Abstract
GATA-2 is a zinc finger transcription factor essential for the development of hematopoiesis. While GATA-2 is generally considered to play an important role in the biology of hematopoietic stem and progenitor cells, its function within these compartments is not well understood. Here we have employed both conditional expression of GATA-2 and conditional activation of a GATA-2/estrogen receptor (ER) chimera to examine the effect of enforced GATA-2 expression in the development and differentiation of hematopoietic progenitors from murine embryonic stem cells. Consistent with the phenotype of GATA-2 null animals, conditional expression of GATA-2 from a tetracycline-inducible promoter enhanced the production of hematopoietic progenitors. Conditional activation of a GATA-2/ER chimera produced essentially opposite effects to those observed with conditional GATA-2 expression. GATA-2 and GATA-2/ER differ in their binding activities and transcriptional interactions from other hematopoietic-associated transcription factors such as c-Myb and PU.1. While we have exploited these differences in activity to explore the transcriptional networks underlying hematopoietic cell fate determination, our results suggest that care should be taken in interpreting results obtained using only chimeric proteins.
Keywords: cell differentiation/GATA-2/hematopoiesis/transcription
Introduction
Hematopoiesis is a sequential cell differentiation process from self-renewing hematopoietic stem cells to mature blood cells via multipotential and lineage-restricted hematopoietic progenitors (Papayannopoulou and Lemischka, 2001). Recent gene targeting experiments have demonstrated that various differentiation stage- or lineage-specific transcription factors play crucial roles during this process (Shivdasani and Orkin, 1996; Orkin, 2001). The differentiation of committed progenitors is especially well characterized, and it is generally believed to be controlled in large part by lineage-specific transcription factors regulating the appearance of lineage-specific molecules (Shivdasani and Orkin, 1996; Tenen et al., 1997). On the other hand, the molecular mechanisms underlying the developmental programs of hematopoietic stem and progenitor cells are largely unknown. Although null mutant analyses have provided a wealth of information as to which genes are indispensable for the appropriate development of immature hematopoietic cells, the functions of the individual genes in these cells remain elusive (Shivdasani and Orkin, 1996; Orkin, 2001).
The GATA factors are a family of transcriptional regulatory proteins characterized by the ability to bind the consensus DNA sequence WGATAR (Orkin, 1992). Three members of the family, GATA-1, GATA-2 and GATA-3, play crucial roles in hematopoiesis. GATA-1 and GATA-3 primarily play roles in relation to lineage-specific transcription. GATA-1 is highly expressed in erythroid cells, mast cells, megakaryocytes and eosinophils (Evans and Felsenfeld, 1989; Tsai et al., 1989; Martin et al., 1990; Romeo et al., 1990). Erythroid precursors lacking GATA-1 fail to differentiate beyond the proerythroblast stage (Weiss et al., 1994; Fujiwara et al., 1996). GATA-3 is expressed in T lymphocytes and is essential for T-cell development (Ko et al., 1991; Landry et al., 1993; Ting et al., 1996). The distribution of another related GATA family member, GATA-2, is broad among hematopoietic cells, with particularly high expression in early hematopoietic progenitors as well as in megakaryocyte and mast cell lineages (Leonard et al., 1993; Mouthon et al., 1993).
Mice lacking GATA-2 clearly demonstrate the crucial role of the gene in both embryonic-type primitive erythropoiesis and adult-type definitive hematopoiesis (Tsai et al., 1994; Tsai and Orkin, 1997). The defects in definitive hematopoiesis are particularly profound in GATA-2–/– mice, which die around embryonic day 10–11 due to a pan-hematopoietic deficit (Tsai et al., 1994). Both the in vitro differentiation induction of GATA-2–/– embryonic stem (ES) cells and the formation of chimeric animals with these cells demonstrate that the production of GATA-2–/– hematopoietic progenitors is severely impaired and that the residual GATA-2–/– progenitors proliferate poorly (Tsai and Orkin, 1997). These data are consistent with the notion that GATA-2 may play a role in the proliferation and survival of hematopoietic progenitors.
GATA-2 function has also been analyzed using forced expression approaches employing both wild-type GATA-2 and conditionally activatable forms based on the fusion of GATA-2 to the ligand-binding domain of the estrogen receptor (GATA-2/ER) (Briegel et al., 1993; Heyworth et al., 1999; Persons et al., 1999). Ligand-inducible GATA-2/ER expression vectors have been introduced into transformed chicken erythroblasts, the factor-dependent mouse hematopoietic progenitor cell line, FDCP-mix, and 5-fluorouracil-treated mouse bone marrow cells. Activ ation of GATA-2/ER by the ligand reduces differentiation and induces proliferation of chicken erythroblasts (Briegel et al., 1993). Activated GATA-2/ER blocks factor- dependent self-renewal and inhibits erythroid differentiation in FDCP-mix cells (Heyworth et al., 1999), and mouse primary bone marrow cells show similar responses to the activated GATA-2/ER in proliferation and differentiation. Mouse hematopoietic stem cells transduced with a GATA-2-expressing retroviral vector exhibit a block in amplification as well as differentiation of transplantable hematopoietic stem cells (Persons et al., 1999).
