Abstract
Background
Studies investigating the costs associated with invasive meningococcal disease (IMD) are scarce, primarily due to the disease rarity, its highly variable prognosis, and the potential for sequelae to develop long after the acute phase. We conducted a retrospective longitudinal cohort study to estimate the healthcare costs associated with IMD in France in the short (one month), medium (two years), and long (up to 12 years) term.
Methods
Using the National Health Data System (SNDS), we extracted data for all individuals hospitalised with a diagnosis of IMD (IMD+) between 1 January 2008 and 31 December 2018. Each IMD + individual was matched to up to four individuals without IMD. Among IMD + individuals discharged alive (exposed individuals), a K-modes clustering method using 64 healthcare resource utilisation (HCRU) variables to identify those with high HCRU levels (EIC+, for Exposed Individuals with Care), who served as a proxy for individuals with IMD sequelae. Additional costs associated with exposure were estimated using generalized estimating equations (GEEs). The cost analysis was conducted from the perspective of the National Health Insurance System.
Results
Of the 5,770 IMD + individuals (52.6% male; 29.7% aged < 5 years; 27.4% with comorbidities), 4,502 were exposed individuals (52.2% male; 30.9% aged < 5 years; 26.4% with comorbidities), of whom 1,032 were EIC+ (40.4% male; 7.9% aged < 5 years; 30.0% aged ≥ 65 years; 62.8% with comorbidities). The mean per capita costs of the index hospitalisation were €10,599 (SD: €16,931). These costs were €2,400 (SD: €10,565) in the short term (excluding the index hospitalisation), €9,304 (SD: €51,785) in the medium term, and €37,718 (SD: €114,143) in the long term. They were, respectively, 19.2 (aOR; 95%CI: 17.9–20.5), 1.3 (1.2–1.4), and 2.2 (2.0-2.5) times higher than those of the matched unexposed individuals. When the index hospitalisation was included, short-term costs were 86.3 (aOR; 95%CI: 81.2–91.7) times higher.
Conclusions
The healthcare costs associated with IMD extend well beyond hospital discharge. The long-term management of sequelae significantly increases the economic burden of the disease, emphasising the importance of effective preventive strategies for IMD. The clustering method used in this study could facilitate the identification of IMD sequelae in real-world data.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-11954-8.
Keywords: Cluster analysis; Cost of illness; France; Meningitis, meningococcal; Sequelae
Background
Invasive meningococcal disease (IMD) is a rare but life-threatening infection caused by Neisseria meningitidis that invades the bloodstream and/or central nervous system. It is characterised by a fatality rate ranging from 10% to 40% [1]. In France, approximately 500 to 600 incident cases across all age groups are reported annually [2], with a case fatality rate of 12.9% accounting for deaths occurring during and after hospitalisation (median follow-up of 2.8 years) [3]. IMD is also associated with lifelong complications: up to half of survivors develop sequelae, and one in five experience permanent sequelae such as hearing loss, amputation, or physical or speech disabilities [4–6]. In a systematic and clinical review of the literature analysing data from 66 observational studies and 34 economic studies, 44 sequelae (30 physical/neurological and 14 psychosocial/behavioural) were identified and up to 40% of IMD survivors experienced both short- and long-term complications [7]. Due to its poor outcomes, IMD is a serious public health concern worldwide.
The economic burden of IMD is poorly characterised [8]. This is partly because the disease is rare, and its prognosis varies a lot from patient to patient. This makes it difficult to get enough patients together to do accurate cost-of-illness studies [5]. It is also difficult to link certain outcomes, such as cognitive impairment or school failure, to IMD, partly because these outcomes become apparent only several years after the disease [9]. Finally, the cost of managing IMD is likely to evolve over time.
Country-specific large healthcare databases facilitate the collection of a huge amount of real-world data over time. Several studies using these large databases have been conducted to assess the burden of IMD [3, 4, 10, 11]. In France, two databases have been used for this purpose: the Medicalisation of Information Systems Programme (Programme de Médicalisation des Systèmes d’Information, PMSI) whose goal is to aggregate and standardise medical data collected during hospital stays [6] and the National Health Data System (Système National des Données de Santé, SNDS) which includes the PMSI [3, 5]. In a study using the SNDS database, Weil-Olivier et al. [3] reported that the economic burden of IMD was mainly driven by the management of sequelae, and their evaluation of the economic burden was restricted to a 5-year period (2012 to 2017). Furthermore, IMD sequelae were identified using hospital discharge summaries or data collected within three months post-hospital discharge, except for a subset of sequelae (e.g., epilepsy, hearing loss, and attention-deficit hyperactivity disorder) where the time windows were determined based on expert opinion.
Given the limited studies on the topic and their inherent limitations, we conducted SEQIIM (formed from the French words SÉQuelles and IIM), a cohort study using the SNDS database. Our study aimed to provide a more accurate estimation of healthcare costs associated with IMD. We included all individuals hospitalised with a diagnosis of IMD over a 11-year period starting from 1 January 2008. To address the challenge of identifying among these individuals those with IMD sequelae, we implemented an innovative approach based on healthcare resource utilisation (HCRU) during the first two years following hospital discharge. To the best of our knowledge, no previous cost-of-illness study in the scientific literature has been performed using a similar method.
Methods
Data sources
Our study is a retrospective longitudinal cohort study conducted using the SNDS database (https://www.snds.gouv.fr/SNDS/Accueil). The SNDS serves as a comprehensive database linking the National Inter-Regimen Health Insurance Information System (Système National d’Information Inter-Régimes de l’Assurance Maladie, SNIIRAM) with the PMSI. The SNDS database integrates and makes available information on both in-hospital (public and private sectors) and out-of-hospital healthcare use of the French population (i.e., > 66 million people) covered by the various compulsory health insurance schemes. Additionally, it integrates data from the Centre for Epidemiology on Medical Causes of Death (Centre d’épidémiologie sur les causes médicales de décès, CépiDC). Individuals are identified by a unique identifier enabling linkage across consistent data sets within the database. Demographic information, such as date of birth, sex, and place of residence, is also available [12].
