Abstract
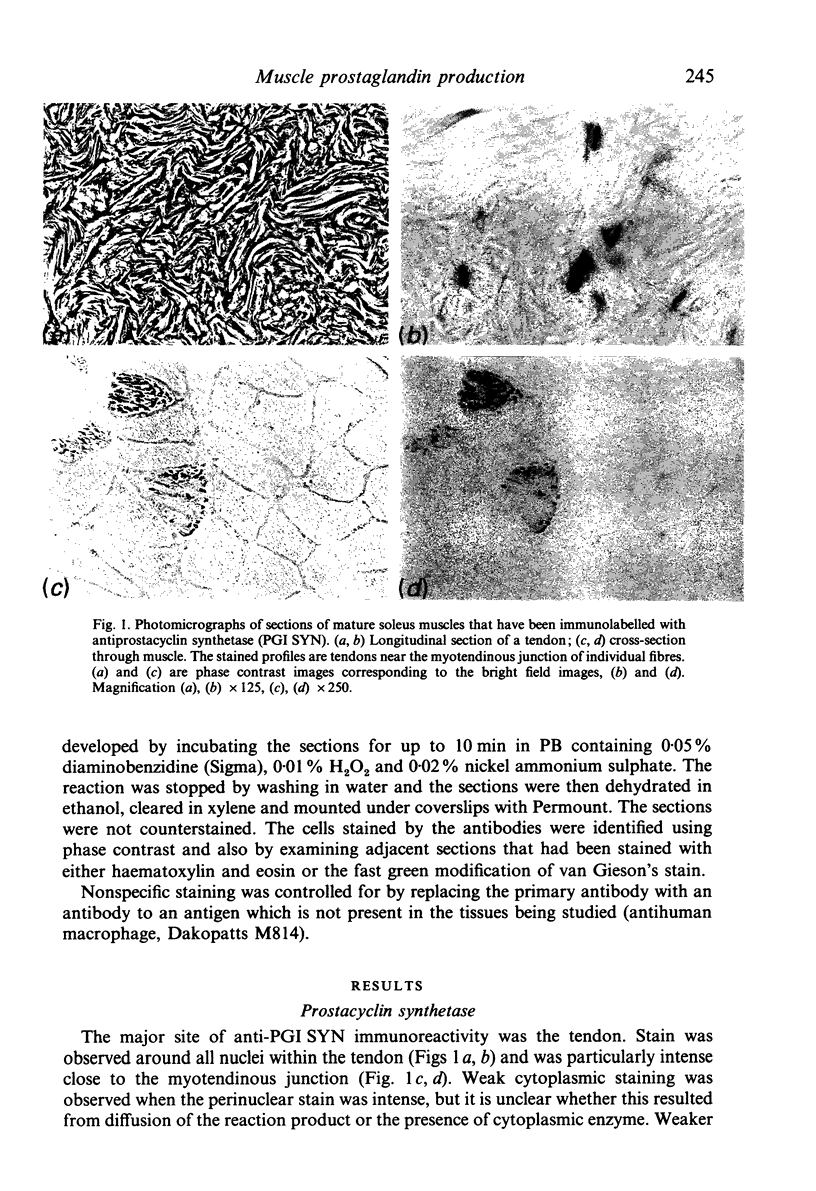
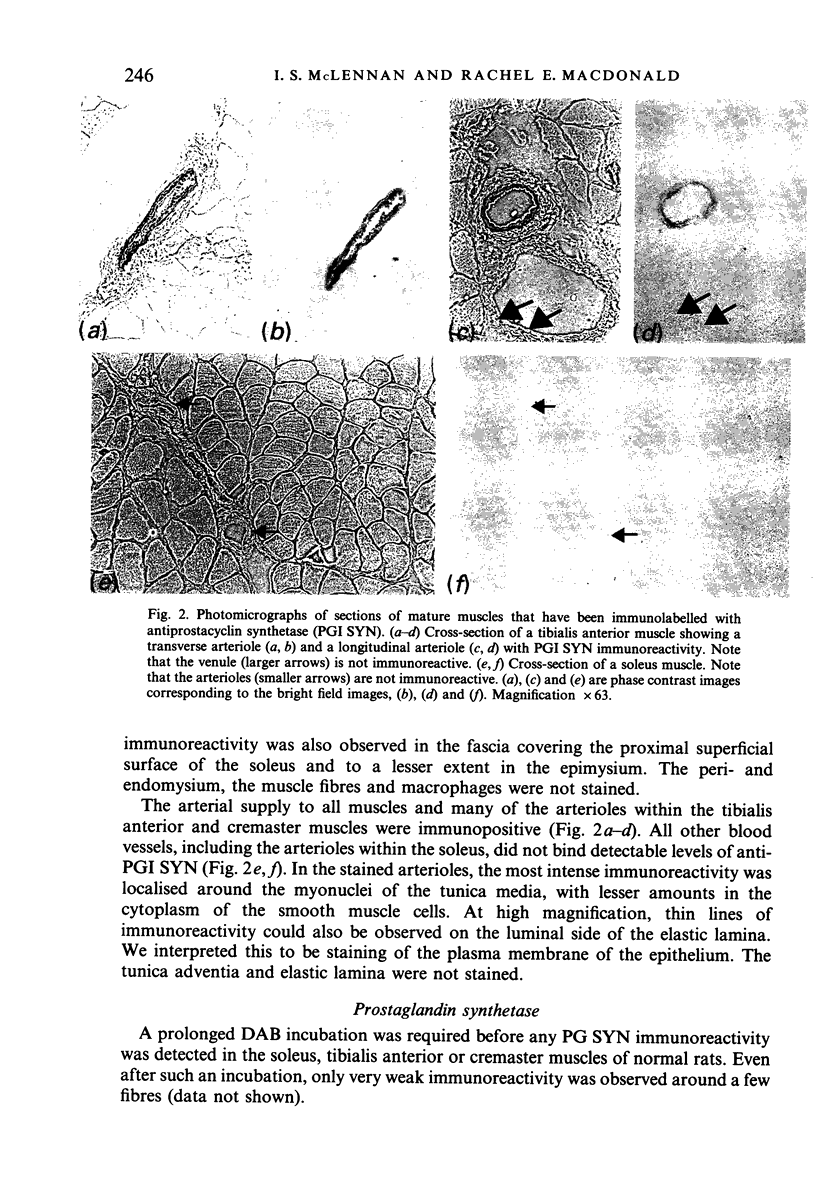
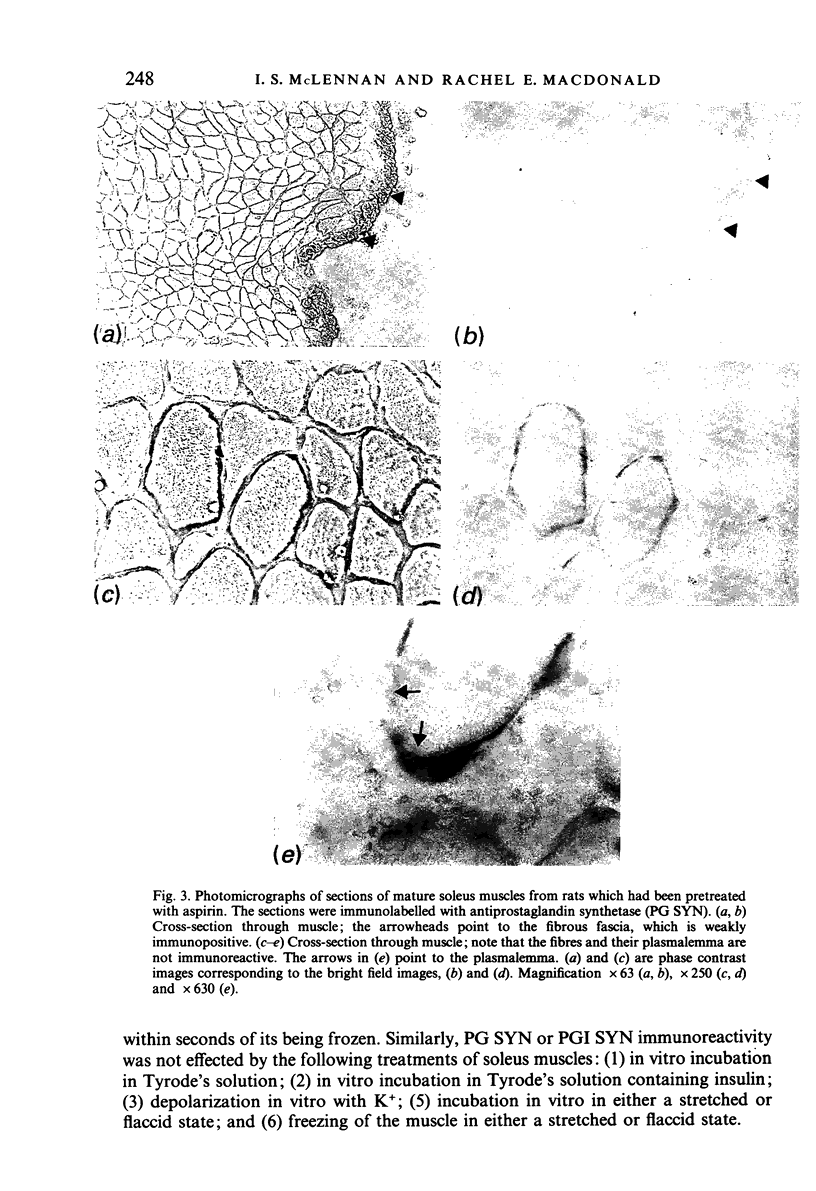
Mature skeletal muscles produce appreciable quantities of prostacyclin (PGI2) and smaller amounts of PGF2 alpha and PGE2, but the sources of these prostaglandins within skeletal muscle are unknown. Monoclonal antibodies to prostaglandin synthetase and prostacyclin synthetase were used to determine which muscle cells produce prostaglandins. The antibody to prostacyclin synthetase stained the tendon, fascia, epimysium and the arteries leading to the muscles. The endothelia of arterioles were also stained in the tibialis anterior and cremaster but not in the soleus muscles. Only trace levels of immunoreactivity were observed with the antibody to prostaglandin synthetase in normal muscles. However, immunoreactivity was observed in the muscles of rats that had been pretreated with aspirin, a drug that inhibits and stabilises prostaglandin synthetase. In muscles of the aspirin-treated rats, all cell types that were stained by the antiprostacyclin synthetase also reacted weakly with the antibody to prostaglandin synthetase. In addition, some cells in the endomysium were strongly stained with the antiprostaglandin synthetase but not with the antiprostacyclin synthetase. We conclude that (1) at least one aspect of the regulation of blood flow in the microcirculation of slow muscles is different from that of fast muscles, (2) that the tendon and connective tissue is the major source of PGI2 in mature skeletal muscles, and (3) that the prostaglandin-dependent effects of insulin and some other stimuli on skeletal muscle may be mediated by the muscle's arterioles or connective tissue.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L., Moncada S., Pearson J. D., Salmon J. A., Trevethick M. A. Effects of isolation and culture on prostaglandin synthesis by porcine aortic endothelial and smooth muscle cells. J Cell Physiol. 1982 Jan;110(1):9–16. doi: 10.1002/jcp.1041100103. [DOI] [PubMed] [Google Scholar]