Taken together, these forced expression data argue for GATA-2 playing a critical role in the cell fate decisions of hematopoietic stem cells and progenitors, but the differences in results obtained using these different cell systems and different GATA-2 molecules are confusing and not necessarily consistent in every case with the phenotype of the GATA-2–/– mice.
In this report, we have examined the role of GATA-2 in the development and differentiation of hematopoietic progenitors from murine ES cells in vitro (Nakano et al., 1994, 1996; Nakano, 1995). In this system, co-cultivation of ES cells on the stromal cell line OP9 gives rise to primitive erythrocytes and definitive multipotential hematopoietic progenitor cells after 5 days of differentiation induction. Using a tetracycline (Tet)-regulatable expression system, we conditionally activated exogenous GATA-2 expression at day 5 of culture and observed enhanced hematopoiesis as a result (Gossen and Bujard, 1992; Era and Witte, 2000). In marked contrast, conditional activation of a GATA-2/ER chimeric molecule by addition of β-estradiol produced the opposite effect. Critically, these results were obtained in the same in vitro differentiation assay system and thus the differences in biological output could be attributed directly to the differences in the two GATA-2 molecules used. Key differences in the molecular properties of GATA-2 and GATA-2/ER presumably underlying these differences in biological activity are described.
Results
Tet-regulatable GATA-2 expression
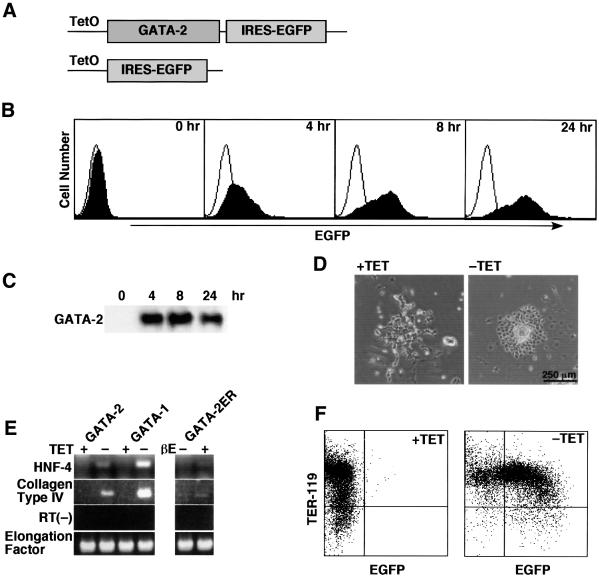
Since ES cell clones constitutively expressing GATA-2 could not be established in our hands (data not shown), we switched to using a conditional expression system. Thus, a Tet-regulatable bicistronic vector encoding GATA-2 and the enhanced green fluorescent protein (EGFP) was introduced into ES cells (Figure 1A). ES cell lines were established in the presence of tetracycline to avoid the expression of exogenous GATA-2. Conditional activation of exogenous GATA-2 and EGFP expression was achieved by withdrawal of tetracycline. Conditional expression of these genes in ES cells was verified by western blot analysis of GATA-2 and fluorescence-activated cell sorting (FACS) analysis of EGFP expression. As shown in Figure 1B and C, significant expression of GATA-2 and EGFP was achieved at 4 h after the cells were deprived of tetracycline, and continued thereafter. The expression of GATA-2 in ES cells induced endodermal differentiation (Figure 1D) and a block in proliferation (data not shown) of ES cells, thus demonstrating that conditional expression of GATA-2 is essential for this experiment. Tet-regulatable expression of GATA-2 and EGFP could be demonstrated not only in ES cells but also in erythroid lineage cells derived from them. Thus, about three-quarters of the TER-119-positive erythroid cells showed EGFP expression (Figure 1F), and Tet-regulated GATA-2 expression was also evident in western blot analyses of cells at day 8 of differentiation induction (data not shown).
Fig. 1. Conditional expression of GATA-2 and EGFP. (A) Constructs of GATA-2/IRES–EGFP and control IRES–EGFP. TetO stands for the tetracycline-responsive element. (B and C) FACS and western blot analyses of the time course of EGFP and GATA-2 expression in ES cells after being deprived of tetracycline. (D) Morphological change of ES cells by depriving them of tetracycline. (E) Expression of primitive endoderm differentiation markers, HNF-4 and collagen type IV, 4 days after the expression of GATA-2, GATA-1 and activated GATA-2/ER. Expression of GATA-2 and GATA-1 was induced by depriving the cells of tetracycline. The activated GATA-2/ER was obtained by depriving the cells of tetracycline and by the addition of β-estradiol (βE). The primer set for collagen type IV was used for the RT(–) control. (F) Expression of EGFP in TER-119-expressing erythroid cells induced from ES cells. The cells were deprived of tetracycline from day 5 of induction and the data were obtained 3 days later.
