Abstract
A new quantitative approach to study cell membrane electrofusion has been developed. Erythrocyte ghosts were brought into close contact using dielectrophoresis and then treated with one square or even exponentially decaying fusogenic pulse. Individual fusion events were followed by lateral diffusion of the fluorescent lipid analogue 1,1'-dihexadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (Dil) from originally labeled to unlabeled adjacent ghosts. It was found that ghost fusion can be described as a first-order rate process with corresponding rate constants; a true fusion rate constant, k(f), for the square waveform pulse and an effective fusion rate constant, k(ef), for the exponential pulse. Compared with the fusion yield, the fusion rate constants are more fundamental characteristics of the fusion process and have implications for its mechanisms. Values of k(f) for rabbit and human erythrocyte ghosts were obtained at different electric field strength and temperatures. Arrhenius k(f) plots revealed that the activation energy of ghost electrofusion is in the range of 6-10 kT. Measurements were also made with the rabbit erythrocyte ghosts exposed to 42 degrees C for 10 min (to disrupt the spectrin network) or 0.1-1.0 mM uranyl acetate (to stabilize the bilayer lipid matrix of membranes). A correlation between the dependence of the fusion and previously published pore-formation rate constants for all experimental conditions suggests that the cell membrane electrofusion process involve pores formed during reversible electrical breakdown. A statistical analysis of fusion products (a) further supports the idea that electrofusion is a stochastic process and (b) shows that the probability of ghost electrofusion is independent of the presence of Dil as a label as well as the number of fused ghosts.
Full text
PDF

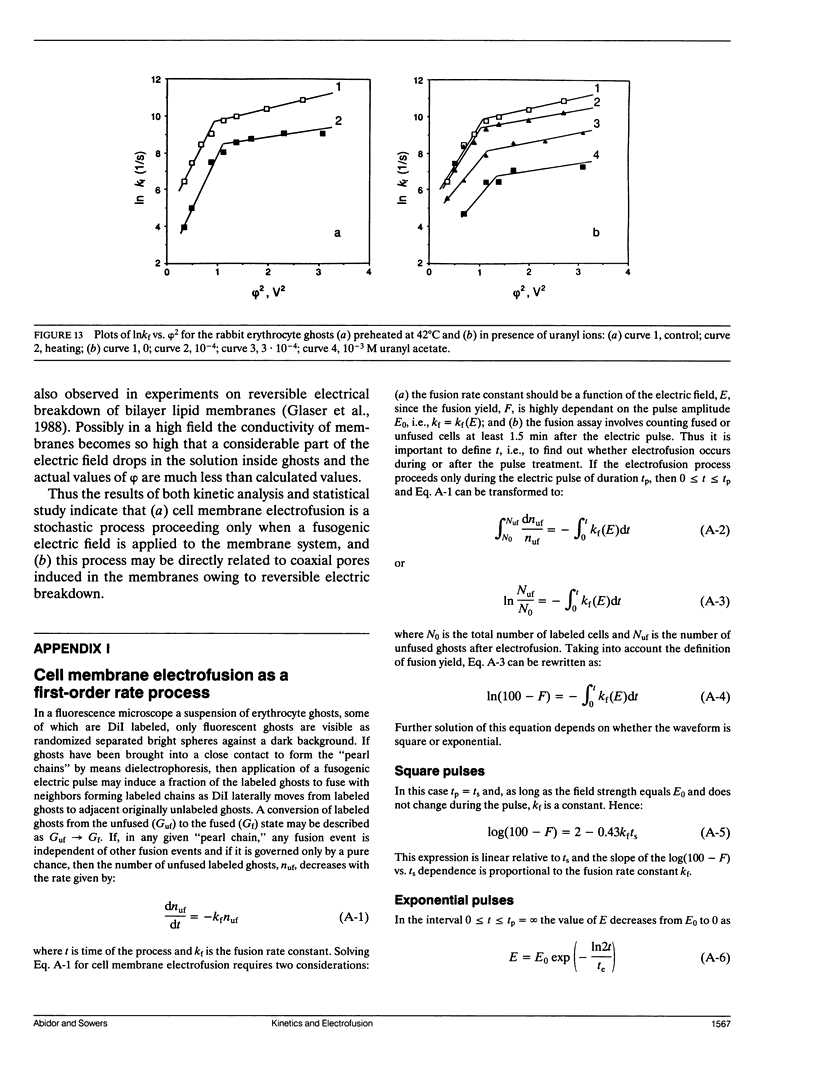


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Beckers F., Zimmermann U. Reversible electrical breakdown of lipid bilayer membranes: a charge-pulse relaxation study. J Membr Biol. 1979 Jul 16;48(2):181–204. doi: 10.1007/BF01872858. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Sowers A. E. Evidence that the spectrin network and a nonosmotic force control the fusion product morphology in electrofused erythrocyte ghosts. Biophys J. 1991 Nov;60(5):1026–1037. doi: 10.1016/S0006-3495(91)82140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Sukharev S. I., Popov S. V., Pastushenko V. F., Sokirko A. V., Abidor I. G., Chizmadzhev Y. A. The electrical breakdown of cell and lipid membranes: the similarity of phenomenologies. Biochim Biophys Acta. 1987 Sep 3;902(3):360–373. doi: 10.1016/0005-2736(87)90204-5. [DOI] [PubMed] [Google Scholar]
- Coakley W. T., Gallez D. Membrane-membrane contact: involvement of interfacial instability in the generation of discrete contacts. Biosci Rep. 1989 Dec;9(6):675–691. doi: 10.1007/BF01114806. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Jain R. K. Membrane stability. Biochim Biophys Acta. 1984 Dec 4;779(4):437–468. doi: 10.1016/0304-4157(84)90020-0. [DOI] [PubMed] [Google Scholar]
