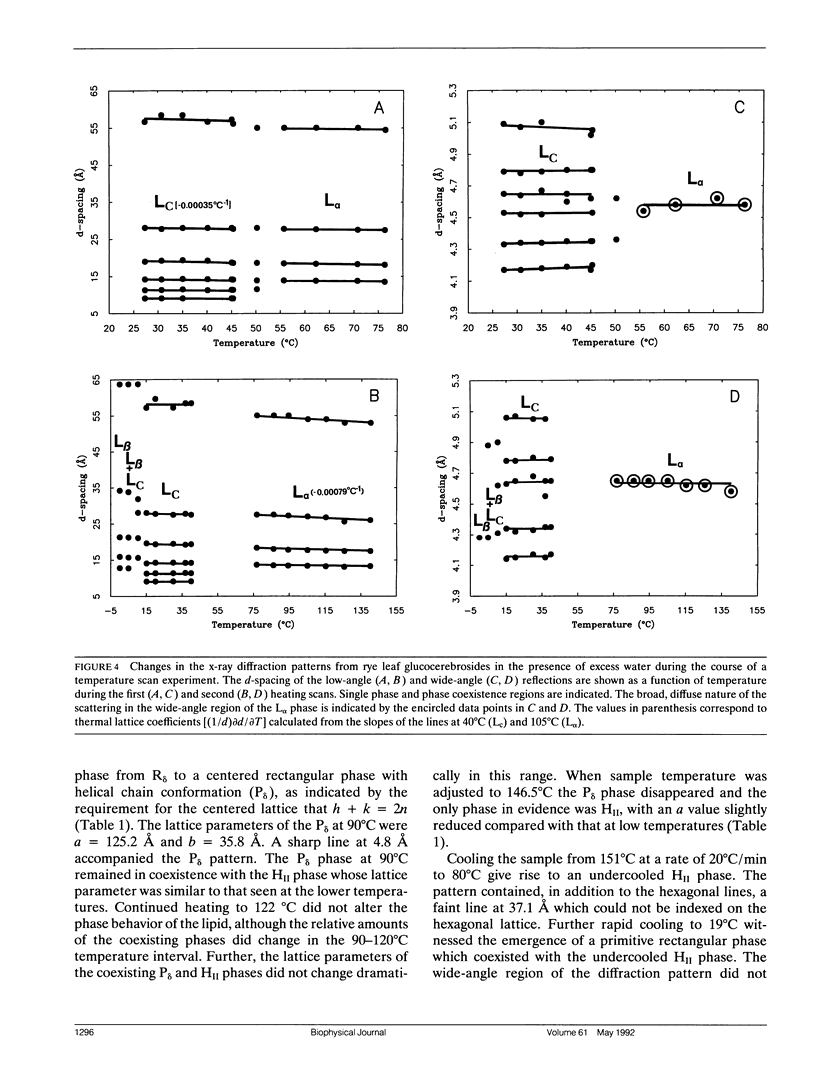
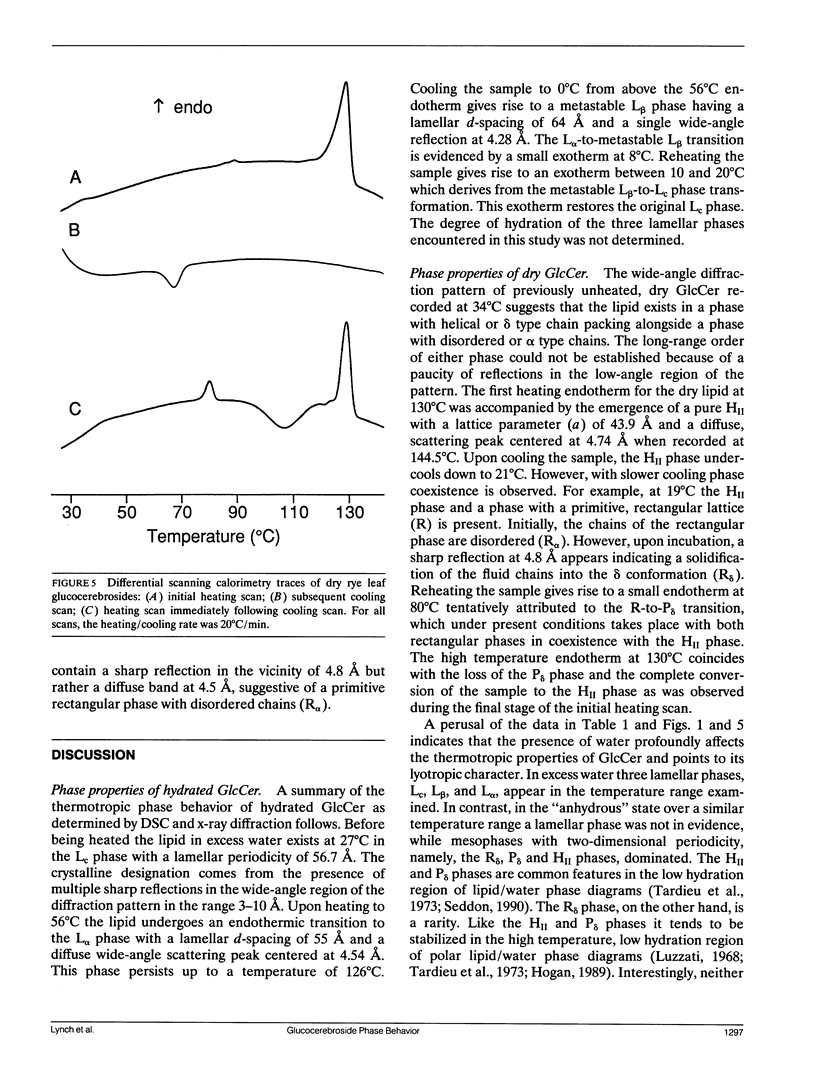
Glucocerebrosides (GlcCer) isolated from the leaves of winter rye (Secale cereale L. cv Puma) differ from the more commonly investigated natural and synthetic cerebrosides, in that greater than 95% of the fatty acids are saturated and monounsaturated hydroxy fatty acids. Isomers of the trihydroxy long chain base hydroxysphingenine (t1(8:18 cis or trans)) and isomers of sphingadienine (d18:2(4trans, 8 cis or trans)) comprise 77% and 17%, respectively, of the total long chain bases. The phase behavior of fully hydrated and dry rye leaf GlcCer was investigated using differential scanning calorimetry (DSC) and x-ray diffraction. On initial heating, aqueous dispersions of GlcCer exhibit a single endothermic transition at 56 degrees C and have an enthalpy (delta H) of 46 J/g. Cooling to 0 degrees C is accompanied by a small exothermic transition (delta H = -8 J/g) at 8 degrees C. On immediate reheating, a broad exothermic transition (delta H = -39 J/g) is observed between 10 and 20 degrees C in addition to a transition at 56 degrees C. These transitions are not reversible, and the exothermic transition rapidly diminishes when the sample is held at low temperature. Using x-ray diffraction, it was determined that the endotherm at 56 degrees C represents a transition from a highly ordered lamellar crystalline phase (Lc) with a d-spacing of 57 A and a series of wide-angle reflections in the 3-10 A range, to a lamellar liquid crystalline (L alpha) phase having a d-spacing of 55 A and a diffuse wide-angle scattering peak centered at 4.7 A. Cooling leads to the formation of a metastable gel phase (L beta) with a d-spacing of 64.0 A and a single broad reflection at 4.28 A. Subsequent warming to above 15 degrees C restores the original Lc phase. Thus, rye GlcCer in excess water exhibit a series of irreversible transitions and gel phase metastability. Dry GlcCer undergo an initial heating endothermic transition at 130 degrees C, which is ascribed to a transformation into the HII phase from a two phase state characterized by the coexistence of phases with disordered (alpha) and helical (delta) type chain conformations but of unknown lattice identity: An exotherm at 67.5 degrees C observed upon subsequent cooling is of unknown origin. Since an undercooled HII phase persists down to 19 degrees C, the exotherm may derive in part from an alpha-to-delta type chain packing conformational change especially under slow cooling conditions. Upon