Abstract
This paper presents a review of our current understanding of the nature of the thymic microenvironment, after briefly considering the major role of the gland. The epithelial cells and their products are of fundamental importance, and other cells of the macrophage series are implicated in most functional events. The embryological origin of the epithelium is still not clear, although disease conditions would suggest a single origin. Immigration and emigration of thymocytes is considered, and also the passage of antigens into the gland. The events within the thymus are under the control of the CNS acting through the innervation or via hormonal pathways. Both of these areas are considered in detail, especially thymic hormone origins, functions and interactions.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspinall R., Kampinga J., van den Bogaerde J. T-cell development in the fetus and the invariant series hypothesis. Immunol Today. 1991 Jan;12(1):7–10. doi: 10.1016/0167-5699(91)90105-3. [DOI] [PubMed] [Google Scholar]
- BURNET F. M., HOLMES M. C. Immunological function of thymus and bursa of Fabricius. Thymus lesions in an auto-immune disease of mice. Nature. 1962 Apr 14;194:146–147. doi: 10.1038/194146a0. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M., Goldstein A. L., Guha A., White A. Appearance of T-cell markers in bone marrow rosette-forming cells after incubation with thymosin, a thymic hormone. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2734–2738. doi: 10.1073/pnas.68.11.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J., Bardenne M., Pleau J., Rosa J. Biochemical characterisation of a serum thymic factor. Nature. 1977 Mar 3;266(5597):55–57. doi: 10.1038/266055a0. [DOI] [PubMed] [Google Scholar]
- Basham T. Y., Merigan T. C. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983 Apr;130(4):1492–1494. [PubMed] [Google Scholar]
- Beletskaya L. V., Gnesditskaya E. V. Detection of squamous epithelial intercellular substance antigen(s) in Hassall's corpuscles of human and animal thymus. Scand J Immunol. 1980;12(2):93–98. doi: 10.1111/j.1365-3083.1980.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Berrih S., Arenzana-Seisdedos F., Cohen S., Devos R., Charron D., Virelizier J. L. Interferon-gamma modulates HLA class II antigen expression on cultured human thymic epithelial cells. J Immunol. 1985 Aug;135(2):1165–1171. [PubMed] [Google Scholar]
- Besedovsky H., Sorkin E., Felix D., Haas H. Hypothalamic changes during the immune response. Eur J Immunol. 1977 May;7(5):323–325. doi: 10.1002/eji.1830070516. [DOI] [PubMed] [Google Scholar]
- Biachi E., Pierpaoli W., Sorkin E. Cytological changes in the mouse anterior pituitary after neonatal thymectomy: a light ane electron microscopical study. J Endocrinol. 1971 Sep;51(1):1–6. doi: 10.1677/joe.0.0510001. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Harbour-McMenamin D., Smith E. M. Peptide hormones shared by the neuroendocrine and immunologic systems. J Immunol. 1985 Aug;135(2 Suppl):858s–861s. [PubMed] [Google Scholar]
- Bockman D. E., Kirby M. L. Neural crest interactions in the development of the immune system. J Immunol. 1985 Aug;135(2 Suppl):766s–768s. [PubMed] [Google Scholar]
- Bofill M., Janossy G., Willcox N., Chilosi M., Trejdosiewicz L. K., Newsom-Davis J. Microenvironments in the normal thymus and the thymus in myasthenia gravis. Am J Pathol. 1985 Jun;119(3):462–473. [PMC free article] [PubMed] [Google Scholar]
- Bordigoni P., Faure G., Bene M. C., Dardenne M., Bach J. F., Duheille J., Olive D. Improvement of cellular immunity and IgA production in immunodeficient children after treatment with synthetic serum thymic factor (FTS). Lancet. 1982 Aug 7;2(8293):293–297. doi: 10.1016/s0140-6736(82)90271-9. [DOI] [PubMed] [Google Scholar]
- Brekelmans P., van Ewijk W. Phenotypic characterization of murine thymic microenvironments. Semin Immunol. 1990 Jan;2(1):13–24. [PubMed] [Google Scholar]