Study populations
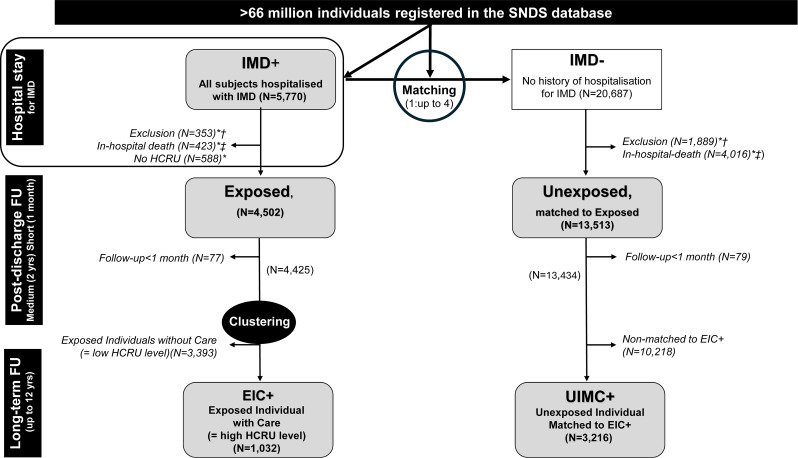
All individuals admitted to a French medical hospital department with a diagnosis of IMD between 1 January 2008 and 31 December 2018 were identified in the SNDS database (Fig. 1). The diagnosis of IMD was based on specific diagnostic codes (A39.0-A39.4 and A39.9) of the International Classification of Diseases version 10 (ICD-10). These individuals were referred to as IMD + individuals thereafter. Each IMD + individual was randomly matched to up to four individuals from the SNDS database with no history of IMD hospitalisation regardless of whether they had been hospitalised for other reasons (IMD-).
Fig. 1.
Study flowchart. EIC+: Exposed Individuals with Care; HCRU: HealthCare Resource Utilisation; IMD: Invasive Meningococcal Disease; UIMC+: Unexposed Individuals Matched to EIC+. * Non-exclusive; † with one exclusion criterion; ‡ on index date. Exclusion criteria: affiliation with a settlement organisation in Mayotte or insufficient identification of the individuals in the SNDS database. IMD+: admitted to hospital for IMD between 1 January 2008, and 31 December 2018; IMD-: not admitted to hospital for IMD between 1 January 2008, and 31 December 2018; Exposed: IMD + discharged alive from the hospital; Unexposed: matched on age, sex, socioeconomic status, and postcode of the municipality to exposed individuals; EIC+: individuals with a high HCRU level as determined by the cluster analysis (see Additional material); UIMC+: among unexposed individuals those matched to EIC+. Short term (1 month): from the index date to 30 days later; Medium term (2 years): during the 23 months after the end of the short-term follow-up; Long term (up to 12 years): from 2 years after the index date until end of study or death. Index date: date of the hospital discharge for exposed individuals. This date served as the index date for matched unexposed individuals
Among IMD + individuals, those who were discharged alive from the hospital and had available HCRU data were referred to as exposed individuals. In the Exposed group, individuals with a high HCRU level according to a clustering method were referred to as EIC+ (Exposed Individuals with Care). Among IMD − individuals, those matched to exposed individuals were referred as unexposed individuals, and those matched to EIC + were referred as UIMC+ (Unexposed Individuals Matched to Exposed Individuals with Care). Age, gender, date of hospitalisation (exposed) or nearest date of care (unexposed), socioeconomic status based on CSS (French healthcare program providing financial aid for medical expenses), and postcode of residence were used as matching variables.
Data collection
Demographic and HCRU data were extracted from the SNDS database on an anonymous and individual basis. The index date was defined as the date of the hospital discharge for exposed individuals, which also served as the index date for matched unexposed individuals. The short-term follow-up began on the index date and lasted 30 days (1 month). The medium-term follow-up started immediately afterward and continued for 23 months (2 years). The long-term follow-up began after the medium-term follow-up and continued until 31 December 2019, or until censored by death, whichever occurred first.
Healthcare costs were assessed at three consecutive time points: during the hospital stay for IMD + individuals; in the short and the medium terms for exposed and unexposed individuals; in the long term for EIC + and UIMC+ (Fig. 1). For both exposed and unexposed individuals, data were collected from the year before the index date to the end of the long-term follow-up period which could reach a maximum of 12 years.
Endpoints
The primary endpoints were (1) additional costs in the short and medium terms for exposed individuals compared to unexposed individuals; and (2) additional costs in the long term in EIC + compared to UIMC+. Secondary endpoints included IMD hospital costs incurred at the time of exposure (assessed only in IMD + individuals, by definition).
Clustering
A K-modes clustering analysis for categorical data was performed to classify exposed individuals into two mutually exclusive groups. The clustering analysis incorporated age (9 categories: <1, 1–5, 5–10, 10–15, 15–20, 20–25, 25–45, 45–65, or ≥ 65 years), gender (male or female), universal healthcare coverage (yes or no); and 64 derived variables assessing HCRU (for each resource, its utilisation or non-utilisation within the two years following the index date). Exposed individuals with high HCRU level (i.e., Exposed Individuals with Care, EIC+) served as proxy for IMD-exposed individuals with sequelae. Based on literature and authors’ advice, it was hypothesised about 30% of exposed individuals would belong to the EIC + group.
The cluster analysis was validated in several ways. Firstly, we relied on graphical within-cluster dissimilarity (cost function) to guide the selection of the number of clusters. Secondly, we performed a two-level cross-analyse to assess the consistency, stability, and interpretability of the resulting groups. We verified that most of individuals with at least one of 16 well known and easily identified sequelae through ICD-10 codes were included in the EIC + group, indicating that the EIC + group included most patients with easily identified sequelae. Then, a sensitivity analysis compared the global partitioning derived from the clustering analysis to that of clustering analyses performed on four subpopulations of exposed individuals, being hypothesised that most individuals with high HCRU level identified in each of the four subpopulations were also identified in the EIC + group. The four populations were: (1) individuals aged 15–25 years; (2) individuals without comorbidities; (3) females; (4) females without comorbidities. This analysis showed the robustness and the stability of the clustering analysis. Further details are provided in Additional Material.
Analysis
All statistical analyses were performed using SAS Guide software, Version 9.4 (SAS Institute, Cary, North Carolina, USA). Continuous variables are presented as mean values with their standard deviation (SD). Categorical variables are presented as frequency counts and percentages. Missing data were not replaced. The significance threshold was set at 0.05.