- Dietze G., Wicklmayr M., Böttger I., Mayer L. Insulin action on glucose uptake into skeletal muscle: inhibition of endogenous biosynthesis of prostaglandins. FEBS Lett. 1978 Aug 15;92(2):294–298. doi: 10.1016/0014-5793(78)80773-x. [DOI] [PubMed] [Google Scholar]
- Doherty M. Soft tissue injections in the surgery. Practitioner. 1989 Oct 8;233(1476):1305–1305. [PubMed] [Google Scholar]
- Faber J. E., Harris P. D., Joshua I. G. Microvascular response to blockade of prostaglandin synthesis in rat skeletal muscle. Am J Physiol. 1982 Jul;243(1):H51–H60. doi: 10.1152/ajpheart.1982.243.1.H51. [DOI] [PubMed] [Google Scholar]
- Fagan J. M., Goldberg A. L. Inhibitors of protein and RNA synthesis cause a rapid block in prostaglandin production at the prostaglandin synthase step. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2771–2775. doi: 10.1073/pnas.83.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangos J. A., Eskin S. G., McIntire L. V., Ives C. L. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985 Mar 22;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Kerry P. J. 6-Oxo-prostaglandin F1a production by guinea-pig skeletal muscle in vitro: the effects of oestradiol and progesterone. Prostaglandins Leukot Med. 1985 Mar;17(3):283–289. doi: 10.1016/0262-1746(85)90117-9. [DOI] [PubMed] [Google Scholar]
- Koller A., Kaley G. Endothelium regulates skeletal muscle microcirculation by a blood flow velocity-sensing mechanism. Am J Physiol. 1990 Mar;258(3 Pt 2):H916–H920. doi: 10.1152/ajpheart.1990.258.3.H916. [DOI] [PubMed] [Google Scholar]
- Koller A., Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circ Res. 1990 Aug;67(2):529–534. doi: 10.1161/01.res.67.2.529. [DOI] [PubMed] [Google Scholar]
- Leighton B., Budohoski L., Lozeman F. J., Challiss R. A., Newsholme E. A. The effect of prostaglandins E1, E2 and F2 alpha and indomethacin on the sensitivity of glycolysis and glycogen synthesis to insulin in stripped soleus muscles of the rat. Biochem J. 1985 Apr 1;227(1):337–340. doi: 10.1042/bj2270337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan I. S. Characterization of a prostaglandin dysfunction myopathy. Muscle Nerve. 1987 Nov-Dec;10(9):801–809. doi: 10.1002/mus.880100905. [DOI] [PubMed] [Google Scholar]