Enhancement of hematopoietic progenitor formation by GATA-2
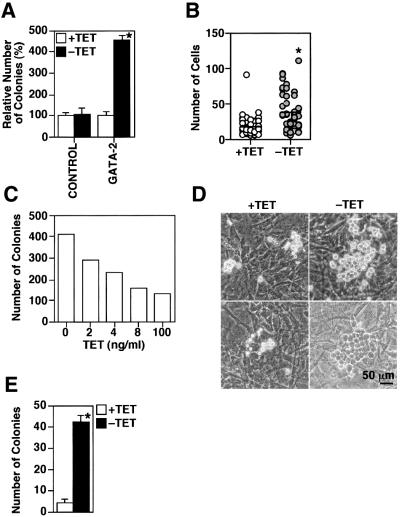
In the OP9 system, primitive erythrocytes and definitive multipotential hematopoietic progenitors develop at day 5 of differentiation induction (Nakano et al., 1994, 1996; Nakano, 1995). GATA-2 expression was therefore induced after day 5 in most experiments to allow examination of its function in hematopoiesis. First, hematopoietic colonies and the cells in individual colonies were counted 2 days after induction of GATA-2 expression. GATA-2 expression brought an ∼4- to 5-fold increase in colony numbers (Figure 2A) and the numbers of the cells in the individual colonies increased 2-fold (Figure 2B). Dose-dependent effects of GATA-2 were examined by the addition of different amounts of tetracycline; gene expression is inversely correlated with the concentration of tetracycline added in the Tet-regulatable system (Gossen and Bujard, 1992). The more GATA-2 was induced, the more hematopoietic colonies appeared (Figure 2C), indicating that the increase was dependent on GATA-2 function. Immature hematopoietic cells grow underneath stroma cells and look darker. This phenomenon is known as ‘pseudoemperipolesis’ and is indicative of the immaturity of hematopoietic cells (Figure 2D). The number of the colonies showing pseudoemperipolesis was increased by 7- to 10-fold by GATA-2 expression (Figure 2E). A block or delay in differentiation by overexpression of GATA-2 might bring about the increase of immature hematopoietic cells. Taken together, expression of GATA-2 increased the number of immature hematopoietic cells up to 10-fold.
Fig. 2. Effect of induced GATA-2 on immature hematopoietic colony formation at day 7 of induction. A total of 105 day 5 induced cells were seeded onto the OP9 stroma cell layer and the cells were cultured in the presence or absence of tetracycline. All the analyses were carried out at day 7 of induction. (A) Relative number of colonies in control and Tet-regulated GATA-2 clones. Data are shown as the mean ± SD of six samples (*P < 0.01 by t-test). (B) Numbers of cells in individual colonies in the presence or absence of tetracycline. GATA-2 increased the number of cells significantly (*P < 0.01 by t-test). (C) Numbers of colonies in the culture containing various concentrations of tetracycline. (D) Typical hematopoietic colonies in the presence (left panels) or absence (right panels) of tetracycline. A colony grown underneath the OP9 stroma cells with a cobble stone appearance (pseudoemperipolesis) is shown in the right lower panel. (E) Number of colonies showing pseudoemperipolesis. Data are shown as the mean ± SD of six samples (*P < 0.01 by t-test).
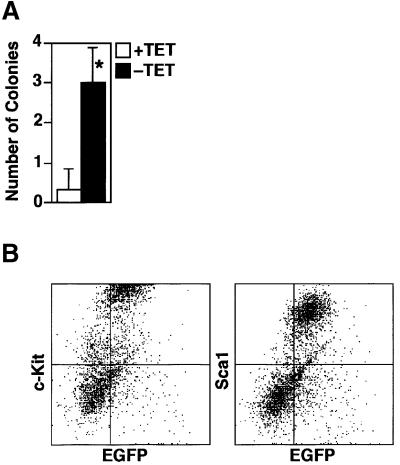
After day 14 of induction of differentiation, the vast majority of wild-type cells in the induced cultures complete terminal differentiation and are detached from the stroma. Subsequently, there were very few hematopoietic colonies at day 15 of induction. However, significant numbers of hematopoietic colonies remained at day 15 of induction when GATA-2 was conditionally expressed (Figure 3A). Furthermore, FACS analysis showed that a significant percentage of the GATA-2-expressing cell population expressed surface markers of immature hematopoietic cells such as c-Kit and Sca-1 at day 18 (Figure 3B).
Fig. 3. Number of hematopoietic colonies at day 15 and surface marker profile of day 18 induced cells. (A) Hematopoietic colonies at day 15 of induction. Data are shown as the mean ± SD of six samples (*P < 0.01 by t-test). (B) c-Kit and Sca-1 expression on the cells persistent at day 18 of induction with the expression of GATA-2. The majority of EGFP-positive cells expressed c-Kit and Sca-1, markers of immature hematopoietic cells.