- Bulloch K., Moore R. Y. Innervation of the thymus gland by brain stem and spinal cord in mouse and rat. Am J Anat. 1981 Oct;162(2):157–166. doi: 10.1002/aja.1001620207. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Buchner V., Pecht M., Trainin N. Thymic humoral factor gamma 2: purification and amino acid sequence of an immunoregulatory peptide from calf thymus. Biochemistry. 1988 May 31;27(11):4066–4071. doi: 10.1021/bi00411a024. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Schreyer M. Immunohistochemical localization of host and donor-derived cells in the regenerating thymus of radiation bone marrow chimeras. Thymus. 1984;6(1-2):15–26. [PubMed] [Google Scholar]
- Chambers D. J., Karimzandi N., Braimbridge M. V., Dunham J., Brooks F., Quiney J., Slavin B. Hormonal and electrolyte responses during and after open heart surgery. Thorac Cardiovasc Surg. 1984 Dec;32(6):358–364. doi: 10.1055/s-2007-1023423. [DOI] [PubMed] [Google Scholar]
- Chaudhri G., Clark I. A., Ceredig R. Proliferation in vitro of Lyt2-,L3T4- thymocytes shows responsiveness to interleukin 1. Clin Exp Immunol. 1988 Jul;73(1):51–56. [PMC free article] [PubMed] [Google Scholar]
- Chilosi M., Iannucci A., Fiore-Donati L., Tridente G., Pampanin M., Pizzolo G., Ritter M., Bofill M., Janossy G. Myasthenia gravis: immunohistological heterogeneity in microenvironmental organization of hyperplastic and neoplastic thymuses suggesting different mechanisms of tolerance breakdown. J Neuroimmunol. 1986 May;11(3):191–204. doi: 10.1016/0165-5728(86)90003-2. [DOI] [PubMed] [Google Scholar]
- Clarke A. G., Kendall M. D. Histological changes in the thymus during mouse pregnancy. Thymus. 1989;14(1-3):65–78. [PubMed] [Google Scholar]
- Cordier A. C., Haumont S. M. Development of thymus, parathyroids, and ultimo-branchial bodies in NMRI and nude mice. Am J Anat. 1980 Mar;157(3):227–263. doi: 10.1002/aja.1001570303. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C., Engel W. K., McClure J. E., Goldstein A. L., Askanas V. Immunocytochemical localization of thymosin-alpha 1 in thymic epithelial cells of normal and myasthenia gravis patients and in thymic cultures. J Neurol Sci. 1981 May;50(2):239–247. doi: 10.1016/0022-510x(81)90170-2. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Charreire J., Bach J. F. Alterations in thymocyte surface markers after in vivo treatment by serum thymic factor. Cell Immunol. 1978 Aug;39(1):47–54. doi: 10.1016/0008-8749(78)90081-3. [DOI] [PubMed] [Google Scholar]
- Dargemont C., Dunon D., Deugnier M. A., Denoyelle M., Girault J. M., Lederer F., Lê K. H., Godeau F., Thiery J. P., Imhof B. A. Thymotaxin, a chemotactic protein, is identical to beta 2-microglobulin. Science. 1989 Nov 10;246(4931):803–806. doi: 10.1126/science.2683083. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Garrett T. J. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med. 1972 Nov 1;136(5):1098–1116. doi: 10.1084/jem.136.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris N., Mocchegiani E. Endocrine control of thymic serum factor production in young-adult and old mice. Cell Immunol. 1985 Apr 1;91(2):325–335. doi: 10.1016/0008-8749(85)90230-8. [DOI] [PubMed] [Google Scholar]
- Fabris N., Mocchegiani E., Mariotti S., Pacini F., Pinchera A. Thyroid function modulates thymic endocrine activity. J Clin Endocrinol Metab. 1986 Mar;62(3):474–478. doi: 10.1210/jcem-62-3-474. [DOI] [PubMed] [Google Scholar]
- Fabris N., Pierpaoli W., Sorkin E. Hormones and the immunological capacity. 3. The immunodeficiency disease of the hypopituitary Snell-Bagg dwarf mouse. Clin Exp Immunol. 1971 Aug;9(2):209–225. [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Anderson S. K., Marrack P., Kappler J. Expression of antigen-specific, major histocompatibility complex-restricted receptors by cortical and medullary thymocytes in situ. Cell. 1985 Dec;43(2 Pt 1):543–550. doi: 10.1016/0092-8674(85)90183-7. [DOI] [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y., Bellinger D. L., Carlson S. L., Ackerman K. D., Madden K. S., Olschowki J. A., Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987 Dec;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Fenske M., Wuttke W. Effects of intraventricular 6-hydroxydopamine injections on serum prolactin and LH levels: absence of stress-induced pituitary prolactin release. Brain Res. 1976 Mar 5;104(1):63–70. doi: 10.1016/0006-8993(76)90647-8. [DOI] [PubMed] [Google Scholar]