reheating from low temperatures to 65 degrees C, a phase with a two-dimensional, primitive rectangular lattice and delta-like chain packing (R8 phase) in coexistence with the HI, phase emerges. With continued heating to 90 degrees C these coexisting phases give way to a phase with a two-dimensional, centered rectangular lattice and delta-like chain packing (P8phase) which again coexists with the HI, phase. Above 130 degrees C, the Pb phase disappears and the sample converts completely to the HI, phase as observed upon initial heating. These results indicate that the mesomorphic behavior of rye leaf GIcCer is distinct from that of other cerebrosides.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barenholz Y., Suurkuusk J., Mountcastle D., Thompson T. E., Biltonen R. L. A calorimetric study of the thermotropic behavior of aqueous dispersions of natural and synthetic sphingomyelins. Biochemistry. 1976 Jun 1;15(11):2441–2447. doi: 10.1021/bi00656a030. [DOI] [PubMed] [Google Scholar]
- Bouchon B., Portoukalian J., Orgiazzi J., Bornet H. Selective enrichment of phytosphingosine in glycosphingolipids of isolated human thyrocytes as compared to the whole thyroid. Biochem Biophys Res Commun. 1987 Mar 30;143(3):827–831. doi: 10.1016/0006-291x(87)90323-8. [DOI] [PubMed] [Google Scholar]
- Bunow M. R. Two gel states of cerebrosides. Calorimetric and Raman spectroscopic evidence. Biochim Biophys Acta. 1979 Sep 28;574(3):542–546. doi: 10.1016/0005-2760(79)90250-9. [DOI] [PubMed] [Google Scholar]
- Caffrey M. Kinetics and mechanism of the lamellar gel/lamellar liquid-crystal and lamellar/inverted hexagonal phase transition in phosphatidylethanolamine: a real-time X-ray diffraction study using synchrotron radiation. Biochemistry. 1985 Aug 27;24(18):4826–4844. doi: 10.1021/bi00339a017. [DOI] [PubMed] [Google Scholar]
- Cahoon E. B., Lynch D. V. Analysis of Glucocerebrosides of Rye (Secale cereale L. cv Puma) Leaf and Plasma Membrane. Plant Physiol. 1991 Jan;95(1):58–68. doi: 10.1104/pp.95.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Freire M. C., Freire E., Barenholz Y., Biltonen R. L., Thompson T. E. Thermotropic behavior of monoglucocerebroside--dipalmitoylphosphatidylcholine multilamellar liposomes. Biochemistry. 1979 Feb 6;18(3):442–445. doi: 10.1021/bi00570a008. [DOI] [PubMed] [Google Scholar]
- Curatolo W. Glycolipid function. Biochim Biophys Acta. 1987 Jun 24;906(2):137–160. doi: 10.1016/0304-4157(87)90009-8. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Jungalwala F. B. Phase behavior of galactocerebrosides from bovine brain. Biochemistry. 1985 Nov 5;24(23):6608–6613. doi: 10.1021/bi00344a046. [DOI] [PubMed] [Google Scholar]