Effect of GATA-2 on differentiation of erythrocytes and megakaryocytes
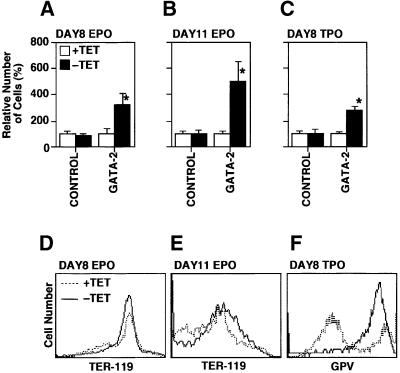
Forced expression experiments using GATA-2 and GATA-2/ER have yielded effects on both erythroid and megakaryocytic differentiation. Thus we examined the effect of enforced expression of GATA-2 on the production of erythrocytes and megakaryocytes by the addition of erythropoietin (EPO) and thrombopoietin (TPO) from day 5 of differentiation induction in our ES cell system. Differentiation induction at days 8 and 11 in the presence of EPO represented the differentiation of yolk sac-type primitive erythrocytes and adult-type definitive erythrocytes, respectively (Nakano et al., 1996). Megakaryocyte production was examined at day 8 of induction in the presence of TPO (Era et al., 2000). Total cell numbers at days 8 and 11 in the presence of EPO and those at day 8 in the presence of TPO are shown in Figure 4A–C. Cell surface expression patterns of an erythroid marker, TER-119 (Ikuta et al., 1990), and a megakaryocyte marker, platelet glycoprotein V (GPV) (Takada et al., 1995; Sato et al., 2000), on the cells induced in the presence of EPO and TPO, respectively, are shown in Figure 4D–F. Enforced expression of GATA-2 increased the number of primitive erythrocytes, definitive erythrocytes and megakaryocytes by ∼4-, ∼7- and ∼7-fold, respectively. These results clearly demonstrate that GATA-2 did not block the differentiation of erythroid and megakaryocyte lineages, but rather increased the production of these cells significantly.
Fig. 4. Numbers and lineage marker profile of the cells induced from ES cells in the presence of EPO or TPO. (A–C) Numbers of cells at days 8 and 11 in the presence of EPO or TPO as described. GATA-2 expression increased the numbers of cell significantly (*P < 0.01 by t-test). Data are shown as the mean ± SD of six samples. (D–F) Surface expression of lineage markers TER-119 and platelet GPV.
Effect of GATA-2 on terminal differentiation of macrophages
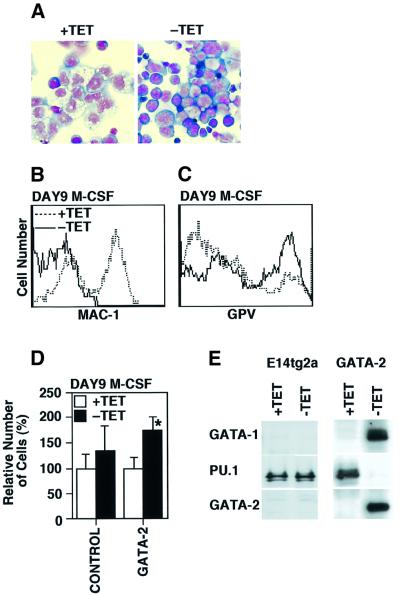
Macrophage colony-stimulating factor (M-CSF) induces preferential differentiation and proliferation of macrophages in the OP9 system (Era et al., 2000). The effects of manipulating GATA-2 expression during macrophage differentiation were analyzed by adding M-CSF from day 5 of induction. Morphological examination showed that none of the induced cells expressed the macrophage phenotype when GATA-2 was conditionally expressed (Figure 5A). Furthermore, almost none of the cells in these cultures express the macrophage-associated cell surface marker Mac-1, whereas more than half the cells in control cultures express Mac-1 by day 9 of differentiation induction (Figure 5B).
Fig. 5. ‘Lineage switch’ from macrophage to megakaryocyte by GATA-2. (A) Morphology of the cells at day 9 of induction in the presence of M-CSF. The characteristics of macrophages were lost in the absence of tetracycline. (B and C) Expression of Mac-1 and platelet GPV in the presence or absence of tetracycline. (D) Cell numbers of control (P > 0.05 by t-test) and GATA-2 clones (*P < 0.01) in the presence or absence of tetracycline at day 9 of induction with M-CSF. (E) Western blot analysis of GATA-1, PU.1 and GATA-2 of control E14tg2a ES cells and GATA-2 clones in the presence or absence of tetracycline.
Experiments of enforced expression of GATA-2 in a couple of cell lines have been reported to result in ‘lineage switch’ to megakaryocytes, which prompted us to examine the expression of megakaryocyte-specific platelet GPV at day 9 of induction in the presence of M-CSF (Visvader and Adams, 1993; Ikonomi et al., 2000). GATA-2 expression increased the numbers of total cells and the percentage of GPV-positive cells significantly (Figure 5C and D). The cells at day 9 of induction were analyzed by western blot analysis to examine the expression of GATA-1, GATA-2 and PU.1. Although control cells expressed PU.1, the cells whose lineage was ‘switched’ by enforced GATA-2 expression did not express PU.1 (Figure 5E); instead, those cells expressed GATA-1 as well as GATA-2.