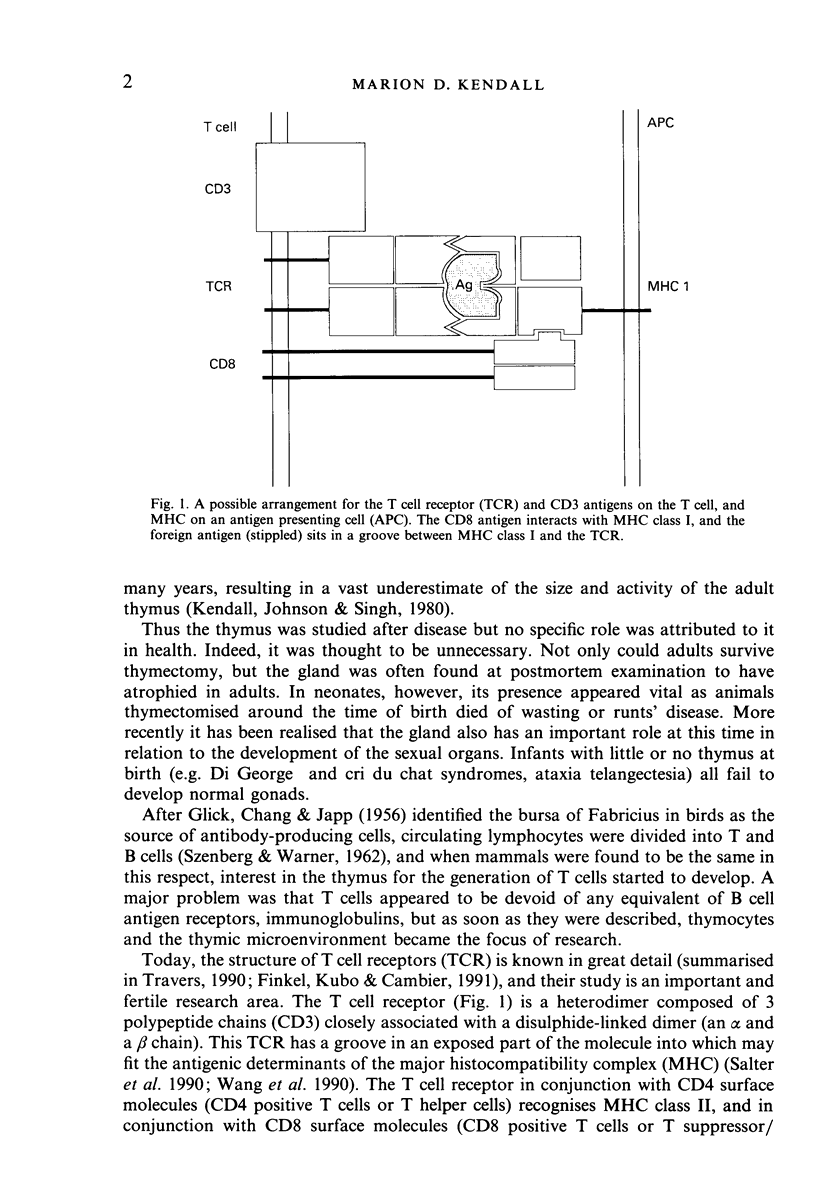
- Finkel T. H., Kubo R. T., Cambier J. C. T-cell development and transmembrane signaling: changing biological responses through an unchanging receptor. Immunol Today. 1991 Feb;12(2):79–85. doi: 10.1016/0167-5699(91)90162-M. [DOI] [PubMed] [Google Scholar]
- Fontaine-Perus J. C., Calman F. M., Kaplan C., Le Douarin N. M. Seeding of the 10-day mouse embryo thymic rudiment by lymphocyte precursors in vitro. J Immunol. 1981 Jun;126(6):2310–2316. [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Gearing A. J., Wadhwa M., Perris A. D. In vivo administration of interleukin 2 stimulates mitosis in thymus and bone marrow. Eur J Immunol. 1986 Sep;16(9):1171–1174. doi: 10.1002/eji.1830160923. [DOI] [PubMed] [Google Scholar]
- Geenen V., Defresne M. P., Robert F., Legros J. J., Franchimont P., Boniver J. The neurohormonal thymic microenvironment: immunocytochemical evidence that thymic nurse cells are neuroendocrine cells. Neuroendocrinology. 1988 Apr;47(4):365–368. doi: 10.1159/000124938. [DOI] [PubMed] [Google Scholar]
- Gnezditskaya E. V., Beletskaya L. V. Immunofluorescence study of keratin of Hassall's corpuscles and epidermis of the human skin. Bull Exp Biol Med. 1974 Oct;77(4):431–433. doi: 10.1007/BF00798107. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Scheid M. P., Boyse E. A., Schlesinger D. H., Van Wauwe J. A synthetic pentapeptide with biological activity characteristic of the thymic hormone thymopoietin. Science. 1979 Jun 22;204(4399):1309–1310. doi: 10.1126/science.451537. [DOI] [PubMed] [Google Scholar]
- Gomariz R. P., Lorenzo M. J., Cacicedo L., Vicente A., Zapata A. G. Demonstration of immunoreactive vasoactive intestinal peptide (IR-VIP) and somatostatin (IR-SOM) in rat thymus. Brain Behav Immun. 1990 Jun;4(2):151–161. doi: 10.1016/0889-1591(90)90017-k. [DOI] [PubMed] [Google Scholar]
- Hall N. R., McGillis J. P., Spangelo B. L., Goldstein A. L. Evidence that thymosins and other biologic response modifiers can function as neuroactive immunotransmitters. J Immunol. 1985 Aug;135(2 Suppl):806s–811s. [PubMed] [Google Scholar]
- Haritos A. A., Tsolas O., Horecker B. L. Distribution of prothymosin alpha in rat tissues. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1391–1393. doi: 10.1073/pnas.81.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Scearce R. M., Lobach D. F., Hensley L. L. Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. J Exp Med. 1984 Apr 1;159(4):1149–1168. doi: 10.1084/jem.159.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Shimizu K., Eisenbarth G. S. Identification of human and rodent thymic epithelium using tetanus toxin and monoclonal antibody A2B5. J Clin Invest. 1983 Jan;71(1):9–14. doi: 10.1172/JCI110755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S. Interleukin 7: effects on early events in lymphopoiesis. Immunol Today. 1989 May;10(5):170–173. doi: 10.1016/0167-5699(89)90175-8. [DOI] [PubMed] [Google Scholar]
- Hersh E. M., Reuben J. M., Rios A., Mansell P. W., Newell G. R., McClure J. E., Goldstein A. L. Elevated serum thymosin alpha 1 levels associated with evidence of immune dysregulation in male homosexuals with a history of infectious diseases or Kaposi's sarcoma. N Engl J Med. 1983 Jan 6;308(1):45–46. doi: 10.1056/nejm198301063080113. [DOI] [PubMed] [Google Scholar]
- Hodgkin P. D., Bond M. W., O'Garra A., Frank G., Lee F., Coffman R. L., Zlotnik A., Howard M. Identification of IL-6 as a T cell-derived factor that enhances the proliferative response of thymocytes to IL-4 and phorbol myristate acetate. J Immunol. 1988 Jul 1;141(1):151–157. [PubMed] [Google Scholar]