Inpatient and outpatient costs were examined for the index hospitalisation period in IMD + individuals only. The additional costs were evaluated in the exposed and unexposed individuals for the short and medium terms and in the EIC + and UIMC + for the long term. The cost analysis perspective was aligned with the French Healthcare Insurance System. The average additional costs due to health resources used by exposed individuals compared to unexposed individuals and EIC + compared to UIMC + were modelled using generalized estimating equations (GEEs) with a gamma distribution and log link function [13]. An unstructured working correlation matrix was specified to account for within-subject correlation over time. The dependent variable was the total cost of follow-up per year. The following independent variables were included as covariates: age, sex, healthcare costs (reimbursed) within the year preceding exposure, and comorbidities of interest within the year preceding exposure and at exposure (please refer to Additional Material). While the outcome variable is a count, and the rate is expressed as count per unit of time (low, short, long-term), the logarithm of person-year was included as an offset in the model. This ensures that costs were not disproportionately influenced by differences in follow-up time between individuals.
Ethical considerations
Data on the SNDS database were pseudo-anonymised using irreversible double encryption. At the time of the study, access to the SNDS database was regulated by the Ethics and Scientific Committee for Research, Studies and Evaluations in the Health Sector (CESREES in French for Comité Éthique et Scientifique pour les Recherches, les Études et les Évaluations dans le domaine de la Santé). The use of the SNDS database was also regulated by the French National Commission on Informatics and Liberty (CNIL in French for Commission Nationale de l’Informatique et des Libertés). The study protocol was approved by the CESREES on 8 October 2020 (No. TPS2483887) and by the CNIL on 1 February 2021 (CNIL, MLD/VCS/AR212214, No. 920458). Informed consent was waived by the Commission Nationale de l’Informatique et des Libertés (CNIL in French) as this study was retrospective on a pseudo-anonymised database and did not influence care.
Results
Study population
We collected data from 5,770 individuals hospitalised with a diagnosis of IMD between 1 January 2008 and 31 December 2018. These patients, referred to as IMD + individuals, were mainly males (52.6%). Approximately 30% were younger than 5 years old, and 20% were between 15 and 25 years old. Less than 30% reported at least one comorbidity. Of these 5,770 IMD + individuals, 7.3% died during the hospital stay, 13.5% during the study period.
After applying the exclusion criteria and removing individuals who were not registered in the SNDS database or for whom there was no linkage between hospital and outpatient care data, a total of 4,502 IMD + individuals were identified as having been discharged alive from the hospital (exposed individuals). These individuals were matched to 13,513 individuals with no history of hospitalisation for IMD (unexposed individuals). Exposed individuals were followed-up for 5.1+/- 3.4 years (mean +/- SD) and unexposed individuals for 5.3+/-3.4 years. Among exposed individuals, 1,032 (22.9%) were EIC + as they presented with a high level of HCRU. These 1,032 EIC + were matched to 3,216 UIMC + (Fig. 1).
Exposed individuals (N = 4,502) had similar characteristics than IMD + individuals, while EIC + differed. EIC+ (N = 1,032) were mainly females (59.6%); less than 8% were under 5 years of age and 30.0% were 65 years or older; more than 50% had at least one comorbidity. The characteristics of exposed and unexposed individuals and EIC + and UIMC + were similar, except for comorbidities which were more frequently reported among exposed than unexposed individuals and EIC + than UIMC+ (Table 1).
Table 1.
Characteristics of individuals according to exposure to IMD and healthcare resource utilisation level
| IMD+ (N = 5,770) |
Exposed (N = 4,502) |
Unexposed (N = 13,513) |
EIC+ (N = 1,032) |
UIMC+ (N = 3,216) |
|
|---|---|---|---|---|---|
| Male (%) | 52.6% | 52.2% | 50.5% | 40.4% | 38.3% |
| Age (years) | |||||
| Mean (SD) | 26.0 (26.0) | 25.2 (25.2) | 25.9 (25.9) | 48.5 (25.8) | 50.0 (25.1) |
| Age Group | |||||
| < 5 year | 29.7% | 30.9% | 32.4% | 7.9% | 7.8% |
| 5–14 | 10.6% | 10.5% | 10.1% | 3.4% | 3.2% |
| 15–24 | 21.6.% | 20.9% | 17.3% | 12.7% | 9.8% |
| 25–64 | 26.4% | 27.6% | 29.0% | 45.9% | 47.6% |
| ≥ 65 years | 11.6% | 10.2% | 11.1% | 30.0% | 31.6% |
| CSS (%) | 19.6% | 17.6% | 17.2% | 10.6% | 10.0% |
| Comorbidity (%) | |||||
| At least one | 27.4% | 26.4% | 16.8% | 62.8% | 39.3% |
| ALD | 21.4% | 21.7% | 10.6% | 55.5% | 23.8% |
| Cardiovascular disease | 20.0% | 18.7% | 13.3% | 52.0% | 34.6% |
| Diabetes | 5.3% | 5.0% | 3.1% | 15.3% | 8.2% |
| Neoplastic pathology | 8.0% | 7.8% | 2.8% | 20.2% | 7.2% |
| Congenital malformation | 2.7% | 2.8% | 1.5% | 3.0% | 0.5% |
| Autoimmune disease | 3.0% | 3.1% | 1.0% | 7.4% | 2.2% |
ALD (affection de longue durée [French]): condition whose severity and/or chronic nature requires prolonged treatment and whose healthcare-related costs are reimbursed (e.g., cancer, autoimmune disease, HIV infection); CSS: French healthcare program providing financial aid for medical expenses; EIC+: Exposed Individual with Care; HCRU: HealthCare Resource Utilisation; IMD: Invasive Meningococcal Disease; SNDS: French National Healthcare Data System; UIMC+: Unexposed Individual Matched to Exposed Individual with Care
IMD+: admitted to hospital for IMD between 1 January 2008, and 31 December 2018; Exposed: IMD + discharged alive from the hospital; EIC+: individuals with a high HCRU level as determined by the cluster analysis (see Additional material); Unexposed: matched on age, sex, socioeconomic status, and postcode of the municipality to exposed individuals; UIMC+: among unexposed individuals those matched to EIC+
Missing data were not replaced. Percentages were calculated on the number of available data
The analysis supporting the partitioning of exposed individuals into EIC + found that individuals with well-known and easily identified sequelae through ICD10 codes (renal insufficiency, mood disorders, and severe neurological disorders) commonly belonged to the EIC + group. The sensitivity analysis demonstrated that, in each subpopulation, 90% or more of the exposed individuals identified as having a high HCRU level belonged to the EIC + group, supporting the robustness of the clustering method across the diverse subpopulations (for further details, please refer to the Additional Material).