Biological effects of GATA-2/ER and GATA-1
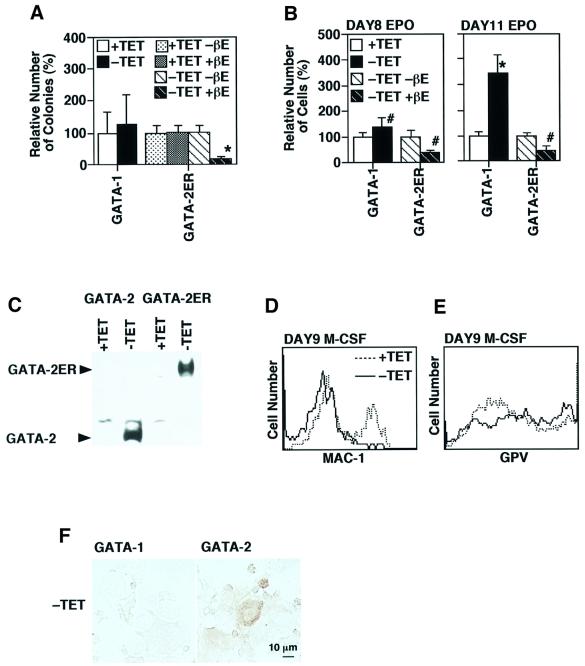
Our data on the function of GATA-2 are quite different, indeed almost opposite to those observed when GATA-2/ER expression was forced in the multipotential progenitor cell line FDCP-mix. In those experiments, activation of GATA-2/ER function by addition of estradiol led to an inhibition of proliferative self-renewal and the differentiation of cells, preferentially down the neutrophil and macrophage pathways. We therefore compared the function of GATA-2 with that of activated GATA-2/ER in the ES cell experimental system. In this series of experiments, we also compared the activity of GATA-2 with that of another GATA factor family member, GATA-1. The efficiency of the induction of differentiation to hematopoietic progenitor cells and various erythroid lineage cells was essentially the same among the control, GATA-1-, GATA-2- and GATA-2/ER-expressing clones in the presence of tetracycline (data not shown). First, the numbers of hematopoietic colonies at day 7 of induction were examined (Figure 6A). GATA-1 increased the numbers a little, which was reproducible but statistically non-significant. On the contrary, the number of hematopoietic colonies was reduced to ∼20% by the activated GATA-2/ER. Neither the addition of β-estradiol without GATA-2/ER expression nor the expression of GATA-2/ER in the absence of β-estradiol affected the numbers of colonies. Also, β-estradiol did not show any effects on GATA-2 function (data not shown).
Fig. 6. Effects of GATA-1 and the activated GATA-2/ER on hematopoietic colony formation and differentiation. (A) Effects on day 7 colony formation. A significant reduction in the cell number by the enforced expression of GATA-2/ER was observed in the presence of β-estradiol (*P < 0.01 by t-test). (B) Effects of GATA-1 and the activated GATA-2/ER on erythrocyte production in the presence of EPO. GATA-1 and the activated GATA-2/ER gave rise to a significant increase and decrease of the erythroid lineage cells, respectively (*P < 0.01, #P < 0.05 by t-test). Data are shown as the mean ± SD of six samples. (C) Western blot analyses of GATA-2 and GATA-2/ER in the day 8 differentiation-induced cells. (D and E) Expression of Mac-1 and platelet GPV at day 9 of culture in the presence of M-CSF. (F) Expression of megakaryocyte-specific acetylcholine esterase by overexpression of GATA-1 and GATA-2.
The effects of GATA-1 and activated GATA-2/ER on erythroid differentiation were examined in the presence of EPO using the same experimental scheme outlined in Figure 4. The production of primitive and definitive erythrocytes was enhanced by GATA-1 as well as by GATA-2 (Figure 6B). On the contrary, not only the production of definitive erythrocytes, but also that of primitive erythrocytes was significantly inhibited by the activated GATA-2/ER (Figure 6B). This difference between GATA-2 and GATA-2/ER is attributable to the functional difference between GATA-2 and GATA-2/ER, since the expression levels of GATA-2 and GATA-2/ER were similar (Figure 6C). We confirmed that the addition of β-estradiol showed no effects on erythrocyte production with or without induced expression of GATA-1 or GATA-2 (data not shown).
GATA-1 also appeared to prevent macrophage differentiation (Figure 6D). However, GATA-1 did not induce a significant increase in the expression of the megakaryocyte marker GPV (Figure 6E) or expression of a megakaryocyte-specific acetylcholine esterase (Figure 6F). Also, no significant increase in megakaryocyte cell numbers was observed (data not shown). It was a little difficult to analyze the effects of the activated GATA-2/ER on this apparent ‘lineage switch’, because hematopoietic colony formation was so severely impaired by activated GATA-2/ER (Figure 6A) and efficient stimulation by M-CSF could not be achieved.
Different biological effects of GATA-1, GATA-2 and activated GATA-2/ER could also be observed in ES cells (Figure 1E). No endodermal colony formation could be observed in control, GATA-2 or GATA-2/ER clones in the presence of tetracycline. In contrast, ∼14% (14.2 ± 3.6%, n = 6) and only ∼1% (1.0 ± 1.0%, n = 6) of colonies expressed endodermal characteristics (P < 0.01 by t-test) by overexpression of GATA-2 and GATA-2ER with β-estradiol, respectively.