- Incefy G. S., Dardenne M., Pahwa S., Grimes E., Pahwa R. N., Smithwick E., O'Reilly R., Good R. A. Thymic activity in severe combined immunodeficiency diseases. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1250–1253. doi: 10.1073/pnas.74.3.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S., Farr A. G., Lafaille J. J., Bonneville M., Takagaki Y., Haas W., Tonegawa S. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990 Feb 22;343(6260):754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- Iwata T., Incefy G. S., Cunningham-Rundles S., Cunningham-Rundles C., Smithwick E., Geller N., O'Reilly R., Good R. A. Circulating thymic hormone activity in patients with primary and secondary immunodeficiency diseases. Am J Med. 1981 Sep;71(3):385–394. doi: 10.1016/0002-9343(81)90164-9. [DOI] [PubMed] [Google Scholar]
- Janković B. D., Isaković K., Knezević Z. Ontogeny of this immuno-neuro-endocrine relationship. Changes in lymphoid tissue of chick embryos surgically decapitated at 33-38 hours of incubation. Dev Comp Immunol. 1978 Jul;2(3):479–491. doi: 10.1016/s0145-305x(78)80009-3. [DOI] [PubMed] [Google Scholar]
- Janossy G., Bofill M., Trejdosiewicz L. K., Willcox H. N., Chilosi M. Cellular differentiation of lymphoid subpopulations and their microenvironments in the human thymus. Curr Top Pathol. 1986;75:89–125. doi: 10.1007/978-3-642-82480-7_3. [DOI] [PubMed] [Google Scholar]
- Jenkinson E. J., Kingston R., Owen J. J. Importance of IL-2 receptors in intra-thymic generation of cells expressing T-cell receptors. Nature. 1987 Sep 10;329(6135):160–162. doi: 10.1038/329160a0. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Torres B. A. Regulation of lymphokine production by arginine vasopressin and oxytocin: modulation of lymphocyte function by neurohypophyseal hormones. J Immunol. 1985 Aug;135(2 Suppl):773s–775s. [PubMed] [Google Scholar]
- KEYNES G. Investigations into thymic disease and tumour formation. Br J Surg. 1955 Mar;42(175):449–462. doi: 10.1002/bjs.18004217502. [DOI] [PubMed] [Google Scholar]
- KEYNES G. The results of thymectomy in myasthenia gravis. Br Med J. 1949 Sep 17;2(4628):611–616. doi: 10.1136/bmj.2.4628.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserling E., Stein H., Müller-Hermelink H. K. Interdigitating reticulum cells in the human thymus. Cell Tissue Res. 1974;155(1):47–55. doi: 10.1007/BF00220283. [DOI] [PubMed] [Google Scholar]
- Kampinga J., Berges S., Boyd R. L., Brekelmans P., Colić M., van Ewijk W., Kendall M. D., Ladyman H., Nieuwenhuis P., Ritter M. A. Thymic epithelial antibodies: immunohistological analysis and introduction of nomenclature. Thymus. 1989;13(3-4):165–173. [PubMed] [Google Scholar]
- Kato S. Intralobular lymphatic vessels and their relationship to blood vessels in the mouse thymus. Light- and electron-microscopic study. Cell Tissue Res. 1988 Jul;253(1):181–187. doi: 10.1007/BF00221753. [DOI] [PubMed] [Google Scholar]
- Kendall M. D., Fitzpatrick F. T., Greenstein B. D., Khoylou F., Safieh B., Hamblin A. Reversal of ageing changes in the thymus of rats by chemical or surgical castration. Cell Tissue Res. 1990 Sep;261(3):555–564. doi: 10.1007/BF00313535. [DOI] [PubMed] [Google Scholar]
- Kendall M. D., Schuurman H. J., Fenton J., Broekhuizen R., Kampinga J. Implantation of cultured thymic fragments in congenitally athymic (nude) rats. Ultrastructural characteristics of the developing microenvironment. Cell Tissue Res. 1988 Nov;254(2):283–294. doi: 10.1007/BF00225801. [DOI] [PubMed] [Google Scholar]
- Kendall M. D. The morphology of perivascular spaces in the thymus. Thymus. 1989;13(3-4):157–164. [PubMed] [Google Scholar]
- Kendall M. D., al-Shawaf A., Zaidi S. A. The cholinergic and adrenergic innervation of the rat thymus. Adv Exp Med Biol. 1988;237:255–261. doi: 10.1007/978-1-4684-5535-9_39. [DOI] [PubMed] [Google Scholar]