In-hospital healthcare resource use and associated costs at the time of exposure
IMD + individuals (N = 5,770) were usually hospitalised for meningococcal meningitis (ICD-10 code A39.0, 66.6%) or acute meningococcaemia (ICD-10 code A39.2, 25.7%). The mean length of their hospital stay was 12 days (SD: 13 days). During the hospital stay, 44.6% were referred to an intensive care unit and 23.7% to a continuing care unit. From the French Health Insurance System perspective, the overall cost of hospitalisation was €60,486,842 (5,770 IMD + individuals, 2008–2018). The mean per capita costs of the hospital stay were €10,599 (SD: €16,931).
Short-term and medium-term healthcare resource use and additional costs after hospital discharge
Exposed individuals (N = 4,502) used more outpatient healthcare resources than unexposed individuals (N = 13,513). The difference was more marked in the short than in the medium term. In the short term, 47% of exposed individuals had consulted general practitioners (GPs), 19.1%, nurses, and 10.0%, physiotherapists vs. 31.2%, 4.1%, and 5.1% of unexposed individuals, respectively. Exposed individuals had 5 times more contact with nurses and 2 times more contact with physiotherapists than unexposed individuals. Biological tests were performed in 26.2% of exposed individuals vs. 7.3% of unexposed individuals, and drugs and medical devices were prescribed in 74.3% and 22.9% of exposed individuals vs. 46.4% and 7.5% of unexposed individuals. In the medium term, more than 90% of exposed and unexposed individuals had consulted at least one GP. 34.3% of exposed individuals had consulted a nurse and 28.4% a physiotherapist vs. 26.2% and 19.8% of unexposed individuals. Biological tests were performed in 59.9% of exposed individuals vs. 51.1% of unexposed individuals. Drugs were prescribed to almost all individuals, but 61.8% of exposed individuals vs. 56.9% of unexposed individuals had been prescribed at least one medical device. In the medium term, 20.3% of exposed individuals compared to 8.4% of unexposed individuals required medical transport, and 15.2% vs. 10.0% received daily allowance. In the short and medium terms, exposed individuals were more frequently admitted to a medical, surgical, or obstetrical hospital unit than unexposed individuals (18.5% and 37.9% vs. 2.1% and 24.0%, respectively). The mean duration of their hospital stay was 10.4 days in the short term and 7.3 days in the medium term (6.5 and 5.9 days for unexposed individuals). Hospitalisation in rehabilitation was also more frequent in exposed than unexposed individuals (5.3% and 4.9% vs. < 0.1% and 1.0% in the short and medium term, respectively). For psychiatric hospitalisations, a small difference was observed between the exposed and unexposed individuals (1.2% vs. 0.7%) in the medium term (Table 2).
Table 2.
Healthcare resource use in the short and medium terms by group
| Periods | Short term | Medium term | ||
|---|---|---|---|---|
| Individuals | Exposed | Unexposed | Exposed | Unexposed |
| (N = 4,502) | (N = 13,513) | (N = 4,425) | (N = 13,434) | |
| Individuals (%) with at least one… | ||||
| In the community | ||||
| Healthcare professional visit | ||||
| General practitioner (GP) | 47.0% | 31.2% | 91.5% | 90.7% |
| Paediatrician | 5.6% | 5.2% | 12.2% | 13.2% |
| Ophthalmologist | 1.5% | 1.3% | 19.3% | 19.5% |
| ENT | 0.7% | 0.5% | 6.5% | 5.3% |
| Psychiatrist | 0.7% | 0.6% | 2.8% | 2.3% |
| Cardiologist | 0.0% | 0.3% | 3.0% | 3.1% |
| Orthopaedist | 0.0% | 0.4% | 2.6% | 3.0% |
| Neurologist | 0.0% | 0.0% | 1.5% | 0.6% |
| Pulmonologist | 0.0% | 0.0% | 1.2% | 0.8% |
| Plastic surgeon | 0.0% | 0.0% | 0.5% | 0.3% |
| Nurse | 19.1% | 4.1% | 34.3% | 26.2% |
| Physiotherapist | 10.0% | 5.1% | 28.4% | 19.8% |
| Optician | 1.3% | 1.3% | 23.3% | 23.3% |
| Speech therapist | 1.1% | 1.1% | 5.1% | 4.0% |
| Orthoptist | 0.6% | 0.4% | 5.5% | 4.7% |
| Audioprosthetist | 0.0% | 0.0% | 2.7% | 1.2% |
| Orthoprosthetist | 0.0% | 0.0% | 1.4% | 0.3% |
| Other | 2.6% | 2.9% | 28.8% | 29.1% |
| Medical act | ||||
| Medicine | 11.5% | 8.0% | 61.1% | 60.3% |
| Medical techniques | 4.7% | 3.2% | 38.3% | 36.7% |
| Medical imaging | 4.3% | 3.3% | 39.3% | 38.4% |
| US scan | 3.4% | 2.1% | 25.8% | 24.7% |
| Prophylaxis and prevention | 0.5% | 0.6% | 10.4% | 12.5% |
| Dental | 0.4% | 0.7% | 10.6% | 11.4% |
| Surgery | 0.4% | 0.4% | 6.7% | 8.2% |
| Anaesthesia | 0.0% | 0.0% | 0.0% | 0.1% |
| Biological examinations | 26.2% | 7.3% | 59.9% | 51.1% |
| Prescription | ||||
| Drug | 74.3% | 46.4% | 96.5% | 96.0% |
| Medical device | 22.9% | 7.5% | 61.8% | 56.9% |
| Daily Allowance | 15.5% | 1.5% | 15.2% | 10.0% |
| Medical transport | 20.0% | 1.4% | 20.3% | 8.4% |
| In-hospital | ||||
| Hospitalisation (medical, surgical, or obstetrical unit) | 18.5% | 2.1% | 37.9% | 24.0% |
| Ambulatory | 8.5% | 1.0% | 22.5% | 14.0% |
| With overnight stay | 12.4% | 1.2% | 25.6% | 14.1% |
| Medical act | ||||
| Medical | 13.5% | 1.5% | 33.3% | 21.3% |
| Technical | 9.2% | 0.8% | 20.3% | 11.2% |
| Medical imaging | 8.6% | 0.6% | 18.8% | 7.9% |
| US scan | 4.4% | 0.3% | 9.0% | 3.9% |
| Anaesthesia | 1.4% | 0.3% | 8.0% | 6.4% |
| Surgery | 3.5% | 0.5% | 15.5% | 10.7% |
| Drug or medical device (additional list) | ||||
| Drug | 0.9% | 0.1% | 2.5% | 0.3% |
| Medical device | 0.4% | 0.0% | 2.5% | 1.5% |
| Hospitalisation in an after-care and rehabilitation unit | 5.3% | 0.0% | 4.9% | 1.0% |
| Hospitalisation in psychiatry | 0.0% | 0.0% | 1.2% | 0.7% |
ENT: ear-nose-throat; IMD: invasive meningococcal disease; US: ultrasound
Exposed: admitted to hospital for IMD between 1 January 2008, and 31 December 2018 and discharged alive from the hospital; Unexposed: matched on age, sex, socioeconomic status, and postcode of the municipality to exposed individuals
Short term (1 month): from the index date to 30 days later; Medium term (2 years): during the 23 months after the end of the short-term follow-up. Index date: date of the hospital discharge for exposed individuals. This date served as the index date for matched unexposed individuals
Mean healthcare costs in exposed and unexposed individuals for the short and medium terms are shown in Table 3. Costs were higher in exposed than unexposed individuals: in the short term, the difference was estimated at €2,271 per capita (€9,063,558 in total), without the index hospitalisation, and €13,108 per capita (€57,140,107 in total), index hospitalisation included. In the medium term, the difference was estimated at €6,733 per capita (€6,628,967 in total). Increased costs observed in exposed individuals in the short term were mainly due to elevated inpatient costs, even after excluding the index hospitalisation. Index hospitalisation excluded, the mean per capita inpatient costs were estimated at €2,145 (SD: €10,422) in exposed individuals vs. €62 (SD: €1,080) in unexposed individuals, while the mean per capita outpatient costs were estimated €255 (SD: €755) in exposed individuals vs. €67 (SD: €327) in unexposed individuals. The distribution of inpatient and outpatient costs were more balanced in the medium term. Inpatient and outpatient mean per capita costs were equal to €5,747 (SD: €40,897) and €3,557 (SD: €15,795) for exposed individuals vs. €1,083 (SD: €7,048) and €1,488 (SD: €5,206) in unexposed individuals.
