Abstract
The expression of major histocompatibility complex (MHC) antigen and leukocyte common antigen (LCA) was observed in the amoeboid microglial cells in postnatal rat brain. Considerable MHC class I surface antigen was detected at the plasma membrane and its tubular invaginations in the amoeboid microglia in the corpus callosum using the monoclonal antibody OX-18. In early postnatal (2 and 5 day) rats, the OX-18 positive cells were mostly round but a few possessed stout processes. With increasing age (9 and 15 days) the OX-18 positive amoeboid microglia assumed an oval or elongated form. By the time of weaning (21 days) and in older animals, the immunoreactivity was extremely weak and was detectable only on some branched microglia bearing fine processes. The presence of MHC class Ia antigens with OX-3 and OX-6 was hardly detectable except for a few weakly stained cells in the corpus callosum and cavum septum pellucidum in early postnatal rats. The expression of LCA was observed in amoeboid microglial using the monoclonal antibody OX-1 and this followed a similar temporal pattern to that with OX-18. The additional phenotypic features of amoeboid microglial cells in the present study support their monocytic origin. These cells are endowed with MHC class I antigens which may serve as the restriction elements for T lymphocytes, at least in the early postnatal brain.
Full text
PDF


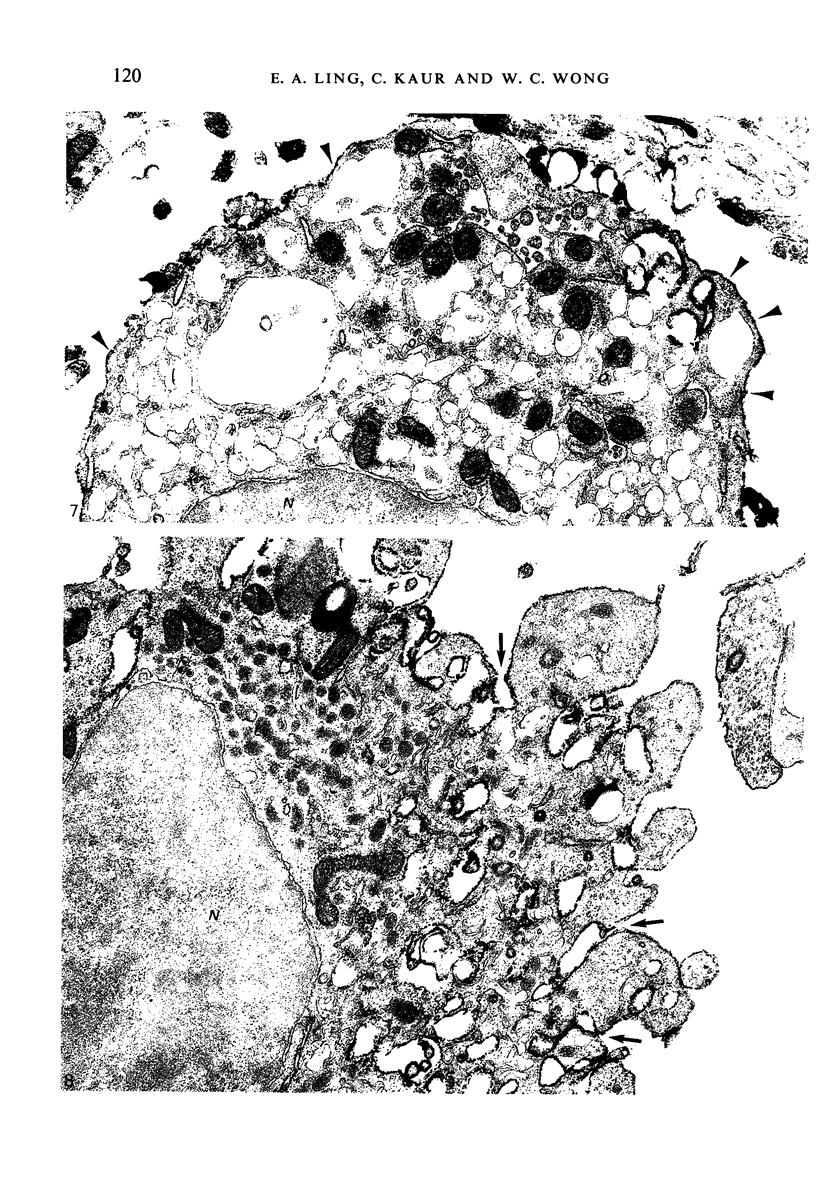

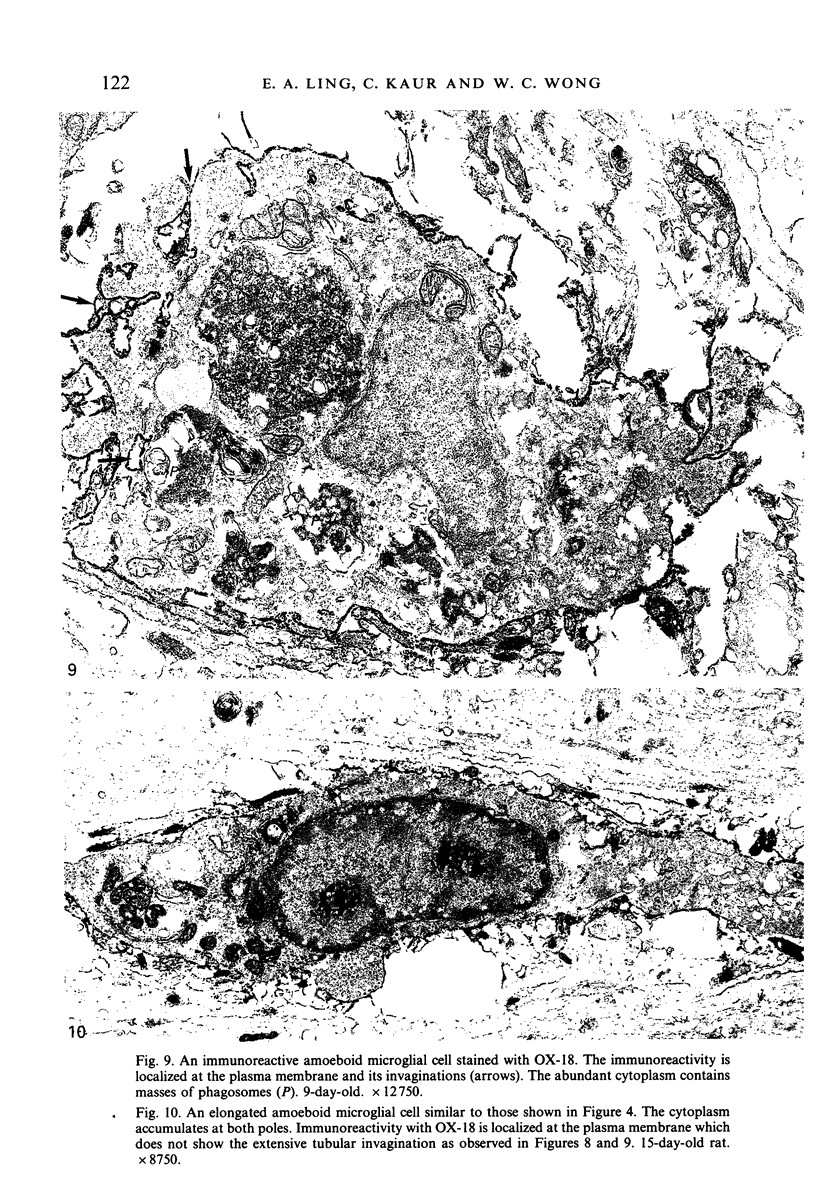



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama H., Itagaki S., McGeer P. L. Major histocompatibility complex antigen expression on rat microglia following epidural kainic acid lesions. J Neurosci Res. 1988;20(2):147–157. doi: 10.1002/jnr.490200202. [DOI] [PubMed] [Google Scholar]
- Akiyama H., McGeer P. L. Microglial response to 6-hydroxydopamine-induced substantia nigra lesions. Brain Res. 1989 Jun 12;489(2):247–253. doi: 10.1016/0006-8993(89)90857-3. [DOI] [PubMed] [Google Scholar]