Different transcriptional and binding activity of GATA-2 and GATA-2/ER
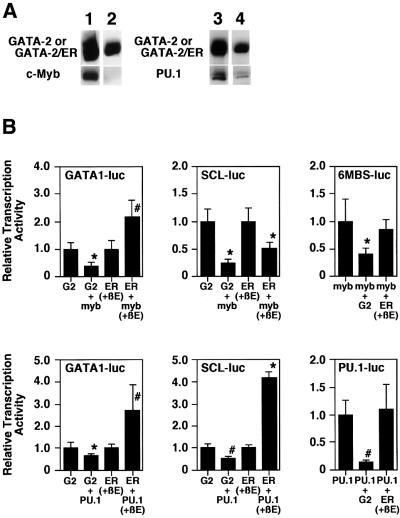
In order to explore further the differences between GATA-2 and GATA-2/ER, we analyzed the physical interaction and transcriptional interference properties of GATA-2 and GATA-2/ER with two other hematopoietic transcription factors, c-Myb and PU.1, both of which interact with GATA-2 (Zhang et al., 1999; our unpublished data). Although co-immunoprecipitation experiments demonstrated that GATA-2 physically bound to c-Myb, no binding of GATA-2/ER to c-Myb could be found in the presence or absence of β-estradiol (Figure 7A). Similarly, the binding of PU.1 to GATA-2/ER was significantly weaker than that to GATA-2. In the PU.1 co-immunoprecipitation experiment, although the amount of precipitated GATA-2/ER was 56% that of GATA-2, the amount of PU.1 co-immunoprecipitated with GATA-2/ER was only 19% of that with GATA-2. Thus, the binding of PU.1 to GATA-2/ER was about one-third of that to GATA-2.
Fig. 7. Differential binding activity and transcriptional activity of GATA-2 and the activated GATA-2/ER. (A) Co-immunoprecipitation analysis of c-Myb and PU.1. 293T cells were transfected with expression plasmids of FLAG-GATA-2 and c-Myb (lane 1), FLAG-GATA-2/ER and c-Myb (lane 2), FLAG-GATA-2 and PU.1 (lane 3) and FLAG-GATA-2/ER and PU.1 (lane 4). β-estradiol was added in the experiments of FLAG-GATA-2/ER (lanes 2 and 4); however, the data were essentially the same in the absence of β-estradiol (data not shown). Total cell lysates were immunoprecipitated with anti-FLAG antibody. The precipitated protein was electrophoresed by SDS–PAGE, blotted and hybridized with the anti-FLAG antibody (upper panels), anti- c-Myb antibody (lower panels of lanes 1 and 2) and anti-PU.1 antibody (lower panels of lanes 3 and 4). (B) Reporter gene analysis of various promoters. Reporter luciferase genes are as described in each panel. G2 and ER stand for the transfection of the GATA-2-expressing plasmid and the GATA-2/ER-expressing plasmid, respectively. When the GATA-2/ER expression plasmid was transfected, β-estradiol was added to activate the protein. An 8 µg aliquot of individual expression plasmids and reporter gene plasmids was transfected into CV-1 cells. Data are shown as the mean ± SD of nine samples (*P < 0.01; #P < 0.05 by t-test).
Like the transcriptional interference observed between GATA-1 and c-Myb, transcriptional activation of GATA-2 and c-Myb is mutually inhibited (unpublished data). Thus, the transcriptional interaction of GATA-2 and GATA-2/ER with c-Myb was analyzed (Figure 7B). When a GATA-1 promoter (GATA1-luc) was used, c-Myb inhibited GATA-2-induced transactivation but synergistically enhanced the transactivation with activated GATA-2/ER. On the other hand, c-Myb inhibited the transactivation of the SCL promoter (SCL-luc) induced by GATA-2 as well as that induced by the activated GATA-2/ER. Analysis of the synthetic Myb-responsive promoter (6MBS-Luc) showed that GATA-2 inhibited c-Myb-dependent activation, while activated GATA-2/ER did not show significant effects.
Mutual transcriptional inhibition between GATA-2 and PU.1 has also been reported (Zhang et al., 1999). Analyses using the GATA-1 and SCL promoters showed that PU.1 inhibited GATA-2-induced activation while it synergized with GATA-2/ER (Figure 7B). In addition, PU.1-induced transactivation of a synthetic PU.1 promoter (PU.1-luc) was inhibited by GATA-2 but was not influenced by activated GATA-2/ER.
Discussion
We have analyzed the function of GATA-2, GATA-2/ER and GATA-1 using a Tet-regulated conditional expression method in the context of the OP9 ES cell differentiation induction system. Expression of GATA-2 in hematopoietic progenitors increased the number of immature hematopoietic cells, while GATA-2/ER had the opposite effect, i.e. it inhibited progenitor colony formation. In addition, macrophage differentiation was inhibited by GATA-2, and a ‘lineage switch’ to megakaryocyte formation was observed.