Table 3.
Healthcare costs in the short, medium, and long terms by group, with additional costs
| Periods | Exposed individuals | Unexposed individuals | Difference | |
|---|---|---|---|---|
| Short term | N | 4,502 | 13,513 | |
| (with index hospitalisation) | Total | €59,944,186 | €2,804,079 | €57,140,107 |
| Mean (SD) per capita | €13,315 (20,528) | €208 (1,500) | €13,108 | |
| Median (Q1-Q3) per capita | €7,537 (4,635–13,355) | €21 (0–75) | €7,516 | |
| Min - Max | 0-325,590 | 0–90,503 | - | |
| Persons-years | 4 019 | 11 727 | ||
| Costs per P-Y | €14 914 | €239 | ||
| Short term - Global | N | 4,502 | 13,513 | |
| (without index hospitalisation) | Total | €10,804,473 | €1,740,915 | €9,063,558 |
| Mean (SD) per capita | €2,400 (10,565) | €129 (1,150) | €2,271 | |
| Median (Q1-Q3) per capita | 88 (22–552) | 15 (0–52) | 73 | |
| Min - Max | 0-324,160 | 0–90,503 | - | |
| Persons-years | 3 684 | 10 750 | ||
| Costs per P-Y | €2 932 | €162 | ||
| Medium term | N | 4,425 | 13,434 | |
| Total | €41,171,752 | €34,542,785 | €6,628,967 | |
| Mean (SD) per capita | €9,304 (51,785,65) | €2,571 (9,580) | €6,733 | |
| Median (Q1-Q3) per capita | 880 (327–3,704) | 526 (220–1566) | 354 | |
| Min - Max | 0–2,070,707 | 0-517,967 | - | |
| Persons-years | 7 865 | 22 951 | ||
| Costs per P-Y | €5 235 | €1 505 | ||
| EIC+ | UIMC+ | Difference | ||
| Long term | N | 844 | 2,640 | |
| Total | €31,834,322 | €31,265,734 | €568,588 | |
| Mean (SD) per capita | €37,718 (114,143) | €11,843 (24,039) | €25,875 | |
| Median (Q1-Q3) per capita | 9,863 (2,166–36,521) | 2,967 (668–11,956) | 6,896 | |
| Min - Max | 0–2,374,352 | 0-292,258 | - | |
| Persons-years | 4 389 | 13 992 | ||
| Costs per P-Y | €7 254 | €2 235 |
EIC+: Exposed Individuals with Care; HCRU: Healthcare Resource Utilisation; IMD: Invasive Meningococcal Disease; SD: Standard Deviation; UIMC+: Unexposed Individuals Matched to EIC+
Difference (additional cost) = Exposed or EIC + values minus Unexposed or UIMC + values
Exposed: admitted to hospital for IMD between 1 January 2008, and 31 December 2018 and discharged alive from the hospital; Unexposed: matched on age, sex, socioeconomic status, and postcode of the municipality to exposed individuals. EIC+: among exposed individuals, those with a high HCRU level as determined by the cluster analysis (see Additional material); UIMC+: among unexposed individuals those matched to EIC+
Short term (1 month): from the index date to 30 days later; Medium term (2 years): during the 23 months after the end of the short-term follow-up; Long term (up to 12 years): from 2 years after the index date until end of study or death. Index date: date of the hospital discharge for exposed individuals. This date served as the index date for matched unexposed individuals
IMD exposure increased short- and medium-term costs. In the month following hospitalisation, the adjusted odds ratios (aORs) were 19.2 (95% confidence interval, CI: 17.9–20.5) index hospitalisation excluded and 86.3 (95%CI: 81.2–91.7) index hospitalisation included. Beyond the first month after exposure, the aOR was 1.3 (95%CI: 1.2–1.4).
Long-term healthcare resource use and additional costs associated to IMD sequelae/a high HCRU level
Exposed Individuals with Care (EIC+, n = 1,032) used more healthcare resources than Unexposed Individuals Matched to EIC+ (UIMC+, n = 3,216), by consulting nurses, physiotherapists, ophthalmologists. Biological tests were carried out in 86.1% of them (vs. 79.2% for UIMC+), and 86.5% of EIC + had a prescription for a medical device (vs. 76.9% for UIMC+). Compared to UIMC+, EIC + were more likely to be hospitalised in a medical, surgical, or obstetric department (65.0% vs. 47.8%), an after-care and rehabilitation unit (12.0% vs. 4.7%), or a psychiatric unit (2.5% vs. 1.4%). During hospitalisation in the medical, surgical, or obstetrical department, the percentage of individuals with at least one medical act, a technical act, a medical imaging, an ultrasound scan, or a surgery was higher in EIC + than UIMC+ (Table 4).
Table 4.