- Khosla S., Ovalle W. K. Morphology and distribution of cystic cavities in the normal murine thymus. Cell Tissue Res. 1986;246(3):531–542. doi: 10.1007/BF00215193. [DOI] [PubMed] [Google Scholar]
- Knutson F., Lundin P. M. The effect of hypophysectomy, adrenalectomy and cortisone on the incorporation of tritiated thymidine in rat organs, with special references to the lymphoid tissues. Acta Endocrinol (Copenh) 1966 Nov;53(3):519–528. doi: 10.1530/acta.0.0530519. [DOI] [PubMed] [Google Scholar]
- Kyewski B. A., Fathman C. G., Rouse R. V. Intrathymic presentation of circulating non-MHC antigens by medullary dendritic cells. An antigen-dependent microenvironment for T cell differentiation. J Exp Med. 1986 Feb 1;163(2):231–246. doi: 10.1084/jem.163.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. Y., Freestone J. A., Goldstein G. Effect of thymopoietin pentapeptide (TP5) on autoimmunity. I. TP5 suppression of induced erythrocyte autoantibodies in C3H mice. J Immunol. 1980 Oct;125(4):1634–1638. [PubMed] [Google Scholar]
- Low T. L., Hu S. K., Goldstein A. L. Complete amino acid sequence of bovine thymosin beta 4: a thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1162–1166. doi: 10.1073/pnas.78.2.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low T. L., Thurman G. B., McAdoo M., McClure J., Rossio J. L., Naylor P. H., Goldstein A. L. The chemistry and biology of thymosin. I. Isolation, characterization, and biological activities of thymosin alpha1 and polypeptide beta1 from calf thymus. J Biol Chem. 1979 Feb 10;254(3):981–986. [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Fahrenkrug J., Hökfelt T., Mutt V. Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1651–1655. doi: 10.1073/pnas.77.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL A. H., WHITE R. G. The immunological reactivity of the thymus. Br J Exp Pathol. 1961 Aug;42:379–385. [PMC free article] [PubMed] [Google Scholar]
- Magni F., Bruschi F., Kasti M. The afferent innervation of the thymus gland in the rat. Brain Res. 1987 Oct 27;424(2):379–385. doi: 10.1016/0006-8993(87)91483-1. [DOI] [PubMed] [Google Scholar]
- McGillis J. P., Hall N. R., Goldstein A. L. Thymosin fraction 5 (TF5) stimulates secretion of adrenocorticotropic hormone (ACTH) from cultured rat pituitaries. Life Sci. 1988;42(22):2259–2268. doi: 10.1016/0024-3205(88)90378-5. [DOI] [PubMed] [Google Scholar]
- McGillis J. P., Hall N. R., Vahouny G. V., Goldstein A. L. Thymosin fraction 5 causes increased serum corticosterone in rodents in vivo. J Immunol. 1985 Jun;134(6):3952–3955. [PubMed] [Google Scholar]
- Nakamura H., Ayer-Le Lièvre C. Neural crest and thymic myoid cells. Curr Top Dev Biol. 1986;20:111–115. doi: 10.1016/s0070-2153(08)60658-4. [DOI] [PubMed] [Google Scholar]
- Nance D. M., Hopkins D. A., Bieger D. Re-investigation of the innervation of the thymus gland in mice and rats. Brain Behav Immun. 1987 Jun;1(2):134–147. doi: 10.1016/0889-1591(87)90016-x. [DOI] [PubMed] [Google Scholar]
- Naylor P. H., Naylor C. W., Badamchian M., Wada S., Goldstein A. L., Wang S. S., Sun D. K., Thornton A. H., Sarin P. S. Human immunodeficiency virus contains an epitope immunoreactive with thymosin alpha 1 and the 30-amino acid synthetic p17 group-specific antigen peptide HGP-30. Proc Natl Acad Sci U S A. 1987 May;84(9):2951–2955. doi: 10.1073/pnas.84.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaszynski E. W., Moody T. W., O'Donohue T. L., Goldstein A. L. Thymosin alpha 1-like peptides: localization and biochemical characterization in the rat brain and pituitary gland. Peptides. 1983 Jul-Aug;4(4):463–467. doi: 10.1016/0196-9781(83)90050-5. [DOI] [PubMed] [Google Scholar]