Enhanced hematopoiesis by GATA-2
Conditional enforcement of GATA-2 expression in ES cell-derived hematopoietic cells produced on OP9 stromal cells led to an increase in immature hematopoietic colonies, and this increase was dose dependent with respect to exogenous GATA-2 expression. These data clearly show that GATA-2 can function to increase the proliferation of hematopoietic progenitors. The notion that GATA-2 enhances the production of hematopoietic progenitors is consistent with the phenotype of GATA-2 null mice (Tsai et al., 1994; Tsai and Orkin, 1997), and thus we assume that this effect could be of physiological relevance. However, the molecular mechanisms by which GATA-2 enhances the production of hematopoietic progenitors remain unclear. Using our experimental system, we are now searching for the GATA-2 target genes and the proteins that function synergistically with GATA-2 to increase hematopoietic progenitors.
Persons et al. (1999) have achieved enforced expression of GATA-2 in hematopoietic stem cells through retroviral transduction. In these experiments, GATA-2 blocked the amplification and differentiation of pluripotent hematopoietic stem cells. We speculate that GATA-2 has different functions in hematopoietic stem cells and progenitor cells. Down-regulation of GATA-2 might occur during the transition from a stem cell to progenitors, and this could be a critical step of hematopoietic differentiation. This does not necessarily require actual reduction of GATA-2 expression, as functional inhibition by other transcription factors such as c-Myb or PU.1 might be involved in this process.
Effects of GATA-2 on erythroid and megakaryocyte differentiation
In contrast to control ES cells, forced expression of exogenous GATA-2 resulted in the presence of significant numbers of hematopoietic progenitors at day 15 after induction. This differentiation inhibition was not complete, since the numbers of erythrocytes and megakaryocytes were also increased. The increased numbers of immature hematopoietic cells brought about by exogenous GATA-2 expression can account, to some extent, for the enhanced production of mature erythrocytes and megakaryocytes. However, the enhancement cannot be attributed fully to the increased hematopoietic progenitors for the following reasons. First, not only definitive but also primitive erythrocytes were increased by exogenous GATA-2 expression. Increased primitive erythrocyte development is unlinked to the increase in multipotential hematopoietic progenitors, since primitive erythrocytes are not differentiated from these hematopoietic progenitors, as we have shown previously (Nakano et al., 1996). Secondly, enhanced production of definitive erythrocytes and megakaryocytes was still observed when Tet-regulated GATA-2 expression was switched on from day 8 of induction (data not shown). Thirdly, when Tet-regulated GATA-2 was induced only between days 5 and 8, the extent of the increase of definitive erythrocytes was less than half that observed when exogenous GATA-2 expression was maintained from day 5 to day 12 (data not shown). Our results indicating that enforced GATA-2 expression promotes the terminal differentiation of erythroid cells are at odds with results obtained using a GATA-2/ER chimeric molecule in chicken erythroid progenitors, where enforced expression was shown to inhibit differentiation (Briegel et al., 1993). Also, expression of a GATA-2/ER molecule in multipotential mouse progenitor cells promoted differentiation down the myelomonocytic pathway and blocked differentiation down the erythroid pathway. However, expression of GATA-2/ER in mouse erythroid cell lines promotes their differentiation (T.Enver, unpublished data), emphasizing the importance of cell stage and context in determining the outcome of these manipulations.
‘Lineage switch’ into megakaryocytes
In normal differentiation induction, M-CSF stimulates macrophage differentiation and proliferation. However, forced expression of GATA-2 altered the differentiation process and, instead, induced megakaryocyte-specific marker expression. Several reports have provided evidence that the enforced expression of GATA-2 induces megakaryocytic differentiation of myeloid and erythroid cell lines (Visvader and Adams, 1993; Ikonomi et al., 2000). Our present data are consistent with these reports and provide the first evidence that enforced expression of GATA-2 can cause a lineage alteration of normal hematopoietic cells, including induction of GATA-1. However, this is probably not a lineage switch but rather an effect on commitment. Thus, when GATA-2 was turned on from day 5 of induction, not only loss of Mac-1 expression but also induction of GPV expression occurred. In contrast, when GATA-2 was turned on from day 8 of induction, the former still occurred but the latter did not (data not shown). This suggests that, once commitment to the macrophage lineage is established, the expression of megakaryocyte lineage markers is suppressed by some as yet unknown mechanism, despite the fact that macrophage marker expression is repressed by GATA-2.
One intriguing aspect of the lineage switch is the functional difference between GATA-1 and GATA-2. GATA-2 and subsequent GATA-1 expression abolished Mac-1 expression and induced megakaryocytic marker expression. It has been reported that GATA-1 can induce a megakaryocytic lineage switch in various cell lines (Visvader et al., 1992, 1995; Yamaguchi et al., 1998). However, as shown here, GATA-1 inhibited Mac-1 expression but did not induce megakaryocytic marker expression. There are two possibilities to account for this result. One possibility is that the co-existence of GATA-1 and GATA-2 is necessary for the switch. The other possibility is that the sequential expression of GATA-2 and GATA-1 is important. Considering the sequential expression of GATA-2 and GATA-1 in normal hematopoietic differentiation, the latter possibility seems likely.