Healthcare resource utilisation in the long term by group
| Individuals (%) with at least one | EIC+ | UIMC+ |
|---|---|---|
| (N = 844) | (N = 2,640) | |
| In the community | ||
| Healthcare professional visit | ||
| General practitioner (GP) | 90.5% | 88.3% |
| Ophthalmologist | 32.0% | 33.1% |
| Cardiologist | 11.5% | 10.9% |
| Orthopaedist | 9.2% | 8.9% |
| ENT | 9.5% | 8.8% |
| Psychiatrist | 7.2% | 4.6% |
| Pulmonologist | 4.7% | 3.0% |
| Paediatrician | 4.1% | 3.2% |
| Neurologist | 2.6% | 2.2% |
| Plastic surgeon | 1.3% | 1.2% |
| Nurse | 71.7% | 61.7% |
| Optician | 52.1% | 49.5% |
| Physiotherapist | 52.3% | 39.4% |
| Orthoptist | 13.4% | 10.6% |
| Speech therapist | 9.0% | 4.4% |
| Audioprosthetist | 9.0% | 4.2% |
| Orthoprosthetist | 4.6% | 0.8% |
| Other | 50.6% | 48.9% |
| Medical act | ||
| Medicine | 85.8% | 85.9% |
| Medical techniques | 73.1% | 69.8% |
| Medical imaging | 71.9% | 72.4% |
| US scan | 60.8% | 59.5% |
| Prophylaxis and prevention | 41.6% | 45.5% |
| Dental | 43.1% | 44.6% |
| Surgery | 28.3% | 30.0% |
| Biological examinations | 86.1% | 79.2% |
| Prescription | ||
| Drug | 97.0% | 95.9% |
| Medical device | 86.5% | 76.9% |
| Daily Allowance | 20.5% | 17.5% |
| Medical transport | 47.9% | 26.5% |
| In-hospital | ||
| Hospitalisation in a medical, surgical, or obstetrical unit | 65.0% | 47.8% |
| Ambulatory | 45.6% | 32.3% |
| With overnight stay | 50.8% | 34.2% |
| Medical act | ||
| Medical | 62.6% | 46.4% |
| Technical | 50.2% | 35.6% |
| Medical imaging | 40.3% | 24.9% |
| US scan | 24.9% | 14.8% |
| Anaesthesia | 26.7% | 18.6% |
| Obstetrics | 4.9% | 4.2% |
| Surgery | 38.5% | 27.8% |
| Drug or medical device (additional list) | ||
| Drug | 7.1% | 1.6% |
| Medical device | 8.9% | 6.9% |
| Hospitalisation in an after-care and rehabilitation unit | 12.0% | 4.7% |
| Hospitalisation in psychiatry | 2.5% | 1.4% |
EIC+: Exposed Individual with Care; ENT: ear-nose-throat; HCRU: Healthcare Resource Utilisation; IMD: invasive meningococcal disease; UIMC+: Unexposed Individual Matched to EIC+; US: ultrasound
EIC+: among individuals admitted to hospital for IMD between 1 January 2008, and 31 December 2018 and discharged alive from the hospital, those with a high HCRU level as determined by the cluster analysis (see Additional material); UIMC+: unexposed individuals matched on age, sex, socioeconomic status, and postcode of the municipality to EIC+
Long term (up to 12 years): from 2 years after the index date until end of study or death. Index date: date of the hospital discharge for EIC+. This date served as the index date for UIMC+
Mean long-term healthcare costs in EIC + and UIMC + are presented in Table 3. The difference between EIC + and UIMC + was estimated at €25,875 per capita (€568,588 in total). Mean per capita costs decreased in both groups over time but remained higher in the EIC + than UIMC + group throughout the study (Fig. 2). Mean per capita inpatient and outpatient costs were: €16,532 (SD: €48,288) and €21,186 (SD: €86,483) for EIC + and €5,260 (SD: €14,458) and €6,583 (SD: €13,772) for UIMC+, respectively.
Fig. 2.
Long-term inpatient and outpatient healthcare costs over time by group. Long term (up to 12 years): from 2 years after the index date until end of study or death. Index date: date of the hospital discharge for exposed individuals. This date served as the index date for matched unexposed individuals. Mean costs are presented with 95% confidence intervals
After adjustment on covariates, IMD exposure increased long-term costs: aOR was 2.2 (95%CI: 2.0-2.5), ranging from 1.6 (1.0-2.3) to 3.3 (1.7–6.7) (Fig. 3).
Fig. 3.
Odds ratios (ORs) for medium-term and long-term healthcare costs. The analysis was adjusted for age, sex, diabetes, autoimmune diseases, and cardiovascular or tumoral history
Discussion
Beyond the burden of deaths (7.3% during the hospital stay), our study highlighted the substantial healthcare resource utilisation (HCRU) and costs associated with IMD among individuals discharged alive, which persist well beyond hospital discharge. In the short term, healthcare costs were approximately 20 times higher in exposed individuals compared to matched unexposed individuals, and up to 50 times higher when including the costs of the initial hospitalisation. Over the long term (up to 12 years), annual healthcare costs for individuals with sequelae (EIC+) were twice as high as those of matched unexposed individuals.
From the perspective of the French Health Insurance System, the estimated additional short-term costs (within 1-month post-discharge) amounted to approximately €6.5 million annually, based on around 500 hospitalisations per year and an estimated additional per capita cost of €13,000 (including the index hospitalisation). Over a mean follow-up period of five years, the total costs per hospitalised individual reached €60,000. These findings underscore the importance of accounting for the long-term economic burden of IMD sequelae in France, consistent with the results based on the same French database, SNDS, but using another methodology as reported by Weil-Olivier et al. [5].
Our study also highlighted that the types of healthcare resources utilised evolved over time. During the first month following discharge, exposed individuals had more frequent consultations with paramedical professionals, underwent more biological tests, and received more drug prescriptions compared to unexposed individuals. During the first two years following exposure, readmissions were more frequent, likely due to sequelae treatment. In the long-term, EIC + more often required nursing or physiotherapy care, were more frequently admitted to rehabilitation or psychiatric units (suggesting poorer physical and mental health), and visited GPs or specialists less frequently, as observed in a previous study [14].