- Hayes G. M., Woodroofe M. N., Cuzner M. L. Microglia are the major cell type expressing MHC class II in human white matter. J Neurol Sci. 1987 Aug;80(1):25–37. doi: 10.1016/0022-510x(87)90218-8. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Graft-vs.-host disease elicits expression of class I and class II histocompatibility antigens and the presence of scattered T lymphocytes in rat central nervous system. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2082–2086. doi: 10.1073/pnas.84.7.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M., Clarke S., Koppel H. Transitory macrophages in the white matter of the developing visual cortex. II. Development and relations with axonal pathways. Brain Res. 1983 Dec;313(1):55–66. doi: 10.1016/0165-3806(83)90201-8. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A. Weak HLA and beta 2-microglobulin expression of neuronal cell lines can be modulated by interferon. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6476–6480. doi: 10.1073/pnas.81.20.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Hickey W. F. Monoclonal antibody analysis of MHC expression in human brain biopsies: tissue ranging from "histologically normal" to that showing different levels of glial tumor involvement. J Immunol. 1986 Jun 1;136(11):4054–4062. [PubMed] [Google Scholar]
- Ling E. A., Kaur L. C., Yick T. Y., Wong W. C. Immunocytochemical localization of CR3 complement receptors with OX-42 in amoeboid microglia in postnatal rats. Anat Embryol (Berl) 1990;182(5):481–486. doi: 10.1007/BF00178913. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Penney D., Leblond C. P. Use of carbon labeling to demonstrate the role of blood monocytes as precursors of the 'ameboid cells' present in the corpus callosum of postnatal rats. J Comp Neurol. 1980 Oct 1;193(3):631–657. doi: 10.1002/cne.901930304. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Some aspects of amoeboid microglia in the corpus callosum and neighbouring regions of neonatal rats. J Anat. 1976 Feb;121(Pt 1):29–45. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Fujiwara M. Absence of donor-type major histocompatibility complex class I antigen-bearing microglia in the rat central nervous system of radiation bone marrow chimeras. J Neuroimmunol. 1987 Dec;17(1):71–82. doi: 10.1016/0165-5728(87)90032-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Fujiwara M. In situ detection of class I and II major histocompatibility complex antigens in the rat central nervous system during experimental allergic encephalomyelitis. An immunohistochemical study. J Neuroimmunol. 