Abstract
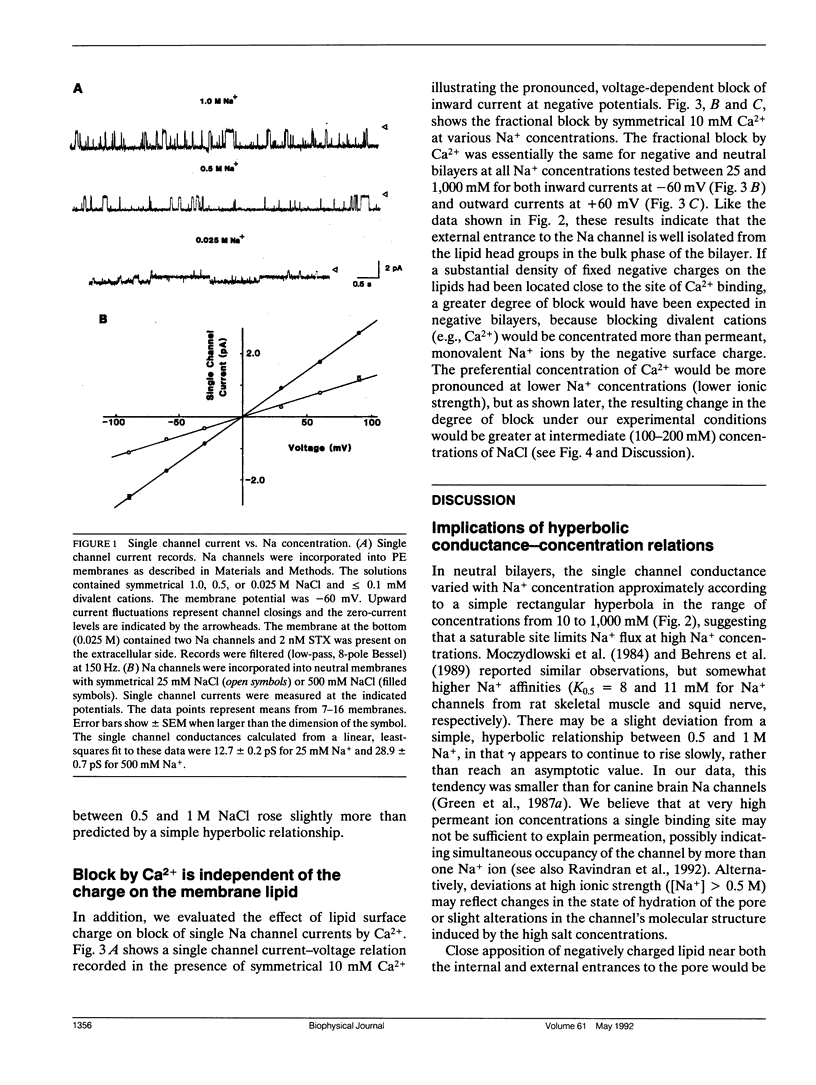
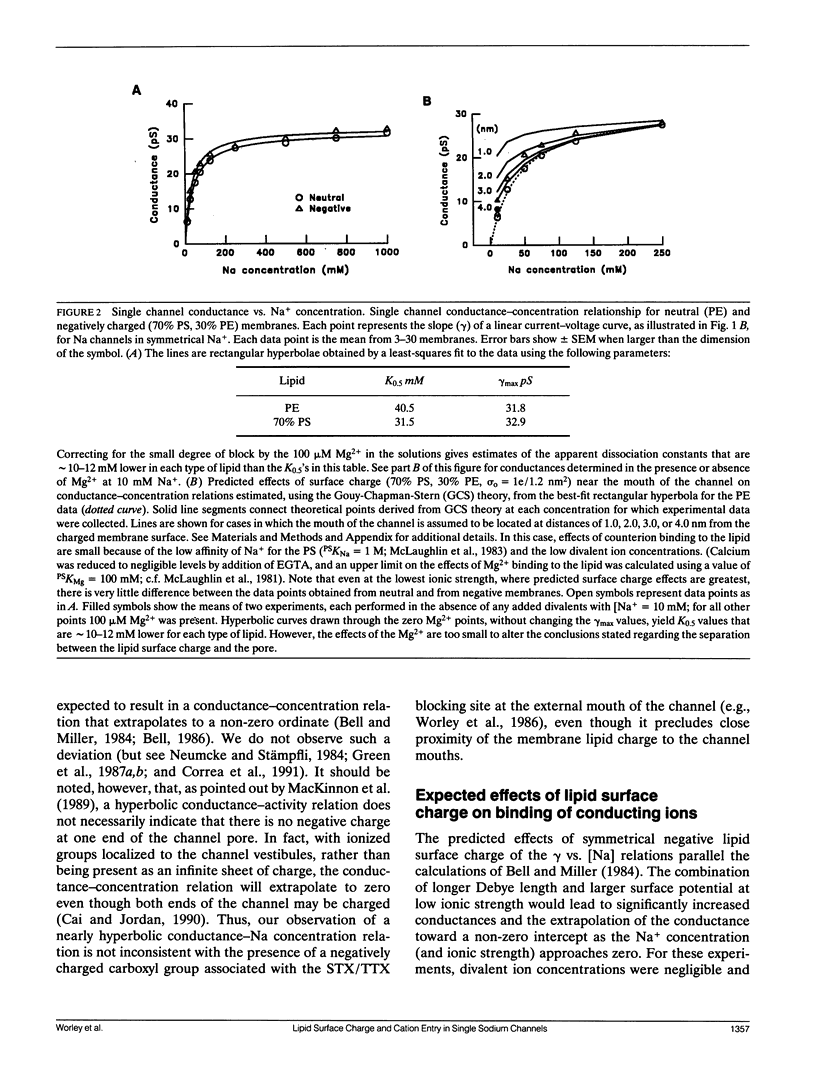
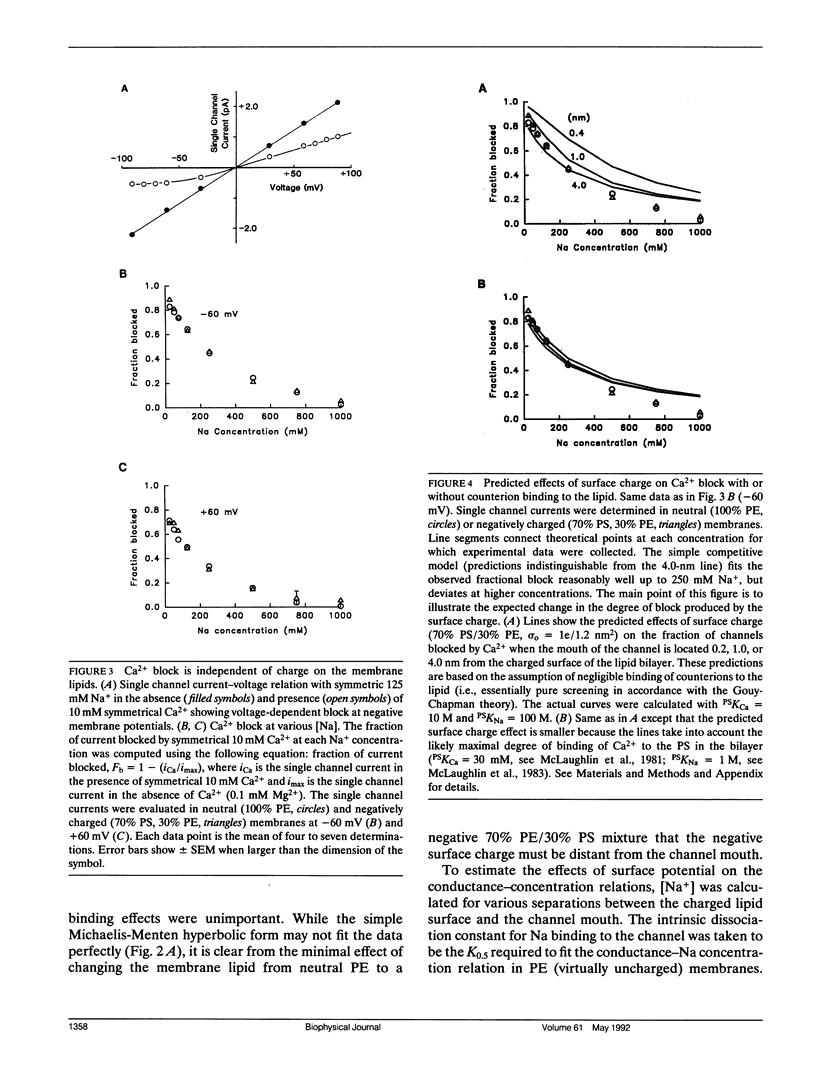
We have studied the effects of membrane surface charge on Na+ ion permeation and Ca2+ block in single, batrachotoxin-activated Na channels from rat brain, incorporated into planar lipid bilayers. In phospholipid membranes with no net charge (phosphatidylethanolamine, PE), at low divalent cation concentrations (approximately 100 microM Mg2+), the single channel current-voltage relation was linear and the single channel conductance saturated with increasing [Na+] and ionic strength, reaching a maximum (gamma max) of 31.8 pS, with an apparent dissociation constant (K0.5) of 40.5 mM. The data could be approximated by a rectangular hyperbola. In negatively charged bilayers (70% phosphatidylserine, PS; 30% PE) slightly larger conductances were observed at each concentration, but the hyperbolic form of the conductance-concentration relation was retained (gamma max = 32.9 pS and K0.5 = 31.5 mM) without any preferential increase in conductance at lower ionic strengths. Symmetrical application of Ca2+ caused a voltage-dependent block of the single channel current, with the block being greater at negative potentials. For any given voltage and [Na+] this block was identical in neutral and negatively charged membranes. These observations suggest that both the conduction pathway and the site(s) of Ca2+ block of the rat brain Na channel protein are electrostatically isolated from the negatively charged headgroups on the membrane lipids.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens M. I., Oberhauser A., Bezanilla F., Latorre R. Batrachotoxin-modified sodium channels from squid optic nerve in planar bilayers. Ion conduction and gating properties. J Gen Physiol. 1989 Jan;93(1):23–41. doi: 10.1085/jgp.93.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. E., Miller C. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys J. 1984 Jan;45(1):279–287. doi: 10.1016/S0006-3495(84)84154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M., Jordan P. C. How does vestibule surface charge affect ion conduction and toxin binding in a sodium channel? Biophys J. 1990 Apr;57(4):883–891. doi: 10.1016/S0006-3495(90)82608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi X., Alvarez O., Latorre R. A three-barrier model for the hemocyanin channel. J Gen Physiol. 