- Pelletier M., Montplaisir S., Dardenne M., Bach J. F. Thymic hormone activity and spontaneous autoimmunity in dwarf mice and their littermates. Immunology. 1976 Jun;30(6):783–788. [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W., Baroni C., Fabris N., Sorkin E. Hormones and immunological capacity. II. Reconstitution of antibody production in hormonally deficient mice by somatotropic hormone, thyrotropic hormone and thyroxin. Immunology. 1969 Feb;16(2):217–230. [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W., Bianchi E., Sorkin E. Hormones and the immunological capacity. V. Modification of growth hormone-producing cells in the adenohypophysis of neonatally thymectomized germ-free mice: an electron microscopical study. Clin Exp Immunol. 1971 Dec;9(6):889–901. [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W., Sorkin E. Hormones and immunologic capacity. I. Effect of heterologous anti-growth hormone (ASTH) antiserum on thymus and peripheral lymphatic tissue in mice. Induction of a wasting syndrome. J Immunol. 1968 Nov;101(5):1036–1043. [PubMed] [Google Scholar]
- Plum J., De Smedt M. Differentiation of thymocytes in fetal organ culture: lack of evidence for the functional role of the interleukin 2 receptor expressed by prothymocytes. Eur J Immunol. 1988 May;18(5):795–799. doi: 10.1002/eji.1830180521. [DOI] [PubMed] [Google Scholar]
- Poo W. J., Conrad L., Janeway C. A., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988 Mar 24;332(6162):378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- Raimond F., Morel E., Bach J. F. Evidence for the presence of immunoreactive acetylcholine receptors on human thymus cells. J Neuroimmunol. 1984 Feb;6(1):31–40. doi: 10.1016/0165-5728(84)90040-7. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Goldstein G., Boyse E. A., Schield M. P. T cell development in normal and thymopentin-treated nude mice. J Exp Med. 1982 Oct 1;156(4):1057–1064. doi: 10.1084/jem.156.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E., Karnovsky M. J. Evidence for a blood-thymus barrier using electron-opaque tracers. J Exp Med. 1972 Sep 1;136(3):466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebar R. W., Miyake A., Low T. L., Goldstein A. L. Thymosin stimulates secretion of luteinizing hormone-releasing factor. Science. 1981 Nov 6;214(4521):669–671. doi: 10.1126/science.7027442. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B., Lehuen A., Papiernik M. IL-2-dependent proliferation of thymic accessory cells. J Immunol. 1988 Feb 15;140(4):1076–1080. [PubMed] [Google Scholar]
- Roszman T. L., Jackson J. C., Cross R. J., Titus M. J., Markesbery W. R., Brooks W. H. Neuroanatomic and neurotransmitter influences on immune function. J Immunol. 1985 Aug;135(2 Suppl):769s–772s. [PubMed] [Google Scholar]
- Rouse R. V., Ezine S., Weissman I. L. Expression of major histocompatibility complex antigens in the thymuses of chimeric mice. Transplantation. 1985 Oct;40(4):422–426. doi: 10.1097/00007890-198510000-00015. [DOI] [PubMed] [Google Scholar]
- Rubinstein A., Novick B. E., Sicklick M. J., Bernstein L. J., Incefy G. S., Naylor P. H., Goldstein A. L. Circulating thymulin and thymosin-alpha 1 activity in pediatric acquired immune deficiency syndrome: in vivo and in vitro studies. J Pediatr. 1986 Sep;109(3):422–427. doi: 10.1016/s0022-3476(86)80111-1. [DOI] [PubMed] [Google Scholar]
- SAINTE-MARIE G. ANTIGEN PENETRATION INTO THE THYMUS. J Immunol. 1963 Dec;91:840–845. [PubMed] [Google Scholar]
- SHREWSBURY M. M., REINHARDT W. O. Effect of pituitary growth hormone on lymphatic tissues, thoracic duct lymph flow, lymph protein and lymphocyte output in the rat. Endocrinology. 1959 Nov;65:858–860. doi: 10.1210/endo-65-5-858. [DOI] [PubMed] [Google Scholar]