- McLennan I. S. Inhibition of prostaglandin synthesis produces a muscular dystrophy-like myopathy. Exp Neurol. 1985 Sep;89(3):616–621. doi: 10.1016/0014-4886(85)90011-1. [DOI] [PubMed] [Google Scholar]
- McMillan D. N., Reeds P. J., Lobley G. E., Palmer R. M. Changes in protein turnover in hypertrophying plantaris muscles of rats: effect of fenbufen--an inhibitor of prostaglandin synthesis. Prostaglandins. 1987 Dec;34(6):841–852. doi: 10.1016/0090-6980(87)90065-7. [DOI] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Nesher R., Karl I. E., Kipnis D. M. Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am J Physiol. 1985 Sep;249(3 Pt 1):C226–C232. doi: 10.1152/ajpcell.1985.249.3.C226. [DOI] [PubMed] [Google Scholar]
- Nowak J., Bohman S. O., Alster P., Berlin T., Cronestrand R., Sonnenfeld T. Biosynthesis of prostaglandins in microsomes of human skeletal muscle and kidney. Prostaglandins Leukot Med. 1983 Jul;11(3):269–279. doi: 10.1016/0262-1746(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Raz A., Wyche A., Siegel N., Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [PubMed] [Google Scholar]
- Reeds P. J., Hay S. M., Glennie R. T., Mackie W. S., Garlick P. J. The effect of indomethacin on the stimulation of protein synthesis by insulin in young post-absorptive rats. Biochem J. 1985 Apr 1;227(1):255–261. doi: 10.1042/bj2270255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds P. J., Palmer R. M. Changes in prostaglandin release associated with inhibition of muscle protein synthesis by dexamethasone. Biochem J. 1984 May 1;219(3):953–957. doi: 10.1042/bj2190953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Rosen G. D., Birkenmeier T. M., Raz A., Holtzman M. J. Identification of a cyclooxygenase-related gene and its potential role in prostaglandin formation. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1358–1365. doi: 10.1016/0006-291x(89)91819-6. [DOI] [PubMed] [Google Scholar]