1981 Dec;78(6):657–681. doi: 10.1085/jgp.78.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Affolter H. Insulation of the conduction pathway of muscle transverse tubule calcium channels from the surface charge of bilayer phospholipid. J Gen Physiol. 1986 Jun;87(6):933–953. doi: 10.1085/jgp.87.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A. M., Latorre R., Bezanilla F. Ion permeation in normal and batrachotoxin-modified Na+ channels in the squid giant axon. J Gen Physiol. 1991 Mar;97(3):605–625. doi: 10.1085/jgp.97.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S., Zinkand W. C., French R. J., Krueger B. K. Effects of membrane surface charge and calcium on the gating of rat brain sodium channels in planar bilayers. J Gen Physiol. 1988 Oct;92(4):431–447. doi: 10.1085/jgp.92.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- French R. J., Worley J. F., 3rd, Krueger B. K. Voltage-dependent block by saxitoxin of sodium channels incorporated into planar lipid bilayers. Biophys J. 1984 Jan;45(1):301–310. doi: 10.1016/S0006-3495(84)84156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W. N., Weiss L. B., Andersen O. S. Batrachotoxin-modified sodium channels in planar lipid bilayers. Characterization of saxitoxin- and tetrodotoxin-induced channel closures. J Gen Physiol. 1987 Jun;89(6):873–903. doi: 10.1085/jgp.89.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W. N., Weiss L. B., Andersen O. S. Batrachotoxin-modified sodium channels in planar lipid bilayers. Ion permeation and block. J Gen Physiol. 1987 Jun;89(6):841–872. doi: 10.1085/jgp.89.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984 Feb 10;259(3):1667–1675. [PubMed] [Google Scholar]
- Hille B. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol. 1975 Nov;66(5):535–560. doi: 10.1085/jgp.66.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971 Dec;58(6):599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger B. K., Ratzlaff R. W., Strichartz G. R., Blaustein M. P. Saxitoxin binding to synaptosomes, membranes, and solubilized binding sites from rat brain. J Membr Biol. 1979 Nov 30;50(3-4):287–310. doi: 10.1007/BF01868894. [DOI] [PubMed] [Google Scholar]