1986 Oct;12(4):265–277. doi: 10.1016/0165-5728(86)90033-0. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Itagaki S., Tago H., McGeer E. G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987 Aug 18;79(1-2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Poltorak M., Freed W. J. Immunological reactions induced by intracerebral transplantation: evidence that host microglia but not astroglia are the antigen-presenting cells. Exp Neurol. 1989 Mar;103(3):222–233. doi: 10.1016/0014-4886(89)90046-0. [DOI] [PubMed] [Google Scholar]
- Steiniger B., van der Meide P. H. Rat ependyma and microglia cells express class II MHC antigens after intravenous infusion of recombinant gamma interferon. J Neuroimmunol. 1988 Aug;19(1-2):111–118. doi: 10.1016/0165-5728(88)90040-9. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Expression of Ia antigen on perivascular and microglial cells after sublethal and lethal motor neuron injury. Exp Neurol. 1989 Aug;105(2):115–126. doi: 10.1016/0014-4886(89)90111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Functional plasticity of microglia: a review. Glia. 1988;1(5):301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Suzumura A., Mezitis S. G., Gonatas N. K., Silberberg D. H. MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: induction of Ia antigen expression by gamma-interferon. J Neuroimmunol. 1987 Jul-Aug;15(3):263–278. doi: 10.1016/0165-5728(87)90121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C. Y., Ling E. A., Wong W. C. Scanning electron microscopy of amoeboid microglial cells in the transient cavum septum pellucidum in pre- and postnatal rats. J Anat. 1983 Mar;136(Pt 2):251–263. [PMC free article] [PubMed] [Google Scholar]
- Weinstein D. L., Walker D. G., Akiyama H., McGeer P. L. Herpes simplex virus type I infection of the CNS induces major histocompatibility complex antigen expression on rat microglia. J Neurosci Res. 1990 May;26(1):55–65. doi: 10.1002/jnr.490260107. [DOI] [PubMed] [Google Scholar]
- Woodroofe M. N., Bellamy A. S., Feldmann M., Davison A. N., Cuzner M. L. Immunocytochemical characterisation of the immune reaction in the central nervous system in multiple sclerosis. Possible role for microglia in lesion growth. J Neurol Sci. 1986 Jul;74(2-3):135–152. doi: 10.1016/0022-510x(86)90100-0. [DOI] [PubMed] [Google Scholar]