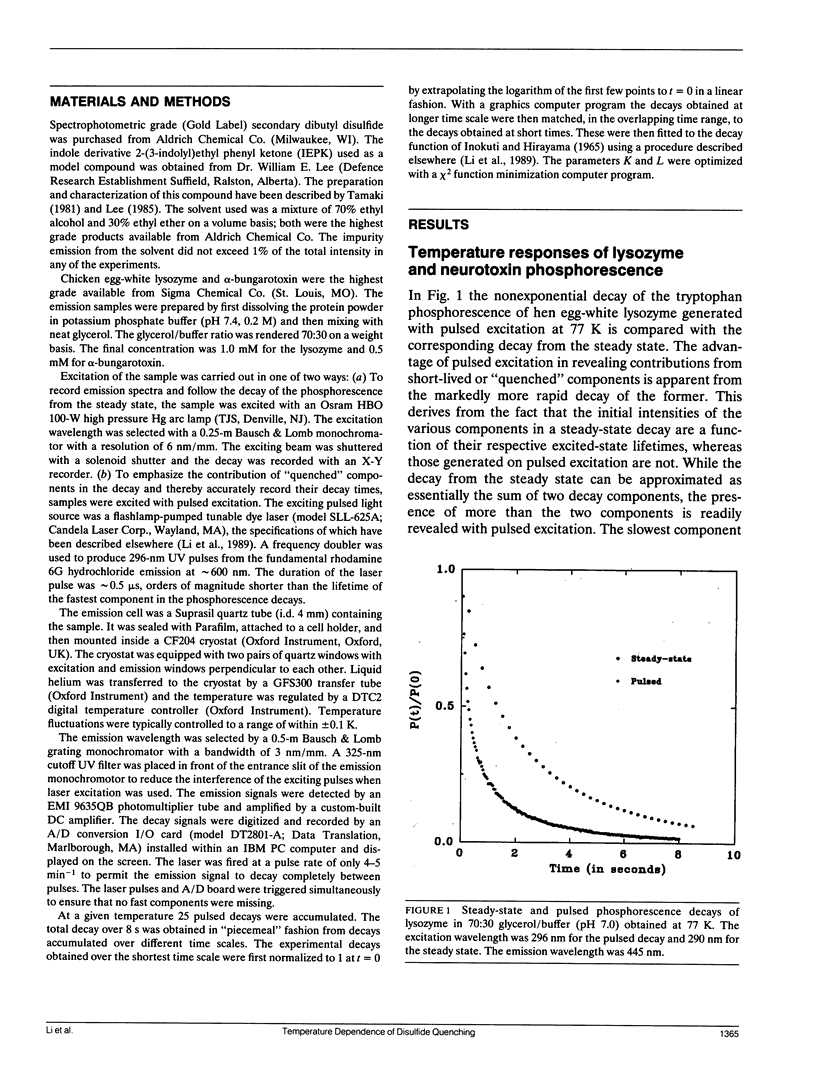
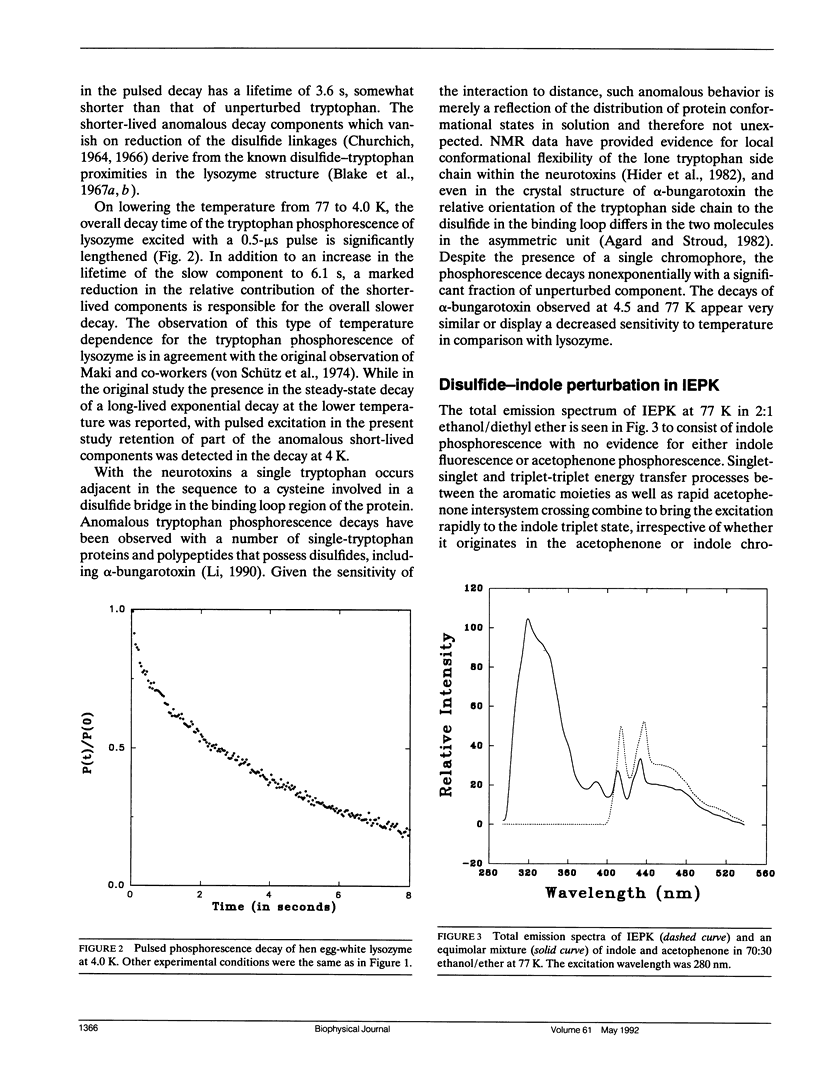
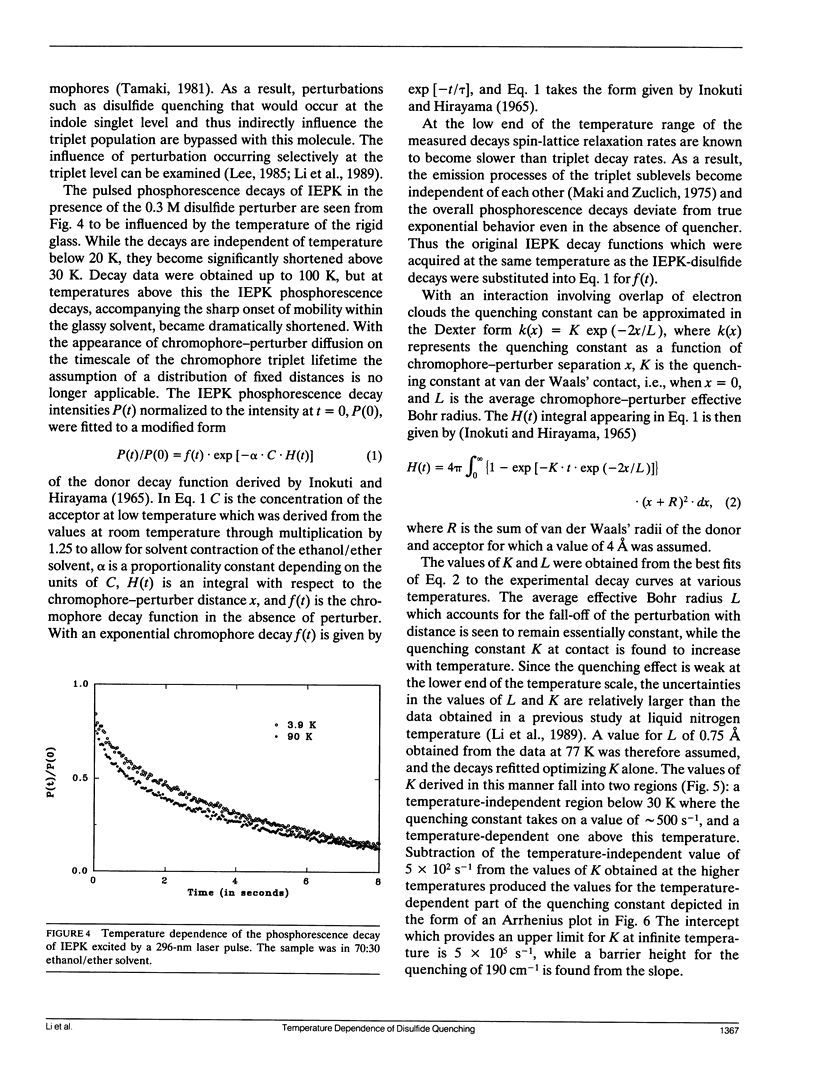
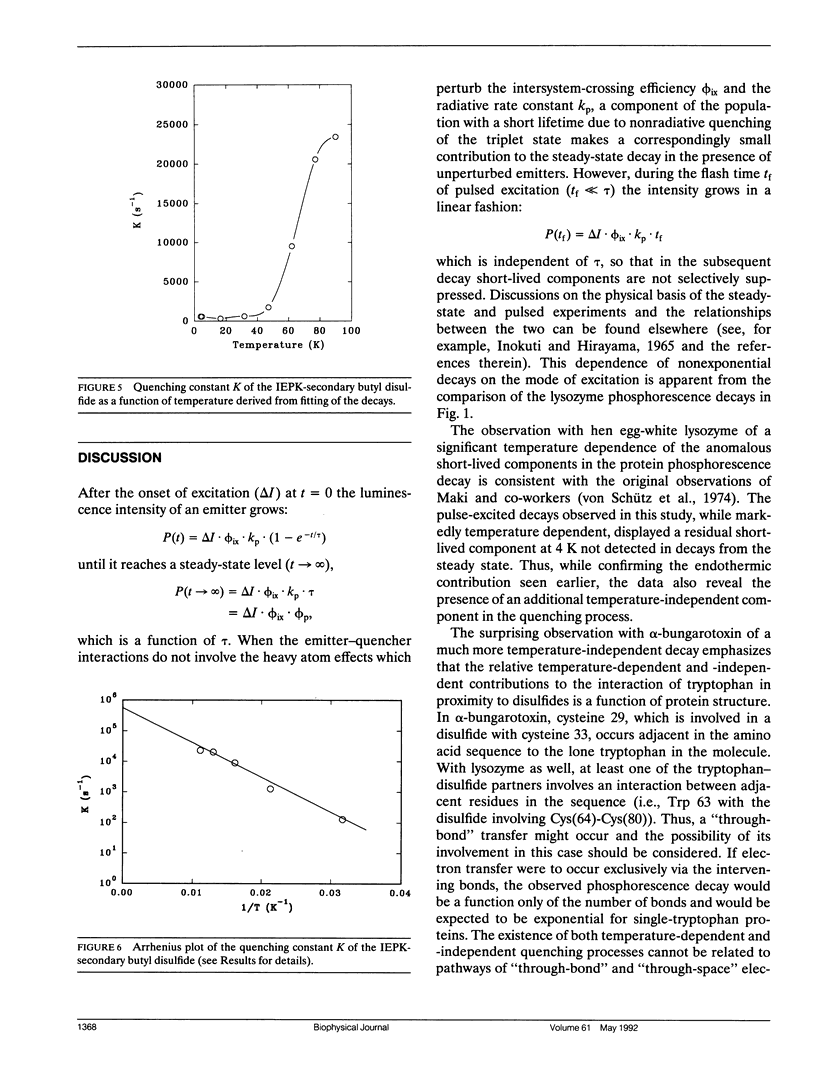
Abstract
Variability in the temperature dependence of disulfide quenching of the tryptophan phosphorescence of globular proteins in rigid glasses is illustrated with lysozyme and α-bungarotoxin. A laser-pulsed phosphorescence study of this short-range interaction with a model indole-disulfide system is described. The perturbation of secondary dibutyl disulfide on the triplet state of the indole moiety in 2-(3-indolyl)ethyl phenyl ketone in rigid media is found to display a bimodal temperature dependence. The quenching rate constant at contact between chromophore and perturber is observed to be temperature independent below 30 K, but to increase with temperature between 30 and 100 K with an activation energy of ∼200 cm-1. The results suggest that the underlying quenching interaction involves a photo-induced one-electron transfer from the excited state of indole to the disulfide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bent D. V., Hayon E. Excited state chemistry of aromatic amino acids and related peptides. III. Tryptophan. J Am Chem Soc. 1975 May 14;97(10):2612–2619. doi: 10.1021/ja00843a004. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Mair G. A., North A. C., Phillips D. C., Sarma V. R. On the conformation of the hen egg-white lysozyme molecule. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):365–377. doi: 10.1098/rspb.1967.0034. [DOI] [PubMed] [Google Scholar]
- CHURCHICH J. E. LUMINESCENCE PROPERTIES OF MURAMIDASE AND REOXIDIZED MURAMIDASE. Biochim Biophys Acta. 1964 Oct 23;92:194–197. doi: 10.1016/0926-6569(64)90295-0. [DOI] [PubMed] [Google Scholar]
- Churchich J. E. Tryptophan residues in native and reoxidized muramidase: luminescence properties. Biochim Biophys Acta. 1966 Jul 13;120(3):406–412. doi: 10.1016/0926-6585(66)90307-4. [DOI] [PubMed] [Google Scholar]
- Cowgill R. W. Fluorescence and protein structure. XVII. On the mechanism of peptide quenching. Biochim Biophys Acta. 1970 Jan 20;200(1):18–25. [PubMed] [Google Scholar]
- DeVault D., Chance B. Studies of photosynthesis using a pulsed laser. I. Temperature dependence of cytochrome oxidation rate in chromatium. Evidence for tunneling. Biophys J. 1966 Nov;6(6):825–847. doi: 10.1016/s0006-3495(66)86698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault D. Quantum mechanical tunnelling in biological systems. Q Rev Biophys. 