- Krueger B. K., Worley J. F., 3rd, French R. J. Single sodium channels from rat brain incorporated into planar lipid bilayer membranes. Nature. 1983 May 12;303(5913):172–175. doi: 10.1038/303172a0. [DOI] [PubMed] [Google Scholar]
- Levinson S. R., Thornhill W. B., Duch D. S., Recio-Pinto E., Urban B. W. The role of nonprotein domains in the function and synthesis of voltage-gated sodium channels. Ion Channels. 1990;2:33–64. doi: 10.1007/978-1-4615-7305-0_2. [DOI] [PubMed] [Google Scholar]
- Loosley-Millman M. E., Rand R. P., Parsegian V. A. Effects of monovalent ion binding and screening on measured electrostatic forces between charged phospholipid bilayers. Biophys J. 1982 Dec;40(3):221–232. doi: 10.1016/S0006-3495(82)84477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R., Latorre R., Miller C. Role of surface electrostatics in the operation of a high-conductance Ca2+-activated K+ channel. Biochemistry. 1989 Oct 3;28(20):8092–8099. doi: 10.1021/bi00446a020. [DOI] [PubMed] [Google Scholar]
- McCarthy M. P., Earnest J. P., Young E. F., Choe S., Stroud R. M. The molecular neurobiology of the acetylcholine receptor. Annu Rev Neurosci. 1986;9:383–413. doi: 10.1146/annurev.ne.09.030186.002123. [DOI] [PubMed] [Google Scholar]
- McLaughlin A., Eng W. K., Vaio G., Wilson T., McLaughlin S. Dimethonium, a divalent cation that exerts only a screening effect on the electrostatic potential adjacent to negatively charged phospholipid bilayer membranes. J Membr Biol. 1983;76(2):183–193. doi: 10.1007/BF02000618. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G., Ciani S. M. Surface charge and the conductance of phospholipid membranes. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1268–1275. doi: 10.1073/pnas.67.3.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Mulrine N., Gresalfi T., Vaio G., McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981 Apr;77(4):445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Alvarez O., Vergara C., Latorre R. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J Membr Biol. 1985;83(3):273–282. doi: 10.1007/BF01868701. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Garber S. S., Miller C. Batrachotoxin-activated Na+ channels in planar lipid bilayers. Competition of tetrodotoxin block by Na+. J Gen Physiol. 1984 Nov;84(5):665–686. doi: 10.1085/jgp.84.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhayeva G. N., Naumov A. P., Nosyreva E. D. Potential-dependent calcium blockage of normal and aconitine-modified sodium channels in frog node of Ranvier. Gen Physiol Biophys. 1985 Aug;4(4):425–427. [PubMed] [Google Scholar]
- Neumcke B., Stämpfli R. Heterogeneity of external surface charges near sodium channels in the nodal membrane of frog nerve. Pflugers Arch. 1984 Jun;401(2):125–131. doi: 10.1007/BF00583872. [DOI] [PubMed] [Google Scholar]
- Ravindran A., Kwiecinski H., Alvarez O., Eisenman G., Moczydlowski E. Modeling ion permeation through batrachotoxin-modified Na+ channels from rat skeletal muscle with a multi-ion pore. Biophys J. 1992 Feb;61(2):494–508. doi: 10.1016/S0006-3495(92)81854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets M. F., Scanley B. E., Hanck D. A., Makielski J. C., Fozzard H. A. Open sodium channel properties of single canine cardiac Purkinje cells. Biophys J. 1987 Jul;52(1):13–22. doi: 10.1016/S0006-3495(87)83183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley J. F., 3rd, French R. J., Krueger B. K. Trimethyloxonium modification of single batrachotoxin-activated sodium channels in planar bilayers. Changes in unit conductance and in block by saxitoxin and calcium. J Gen Physiol. 1986 Feb;87(2):327–349. doi: 10.1085/jgp.87.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z., Narahashi T. Interactions of permeant cations with sodium channels of squid axon membranes. Biophys J. 1985 Sep;48(3):361–368. doi: 10.1016/S0006-3495(85)83792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z., Narahashi T. Voltage-dependent calcium block of normal and tetramethrin-modified single sodium channels. Biophys J. 1984 Jan;45(1):337–344. doi: 10.1016/S0006-3495(84)84159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