The long-term evaluation of sequelae
Unlike previous studies [3–6, 10, 11], our study used a novel approach to identify individuals with sequelae, based on real-world inpatient and outpatient HCRU during the two years after discharge. Identifying IMD sequelae remains challenging due to the absence of a standardised definition in the literature [14]. Grouping data from 66 observational studies and 34 economic evaluations, Shen et al. [7] identified 44 sequelae (30 physical/neurological and 14 psychosocial/behavioural). They highlighted that most studies reported only a limited range of sequalae and that the prevalence of certain complications often diverged from clinical expectations. Traditional approaches rely on the presence of chronic diseases, diagnoses codes, medical procedures, treatments derived from hospitalisation data, or the dispensing of drugs or medical devices used as proxies for sequelae. In contrast, our method integrated more than 60 healthcare variables to identify sequelae without any a priori assumption. This approach was validated through cross-analyses of ICD-10 codes for clearly identifiable sequelae - such as renal failure, mood disorders, motor impairment, and severe neurological conditions [7] - and its robustness was confirmed through sensitivity analyses across four subpopulations.
Strengths and limitations of the study
Our study has several strengths. First, it used data from the SNDS which covers > 99% of the French population, allowing for the exhaustive inclusion of all IMD cases nationwide [3]. Second, each individual hospitalised for IMD was matched to up to 4 individuals without IMD hospitalisation. Third, it included long-term follow-up, extending up to 12 years. By extracting data on all individuals hospitalised for IMD during the study period, we were able to apply inclusion criteria and analyse virtually the entire population of interest. The value of administrative health databases for epidemiological research has been demonstrated previously [3, 6, 15]. Including socioeconomic status and place of residence in the matching process was particularly relevant, given the strong association between low household income and IMD, as well as the uneven distribution of healthcare resources in France [2, 16]. Comorbidities were not included in the initial matching process to avoid overmatching and to preserve the frequency distributions of these comorbidities within both the IMD + and IMD- groups. This approach allows us to observe differences in comorbidity profiles in a non-interventional manner. However, to address potential imbalances, we adjusted for baseline presence of comorbidities in the multivariable GEE models used to estimate total cost differences over time. This model accounts for the correlation within matched sets (1:N) and allows for unequal cluster sizes, helping to reduce the potential bias introduced by variable matching ratios.
Of note, this method is usual in longitudinal cost-analyses. The longitudinal design aligns with standard practices in studies of disease-related expenditure, especially for infectious diseases. An exploratory unsupervised clustering approach using the k-modes algorithm was employed to identify distinct healthcare utilisation trajectories following IMD, with the aim of characterising patients with sequelae-related patterns independently of underreported diagnoses, which are common in administrative healthcare databases. Then, the use of GEE models was appropriate for analysing healthcare reimbursement data [17, 18] without requiring cost-specific IMD-related assumptions. In addition, the clustering was based on sociodemographic and healthcare resource utilisation (HCRU) variables, which are inherently correlated with healthcare cost. Consequently, the observed differences in cost between clusters in the GEE model are not independent of clustering criteria. This cost comparison was exploratory and aims to illustrate the economic burden associated with different post-IMD healthcare trajectories, particularly those with high HCRU; and not to infer a causal relationship between cluster and cost. Further studies using independent clinical outcomes will be needed to confirm the association between sequelae-related trajectories and long-term cost. However, our study also has some limitations, notably those associated with the use of administrative databases. The health characteristics of included individuals were not fully captured, and comorbidities were not used as matching variables, although statistical adjustments were made during the analysis. Potential coding errors cannot be ruled out, and the specific reasons for healthcare utilisation were not available. Additionally, the serogroup was unknown, which prevented cost analyses by serogroup.
Regarding the clustering approach, additional validation steps such as comparing alternative clustering algorithms or using other external validation metrics would have been consistent. Instead, we relied on the within-cluster dissimilarity and clinical interpretability of the clusters, supported by cross-analyses with ICD-10 codes for known IMD sequelae. While this provides some strong internal consistency, it does not replace formal validation. Moreover, baseline comorbidities, which are important drivers of HCRU and costs, were not included in the clustering process to avoid masking natural post-IMD patterns. This may have introduced residual confounding.
Despite these limitations, the robustness of our findings is supported by several factors: (1) the large number of exposed individuals (N = 5,770); (2) the close alignment with the number of IMD cases reported by Santé publique France during the same period (N = 5,924, https://www.santepubliquefrance.fr/); (3) the in-hospital mortality rate (423/4878, 8.6%) was consistent with previous studies (6.0%-8.3%) [3, 7, 10]; (4) the proportion of EIC+ (≈ 23%) was similar to that reported in other studies (23.3% to 25.4%) [3, 4, 7, 10] although a recent US study suggested rates equal to 40%; (5) the length of the index hospital stay was comparable to that observed in Germany [10]; (6) the mean per capita cost of IMD hospitalisation (€10,599) was in line with estimates from Huang et al. (€11,445) [10].
The clustering algorithm was applied to post hospitalisation HCRU data with the aim of identifying distinct long-term care trajectories, including those potentially associated with IMD sequelae. However, a high HCRU does not exclusively reflect sequelae, and that the EIC + cluster may also include individuals with pre-existing chronic conditions or baseline vulnerabilities unrelated to IMD. However, cross-analyses with ICD-10 codes known to be associated with IMD-related sequelae, which were disproportionately represented in EIC + group suggests that this cluster likely includes a substantial proportion of patients with sequelae.
Impact on vaccination policy
IMD is a vaccine-preventable disease, and current European immunisation programmes target the four most prevalent serogroups B, C, W, and Y [19]. Our findings underscore the importance of strengthening immunisation efforts and increasing healthcare provider and patient awareness of vaccine recommendations to reduce the incidence, deaths, long-term sequelae, and economic burden associated with IMD.
Conclusions
Our study, which collected data on virtually all individuals hospitalised for IMD in France between 2008 and 2018 revealed the high economic burden of IMD, primarily due to the management of sequelae. The study emphasised the importance of developing and implementing effective prevention strategies mitigate the impact of IMD caused by the most prevalent serotypes. While the economic burden associated with sequelae decreased over time, it persisted for more than a decade after hospitalisation for IMD. Furthermore, the clustering method employed in our study could help identify sequelae following IMD in real-world data studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Léa Antoniali from Sanofi, Fabienne Péretz and Matthieu Chanard from Abelia Science for their strong and significant contributions to this project and the manuscript writing. Regarding data extraction, we thank the teams of the Direction de la Stratégie, des études et des Statistiques, département Accès, Traitements et Analyse de la Donnée and of the Cellule de la CNAM en Charge de l’accompagnement des Demandes d’extraction at the Caisse Nationale de l’Assurance Maladie.