- Curatolo W. The physical properties of glycolipids. Biochim Biophys Acta. 1987 Jun 24;906(2):111–136. doi: 10.1016/0304-4157(87)90008-6. [DOI] [PubMed] [Google Scholar]
- Curatolo W. Thermal behavior of fractionated and unfractionated bovine brain cerebrosides. Biochemistry. 1982 Apr 13;21(8):1761–1764. doi: 10.1021/bi00537a010. [DOI] [PubMed] [Google Scholar]
- Dahiya R., Brasitus T. A. Distribution of glycosphingolipids and ceramide of rat small intestinal mucosa. Lipids. 1986 Feb;21(2):112–116. doi: 10.1007/BF02534430. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Calhoun W. I., Barenholz Y., Biltonen R. L., Shipley G. G., Thompson T. E. Evidence for metastability in stearoylsphingomyelin bilayers. Biochemistry. 1980 Jan 8;19(1):20–24. doi: 10.1021/bi00542a004. [DOI] [PubMed] [Google Scholar]
- Fernandez-Bermudez S., Loboda-Cacković J., Cacković H., Hosemann R. Structure of cerebrosides I. Phrenosine at 23 degrees C and 66 degrees C. Z Naturforsch C. 1977 May-Jun;32(5-6):362–374. doi: 10.1515/znc-1977-5-608. [DOI] [PubMed] [Google Scholar]
- Freire E., Bach D., Correa-Freire M., Miller I., Barenholz Y. Calorimetric investigation of the complex phase behavior of glucocerebroside dispersions. Biochemistry. 1980 Aug 5;19(16):3662–3665. doi: 10.1021/bi00557a004. [DOI] [PubMed] [Google Scholar]
- Hosemann R., Loboda-Cacković J., Cacković H., Fernandez-Bermúdez S., Baltá-Calleja F. J. Structure of cerebrosides. II. Small angle X-ray diffraction study of cerasine. Z Naturforsch C. 1979 Dec;34(12):1121–1124. doi: 10.1515/znc-1979-1206. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Samuelsson B. E., Steen G. O. Separation of monoglycosylceramides (cerebrosides) of bovine kidney into subgroups and characterization by mass spectrometry. Biochim Biophys Acta. 1973 May 24;306(2):317–328. doi: 10.1016/0005-2760(73)90237-3. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A. Sphingolipid long chain bases. Lipids. 1970 Nov;5(11):878–891. doi: 10.1007/BF02531119. [DOI] [PubMed] [Google Scholar]
- Larsson D., Karlsson D. A. Molecular arrangements in glycosphingolipids. Chem Phys Lipids. 1972 Mar;8(2):152–179. doi: 10.1016/0009-3084(72)90027-8. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Miller I. R., Chapman D. An infrared spectroscopic study of metastable and stable forms of hydrated cerebroside bilayers. Biochim Biophys Acta. 1986 Jul 24;859(2):266–270. doi: 10.1016/0005-2736(86)90222-1. [DOI] [PubMed] [Google Scholar]