- Safieh B., Kendall M. D., Norman J. C., Metreau E., Dardenne M., Bach J. F., Pleau J. M. A new radioimmunoassay for the thymic peptide thymulin, and its application for measuring thymulin in blood samples. J Immunol Methods. 1990 Mar 9;127(2):255–262. doi: 10.1016/0022-1759(90)90076-8. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Benjamin R. J., Wesley P. K., Buxton S. E., Garrett T. P., Clayberger C., Krensky A. M., Norment A. M., Littman D. R., Parham P. A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature. 1990 May 3;345(6270):41–46. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- Savino W., Gagnerault M. C., Bach J. F., Dardenne M. Neuroendocrine control of thymic hormonal production. II. Stimulatory effects of endogenous opioids on thymulin production by cultured human and murine thymic epithelial cells. Life Sci. 1990;46(23):1687–1697. doi: 10.1016/0024-3205(90)90384-4. [DOI] [PubMed] [Google Scholar]
- Savino W., Wolf B., Aratan-Spire S., Dardenne M. Thymic hormone containing cells. IV. Fluctuations in the thyroid hormone levels in vivo can modulate the secretion of thymulin by the epithelial cells of young mouse thymus. Clin Exp Immunol. 1984 Mar;55(3):629–635. [PMC free article] [PubMed] [Google Scholar]
- Scheiff J. M., Cordier A. C., Haumont S. Epithelial cell proliferation in thymic hyperplasia induced by triiodothyronine. Clin Exp Immunol. 1977 Mar;27(3):516–521. [PMC free article] [PubMed] [Google Scholar]
- Schuurman H. J., Vos J. G., Broekhuizen R., Brandt C. J., Kater L. In vivo biological effect of allogeneic cultured thymic epithelium on thymus-dependent immunity in athymic nude rats. Scand J Immunol. 1985 Jan;21(1):21–30. doi: 10.1111/j.1365-3083.1985.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Sideras P., Funa K., Zalcberg-Quintana I., Xanthopoulos K. G., Kisielow P., Palacios R. Analysis by in situ hybridization of cells expressing mRNA for interleukin 4 in the developing thymus and in peripheral lymphocytes from mice. Proc Natl Acad Sci U S A. 1988 Jan;85(1):218–221. doi: 10.1073/pnas.85.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U. Effect of catecholamines on lymphopoiesis in fetal mouse thymic explants. J Anat. 1979 Sep;129(Pt 2):279–292. [PMC free article] [PubMed] [Google Scholar]
- Singh U., Owen J. J. Studies on the maturation of thymus stem cells. The effects of catecholamines, histamine and peptide hormones on the expression of T cell alloantigens. Eur J Immunol. 1976 Jan;6(1):59–62. doi: 10.1002/eji.1830060113. [DOI] [PubMed] [Google Scholar]
- Steinmann G. G. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Imamura S., Ofuji S. Demonstration of epidermis-specific heteroantigens in thymic epithelial cells. Int Arch Allergy Appl Immunol. 1977;55(1-6):58–60. doi: 10.1159/000231909. [DOI] [PubMed] [Google Scholar]
- Timsit J., Safieh B., Gagnerault M. C., Savino W., Lubetzki J., Bach J. F., Dardenne M. Augmentation des taux circulants de thymuline au cours de l'hyperprolactinémie et de l'acromégalie. C R Acad Sci III. 1990;310(1):7–13. [PubMed] [Google Scholar]
- Tollefson L., Bulloch K. Dual-label retrograde transport: CNS innervation of the mouse thymus distinct from other mediastinum viscera. J Neurosci Res. 1990 Jan;25(1):20–28. doi: 10.1002/jnr.490250104. [DOI] [PubMed] [Google Scholar]
- Trainin N., Rotter V., Yakir Y., Leve R., Handzel Z., Shohat B., Zaizov R. Biochemical and biological properties of THF in animal and human models. Ann N Y Acad Sci. 1979;332:9–22. doi: 10.1111/j.1749-6632.1979.tb47093.x. [DOI] [PubMed] [Google Scholar]
- Trainin N., Small M. Studies on some physicochemical properties of a thymus humoral factor conferring immunocompetence on lymphoid cells. J Exp Med. 1970 Nov;132(5):885–897. doi: 10.1084/jem.132.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainin N. Therapeutic prospects of THF--a thymic hormone--in autoimmune processes. Isr J Med Sci. 1988 Dec;24(12):739–740. [PubMed] [Google Scholar]
- Trainin N. Thymic hormones and the immune response. Physiol Rev. 1974 Apr;54(2):272–315. doi: 10.1152/physrev.1974.54.2.272. [DOI] [PubMed] [Google Scholar]