- Glaser R. W., Leikin S. L., Chernomordik L. V., Pastushenko V. F., Sokirko A. I. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta. 1988 May 24;940(2):275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P., Boni L. T., Yeagle P. L. Membrane fusion through point defects in bilayers. Science. 1981 May 22;212(4497):921–923. doi: 10.1126/science.7233185. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Voltage-induced pore formation and hemolysis of human erythrocytes. Biochim Biophys Acta. 1977 Dec 1;471(2):227–242. doi: 10.1016/0005-2736(77)90252-8. [DOI] [PubMed] [Google Scholar]
- Pilwat G., Richter H. P., Zimmermann U. Giant culture cells by electric field-induced fusion. FEBS Lett. 1981 Oct 12;133(1):169–174. doi: 10.1016/0014-5793(81)80497-8. [DOI] [PubMed] [Google Scholar]
- Rols M. P., Teissié J. Electropermeabilization of mammalian cells. Quantitative analysis of the phenomenon. Biophys J. 1990 Nov;58(5):1089–1098. doi: 10.1016/S0006-3495(90)82451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpersu E. H., Kinosita K., Jr, Tsong T. Y. Reversible and irreversible modification of erythrocyte membrane permeability by electric field. Biochim Biophys Acta. 1985 Feb 14;812(3):779–785. doi: 10.1016/0005-2736(85)90272-x. [DOI] [PubMed] [Google Scholar]
- Sowers A. E. A long-lived fusogenic state is induced in erythrocyte ghosts by electric pulses. J Cell Biol. 1986 Apr;102(4):1358–1362. doi: 10.1083/jcb.102.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers A. E. Characterization of electric field-induced fusion in erythrocyte ghost membranes. J Cell Biol. 1984 Dec;99(6):1989–1996. doi: 10.1083/jcb.99.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers A. E. Evidence that electrofusion yield is controlled by biologically relevant membrane factors. Biochim Biophys Acta. 1989 Nov 3;985(3):334–338. doi: 10.1016/0005-2736(89)90422-7. [DOI] [PubMed] [Google Scholar]
- Sowers A. E., Lieber M. R. Electropore diameters, lifetimes, numbers, and locations in individual erythrocyte ghosts. FEBS Lett. 1986 Sep 15;205(2):179–184. doi: 10.1016/0014-5793(86)80893-6. [DOI] [PubMed] [Google Scholar]
- Sowers A. E. The long-lived fusogenic state induced in erythrocyte ghosts by electric pulses is not laterally mobile. Biophys J. 1987 Dec;52(6):1015–1020. doi: 10.1016/S0006-3495(87)83294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger D. A., Hui S. W. Kinetics of ultrastructural changes during electrically induced fusion of human erythrocytes. J Membr Biol. 1986;93(1):43–53. doi: 10.1007/BF01871017. [DOI] [PubMed] [Google Scholar]
- Sukharev S. I., Bandrina I. N., Barbul A. I., Fedorova L. I., Abidor I. G., Zelenin A. V. Electrofusion of fibroblasts on the porous membrane. Biochim Biophys Acta. 1990 May 16;1034(2):125–131. doi: 10.1016/0304-4165(90)90065-5. [DOI] [PubMed] [Google Scholar]
- Teissie J., Rols M. P. Fusion of mammalian cells in culture is obtained by creating the contact between cells after their electropermeabilization. Biochem Biophys Res Commun. 1986 Oct 15;140(1):258–266. doi: 10.1016/0006-291x(86)91084-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electric field-mediated fusion and related electrical phenomena. Biochim Biophys Acta. 1982 Nov 30;694(3):227–277. doi: 10.1016/0304-4157(82)90007-7. [DOI] [PubMed] [Google Scholar]