- Lynch D. V., Steponkus P. L. Plasma Membrane Lipid Alterations Associated with Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1987 Apr;83(4):761–767. doi: 10.1104/pp.83.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito A. J., Akita T., Sweeley C. C. Gas chromatography and mass spectrometry of sphingolipid bases. Characterization of sphinga-4,14-dienine from plasma sphingomyelin. Biochemistry. 1968 Jul;7(7):2609–2614. doi: 10.1021/bi00847a024. [DOI] [PubMed] [Google Scholar]
- Reed R. A., Shipley G. G. Effect of chain unsaturation on the structure and thermotropic properties of galactocerebrosides. Biophys J. 1989 Feb;55(2):281–292. doi: 10.1016/S0006-3495(89)82803-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. A., Shipley G. G. Structure and metastability of N-lignocerylgalactosylsphingosine (cerebroside) bilayers. Biochim Biophys Acta. 1987 Jan 26;896(2):153–164. doi: 10.1016/0005-2736(87)90175-1. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Rochester C. P., Kjellbom P., Andersson B., Larsson C. Lipid composition of plasma membranes isolated from light-grown barley (Hordeum vulgare) leaves: identification of cerebroside as a major component. Arch Biochem Biophys. 1987 Jun;255(2):385–391. doi: 10.1016/0003-9861(87)90406-1. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Atkinson D., Small D. M., Skarjune R. P., Oldfield E., Shipley G. G. X-ray diffraction and calorimetric study of anhydrous and hydrated N-palmitoylgalactosylsphingosine (cerebroside). Biochemistry. 1981 Oct 13;20(21):5957–5966. doi: 10.1021/bi00524a006. [DOI] [PubMed] [Google Scholar]
- Sandstrom R. P., Cleland R. E. Comparison of the lipid composition of oat root and coleoptile plasma membranes: lack of short-term change in response to auxin. Plant Physiol. 1989;90:1207–1213. doi: 10.1104/pp.90.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Steponkus P. L., Uemura M., Balsamo R. A., Arvinte T., Lynch D. V. Transformation of the cryobehavior of rye protoplasts by modification of the plasma membrane lipid composition. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9026–9030. doi: 10.1073/pnas.85.23.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Uemura M. Lipid Composition of Plasma Membranes and Tonoplasts Isolated from Etiolated Seedlings of Mung Bean (Vigna radiata L.). Plant Physiol. 1986 Nov;82(3):807–812. doi: 10.1104/pp.82.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]