- Travers P. Immunology. One hand clapping. Nature. 1990 Nov 29;348(6300):393–394. doi: 10.1038/348393a0. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian K., Audhya T., Goldstein G. Binding of thymopoietin to the acetylcholine receptor. Proc Natl Acad Sci U S A. 1986 May;83(10):3171–3174. doi: 10.1073/pnas.83.10.3171. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Viamontes G. I., Audhya T., Goldstein G. Immunohistochemical localization of thymopoietin with an antiserum to synthetic Cys-thymopoietin28-39. Cell Immunol. 1986 Jul;100(2):305–313. doi: 10.1016/0008-8749(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Vécsei L., Faludi M., Najbauer J. The effect of "facteur thymique serique" (FTS) on catecholamine and serotonin neurotransmission in discrete brain regions of mice. Acta Physiol Hung. 1987;69(1):129–132. [PubMed] [Google Scholar]
- Wang J. H., Yan Y. W., Garrett T. P., Liu J. H., Rodgers D. W., Garlick R. L., Tarr G. E., Husain Y., Reinherz E. L., Harrison S. C. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990 Nov 29;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- Weihe E., Müller S., Fink T., Zentel H. J. Tachykinins, calcitonin gene-related peptide and neuropeptide Y in nerves of the mammalian thymus: interactions with mast cells in autonomic and sensory neuroimmunomodulation? Neurosci Lett. 1989 May 22;100(1-3):77–82. doi: 10.1016/0304-3940(89)90663-0. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Gillan D. J. The possible mediation by cyclic AMP of the stimulation of thymocyte proliferation by vasopressin and the inhibition of this mitogenic action by thyrocalcitonin. J Cell Physiol. 1970 Aug;76(1):65–76. doi: 10.1002/jcp.1040760110. [DOI] [PubMed] [Google Scholar]
- Willcox N., Schluep M., Ritter M. A., Schuurman H. J., Newsom-Davis J., Christensson B. Myasthenic and nonmyasthenic thymoma. An expansion of a minor cortical epithelial cell subset? Am J Pathol. 1987 Jun;127(3):447–460. [PMC free article] [PubMed] [Google Scholar]
- Willoughby J. O., Day T. A. Central catecholamine depletion: effects on physiological growth hormone and prolactin secretion. Neuroendocrinology. 1981 Feb;32(2):65–69. doi: 10.1159/000123132. [DOI] [PubMed] [Google Scholar]
- Wu L., Scollay R., Egerton M., Pearse M., Spangrude G. J., Shortman K. CD4 expressed on earliest T-lineage precursor cells in the adult murine thymus. Nature. 1991 Jan 3;349(6304):71–74. doi: 10.1038/349071a0. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Ransom J., Frank G., Fischer M., Howard M. Interleukin 4 is a growth factor for activated thymocytes: possible role in T-cell ontogeny. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3856–3860. doi: 10.1073/pnas.84.11.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Shawaf A. A., Kendall M. D., Cowen T. Identification of neural profiles containing vasoactive intestinal polypeptide, acetylcholinesterase and catecholamines in the rat thymus. J Anat. 1991 Feb;174:131–143. [PMC free article] [PubMed] [Google Scholar]
- de Maagd R. A., MacKenzie W. A., Schuurman H. J., Ritter M. A., Price K. M., Broekhuizen R., Kater L. The human thymus microenvironment: heterogeneity detected by monoclonal anti-epithelial cell antibodies. Immunology. 1985 Apr;54(4):745–754. [PMC free article] [PubMed] [Google Scholar]
- von Gaudecker B., Larché M., Schuurman H. J., Ritter M. A. Analysis of the fine distribution of thymic epithelial microenvironmental molecules by immuno-electron microscopy. Thymus. 1989;13(3-4):187–194. [PubMed] [Google Scholar]
- von Gaudecker B., Müller-Hermelink H. K. Ontogeny and organization of the stationary non-lymphoid cells in the human thymus. Cell Tissue Res. 1980;207(2):287–306. doi: 10.1007/BF00237813. [DOI] [PubMed] [Google Scholar]
- von Gaudecker B., Steinmann G. G., Hansmann M. L., Harpprecht J., Milicevic N. M., Müller-Hermelink H. K. Immunohistochemical characterization of the thymic microenvironment. A light-microscopic and ultrastructural immunocytochemical study. Cell Tissue Res. 1986;244(2):403–412. doi: 10.1007/BF00219216. [DOI] [PubMed] [Google Scholar]
- von Gaudecker B. The development of the human thymus microenvironment. Curr Top Pathol. 1986;75:1–41. doi: 10.1007/978-3-642-82480-7_1. [DOI] [PubMed] [Google Scholar]