- Smith R. H., Palmer R. M., Reeds P. J. Protein synthesis in isolated rabbit forelimb muscles. The possible role of metabolites of arachidonic acid in the response to intermittent stretching. Biochem J. 1983 Jul 15;214(1):153–161. doi: 10.1042/bj2140153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., DeWitt D. L., Allen M. L. Bimodal distribution of the prostaglandin I2 synthase antigen in smooth muscle cells. J Biol Chem. 1983 May 10;258(9):5922–5926. [PubMed] [Google Scholar]
- Smith W. L., Rollins T. E. Characteristics of rabbit anti-PGH synthase antibodies and use in immunocytochemistry. Methods Enzymol. 1982;86:213–222. doi: 10.1016/0076-6879(82)86192-2. [DOI] [PubMed] [Google Scholar]
- Smith W. L. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989 Apr 15;259(2):315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist H., Forsskåhl B., Kvist M. A promising novel therapy for Achilles peritendinitis: double-blind comparison of glycosaminoglycan polysulfate and high-dose indomethacin. Int J Sports Med. 1987 Aug;8(4):298–303. doi: 10.1055/s-2008-1025673. [DOI] [PubMed] [Google Scholar]
- Templeton G. H., Padalino M., Moss R. Influences of inactivity and indomethacin on soleus phosphatidylethanolamine and size. Prostaglandins. 1986 Mar;31(3):545–559. doi: 10.1016/0090-6980(86)90116-4. [DOI] [PubMed] [Google Scholar]
- Vandenburgh H. H., Hatfaludy S., Sohar I., Shansky J. Stretch-induced prostaglandins and protein turnover in cultured skeletal muscle. Am J Physiol. 1990 Aug;259(2 Pt 1):C232–C240. doi: 10.1152/ajpcell.1990.259.2.C232. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Magata K., Ehara H., Mizuno K., Yamamoto S. Regional distribution of prostaglandin endoperoxide synthase studied by enzyme-linked immunoassay using monoclonal antibodies. Biochim Biophys Acta. 1986 Jun 11;877(1):141–150. doi: 10.1016/0005-2760(86)90129-3. [DOI] [PubMed] [Google Scholar]