1980 Nov;13(4):387–564. doi: 10.1017/s003358350000175x. [DOI] [PubMed] [Google Scholar]
- Grossweiner L. I., Usui Y. Flash photolysis and inactivation of aqueous lysozyme. Photochem Photobiol. 1971 Mar;13(3):195–214. doi: 10.1111/j.1751-1097.1971.tb06106.x. [DOI] [PubMed] [Google Scholar]
- Hider R. C., Drake A. F., Inagaki F., Williams R. J., Endo T., Miyazawa T. Molecular conformation of alpha-cobratoxin as studied by nuclear magnetic resonance and circular dichroism. J Mol Biol. 1982 Jun 25;158(2):275–291. doi: 10.1016/0022-2836(82)90433-8. [DOI] [PubMed] [Google Scholar]
- Li Z., Galley W. C. Evidence for ligand-induced conformational changes in proteins from phosphorescence spectroscopy. Biophys J. 1989 Aug;56(2):353–360. doi: 10.1016/S0006-3495(89)82681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Lee W. E., Galley W. C. Distance dependence of the tryptophan-disulfide interaction at the triplet level from pulsed phosphorescence studies on a model system. Biophys J. 1989 Aug;56(2):361–367. doi: 10.1016/S0006-3495(89)82682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. R., Huang Z. X., Eley C. G., Barker H. A., Williams G., Robinson M. N., Williams R. J. Electron transfer in biology. The function of cytochrome c. Faraday Discuss Chem Soc. 1982;74:311–329. doi: 10.1039/dc9827400311. [DOI] [PubMed] [Google Scholar]
- Ménez A., Montenay-Garestier T., Fromageot P., Hélène C. Conformation of two homologous neurotoxins. Fluorescence and circular dichroism studies. Biochemistry. 1980 Nov 11;19(23):5202–5208. doi: 10.1021/bi00564a008. [DOI] [PubMed] [Google Scholar]
- Saviotti M. L., Galley W. C. Room temperature phosphorescence and the dynamic aspects of protein structure. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4154–4158. doi: 10.1073/pnas.71.10.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuclich J. A., Maki A. H. Protein triplet states. Top Curr Chem. 1975;(54):115–163. doi: 10.1007/BFb0046182. [DOI] [PubMed] [Google Scholar]
- von Schütz J. U., Zuclich J., Maki A. H. Resolution of tryptophan phosphorescence from multiple sites in proteins using optical detection of magnetic resonance. J Am Chem Soc. 1974 Feb 6;96(3):714–718. doi: 10.1021/ja00810a014. [DOI] [PubMed] [Google Scholar]