Abbreviations
- CépiDC
Centre for epidemiology on medical causes of death
- CESREES
Ethics and scientific committee for research, studies and evaluations in the health sector
- CNIL
French national commission on informatics and liberty
- EIC+
Exposed individuals with care
- GEE
Generalized estimating equation
- HCRU
Healthcare resource utilisation
- IMD
Invasive meningococcal disease
- NIP
National immunisation program
- PMSI
Medicalisation of information systems program
- SNDS
National healthcare data system
- SNIIRAM
National inter-regimen health insurance information system
- UIMC+
Unexposed individuals matched to EIC+
Author contributions
BH, XD, CC and LF contributed to the study design, statistical analysis plan, data analysis, interpretation and critically analysis of results and manuscript writing. CC led the writing of the statistical analysis plan, data management, ran the analysis and manuscript writing. AB and TN contributed to the development of outline and manuscript writing. All co-authors reviewed the manuscript and agreed with the content of the submitted version.
Funding
The study was funded by Sanofi. Partnership with IQVIA and scientist committee was done.
Data availability
The data that support the findings of this study are available from the French national health insurance information system. As restrictions apply to the availability of these data which were used under license for the current study, they are not publicly available.
Declarations
Ethics approval and consent to participate
Our study protocol was reviewed and approved by the Comité Éthique et Scientifique pour les Recherches, les Études et les Évaluations dans le domaine de la Santé (CESREES) on 8 October 2020 (No. TPS2483887) and by the Commission Nationale de l’Informatique et des Libertés (CNIL) on 1 February 2021 (CNIL, MLD/VCS/AR212214, No. 920458). Informed consent was waived by the Commission Nationale de l’Informatique et des Libertés (CNIL in French) as this study was retrospective on a pseudo-anonymised database and did not influence care. This study was conducted according to national laws, regulations, and the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
BH and XD declare that they have no competing interests except consulting fees for this study.CC is employed by IQVIA which received funding from Sanofi to run the study.TN, AB and LF are Sanofi employees and may hold shares in the company.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xavier Duval, Email: xavier.duval@aphp.fr.
Bruno Hoen, Email: bruno@hoen.pro.
References
- 1.Martinón-Torres F. Deciphering the burden of meningococcal disease: conventional and Under-recognized elements. J Adolesc Health. 2016;59:S12–20. [DOI] [PubMed] [Google Scholar]
- 2.Taha MK, Weil-Olivier C, Bouée S, Emery C, Nachbaur G, Pribil C, et al. Risk factors for invasive meningococcal disease: a retrospective analysis of the French National public health insurance database. Hum Vaccines Immunotherapeutics. 2021;17:1858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weil-Olivier C, Taha MK, Bouée S, Emery C, Loncle-Provot V, Nachbaur G, et al. Care pathways in invasive meningococcal disease: a retrospective analysis of the French National public health insurance database. Hum Vaccines Immunotherapeutics. 2022;18:2021764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rampakakis E, Vaillancourt J, Mursleen S, Sampalis JS. Healthcare resource utilization and cost of invasive meningococcal disease in Ontario, Canada. Pediatr Infect Dis J. 2019;38:253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weil-Olivier C, Taha MK, Emery C, Bouée S, Beck E, Aris E, et al. Healthcare resource consumption and cost of invasive meningococcal disease in france: A study of the National health insurance database. Infect Dis Ther. 2021;10:1607–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Fievez S, Goguillot M, Marié L, Bénard S, Elkaïm A, et al. A database study of clinical and economic burden of invasive meningococcal disease in France. PLoS ONE. 2022;17:e0267786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen J, Begum N, Ruiz-Garcia Y, Martinon-Torres F, Bekkat-Berkani R, Meszaros K. Range of invasive meningococcal disease sequelae and health economic application - a systematic and clinical review. BMC Public Health. 2022;22:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Santoreneos R, Afzali H, Giles L, Marshall H. Costs of invasive meningococcal disease: A global systematic review. PharmacoEconomics. 2018;36:1201–22. [DOI] [PubMed] [Google Scholar]
- 9.Deng L, Barton B, Lorenzo J, Rashid H, Dastouri F, Booy R. Longer term outcomes following serogroup B invasive meningococcal disease. J Paediatr Child Health. 2021;57:894–902. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Heuer OD, Janßen S, Häckl D, Schmedt N. Clinical and economic burden of invasive meningococcal disease: evidence from a large German claims database. PLoS ONE. 2020;15:e0228020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chacon-Cruz E, Lopatynsky-Reyes EZ, Huerta-Garcia G, Cervantes-Apolinar MY, Guzman-Holst A, Van Oorschot D. Economic burden of meningococcal disease in children and adolescents in Tijuana, Mexico. Hum Vaccin Immunother. 2022;18:2103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanguy-Melac A, Verboux D, Pestel L, Fagot-Campagna A, Tuppin P, Gastaldi-Ménager C. Evolution of health care utilization and expenditure during the year before death in 2015 among people with cancer: French SNDS-based cohort study. Eur J Health Econ. 2021;22:1039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohl AA, Fishman PA, Ciol MA, Williams B, Logerfo J, Phelan EA. A longitudinal analysis of total 3-year healthcare costs for older adults who experience a fall requiring medical care. J Am Geriatr Soc. 2010;58:853–60. [DOI] [PubMed] [Google Scholar]
- 14.Guedes S, Bricout H, Langevin E, Tong S, Bertrand-Gerentes I. Epidemiology of invasive meningococcal disease and sequelae in the united Kingdom during the period 2008 to 2017 - a secondary database analysis. BMC Public Health. 2022;22:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scailteux LM, Droitcourt C, Balusson F, Nowak E, Kerbrat S, Dupuy A, et al. French administrative health care database (SNDS): the value of its enrichment. Therapie. 2019;74:215–23. [DOI] [PubMed] [Google Scholar]
- 16.Bonal M, Padilla C, Chevillard G, Lucas-Gabrielli V. A French classification to describe medical deserts: a multi-professional approach based on the first contact with the healthcare system. Int J Health Geogr. 2024;23:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleine-Budde K, Müller R, Kawohl W, Bramesfeld A, Moock J, Rössler W. The cost of depression - a cost analysis from a large database. J Affect Disord. 2013;147:137–43. [DOI] [PubMed] [Google Scholar]
- 18.Moon S, Choi M. The effect of usual source of care on the association of annual healthcare expenditure with patients’ age and chronic disease duration. Int J Environ Res Public Health. 2018;15:1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh SR, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81:483–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the French national health insurance information system. As restrictions apply to the availability of these data which were used under license for the current study, they are not publicly available.