Comparison between GATA-2 and GATA-2/ER
Fusion of a transcription factor to the ligand-binding domain of the ER is a commonly used strategy to analyze the function of transcription factors. We have used this strategy to analyze the function of GATA-2. GATA-2/ER inhibited the proliferation of hematopoietic progenitors and the terminal differentiation of erythroid lineage cells in our ES cell in vitro differentiation system. Strikingly, the function of GATA-2/ER appears opposite to that of GATA-2. These differences in activity must relate directly to differences in the GATA-2 and GATA-2/ER molecules themselves, since the cellular context is the same in all our experiments. Although reporter gene assays revealed that the chimeric protein could activate transcription effectively, the roles of the chimeric protein in the complex process of cell differentiation may not be predictable from these simple transactivation assays (Schuermann et al., 1993). In particular, cell differentiation has been shown to be regulated by the combinatorial interaction of various transcription factors (Ness and Engel, 1994; Sieweke and Graf, 1998). GATA-2 physically binds to PU.1 and c-Myb, both of which play important roles in hematopoietic differentiation. However, GATA-2/ER could not bind to c-Myb and could bind to PU.1 only weakly. The differences in properties of GATA-2 and GATA-2/ER were not only restricted to their capacity for physical association with other regulatory transcription factors, but also extended to their functional activity on transcription. These different characteristics presumably underlie the divergent biological functions of GATA-2 and its chimeric GATA-2/ER counterpart. The ER ligand-binding domain is widely used to produce ligand-inducible chimeric proteins for experimental analysis. Our results suggest that caution should be exercized in validating the authenticity of their function, and care taken in interpreting results obtained with chimeric proteins alone. However, where they exist, differences in the physical and biological properties of authentic versus chimeric proteins may be exploited to gain additional information about the prevailing networks of regulatory interactions and their role in cell differentiation.
Materials and methods
Establishment of Tet-regulated ES cell clones and differentiation induction
E14tg2a ES cells and their clones were utilized in this study. These cells were maintained as described previously (Niwa et al., 1998). Culture of OP9 stromal cells and in vitro differentiation induction to hematopoietic cells from ES cells on OP9 cells were performed as described previously (Nakano et al., 1994, 1996).
The Tet regulatory system was used to obtain GATA-2-, GATA-1- and GATA-2/ER-regulatable ES cell clones (Gossen and Bujard, 1992). The expression of these genes was driven by a modified version of the Tet system recently developed by Era and Witte (2000). Tet-regulatable GATA-2, GATA-1 and GATA-2/ER constructs were generated by inserting mouse GATA-2, GATA-1 (a kind gift of Dr Yamamoto, Tsukuba University, Tsukuba, Japan) and human GATA-2/ER cDNA into the EcoRI or NotI site of pUHD10-3.IRES–EGFP (Era and Witte, 2000), respectively.
The production of Tet-regulated clones was carried out essentially as described. First, an E14tg2a ES cell clone, ES2-1, which constitutively expresses the modified Tet-regulated transactivator driven by the CAG promoter, was established. Then, the linearized plasmids of bicistronic Tet-regulatable GATA-2-IRES–EGFP, GATA-1-IRES–EGFP and GATA-2/ER-IRES–EGFP genes (30 µg) were co-transfected with the neomycin plasmid pPGKneo (1 µg) into 8 × 106 ES2-1 cells. For gene introduction, electroporation was carried out at 0.23 kV and 500 µF using Gene Pulser II (Bio-Rad, Hercules, CA). The cells were selected by 200 µg/ml G418 (Sigma, St Louis, MO) for 7–10 days in the presence of 1 µg/ml tetracycline (Sigma). The addition of tetracycline is essential to avoid the expression of Tet-regulatable genes in ES cells. The clones whose EGFP expression was tightly regulated by tetracycline were selected. Among these clones, the expression of the desirable genes was examined by western blotting as described below. In addition, at least three clones in which GATA expression was tightly regulated were analyzed. Data of the representative clones are given herein. To activate the GATA-2/ER protein, 2 µM β-estradiol (Sigma) was added.
Flow cytometry
Phycoerythrin (PE)-conjugated anti-mouse Mac-1 (M1.70), Sca-1 (IOT-6A.2) and c-kit (3C1) antibodies were purchased from Immunotech (Marseille, France). Biotinylated anti-GPV (1C2) and TER-119 antibodies were a kind gift of Dr J.Fujimoto (National Children’s Medical Research Center, Tokyo, Japan) and Dr T.Kina (Kyoto University, Kyoto, Japan), respectively. The biotinylated antibodies were visualized by PE-conjugated streptavidin (PharMingen, San Diego, CA). The cells were harvested by mild pipetting, resuspended in Ca, Mg-free phosphate-buffered saline (PBS) containing 5% fetal calf serum, and stained with the PE-conjugated antibodies or the biotinylated antibodies followed by PE-conjugated streptavidin. The stained cells were analyzed by FACSCalibur (Becton Dickinson, Franklin Lakes, NJ).
RT–PCR
Total RNA was recovered by RNeasy mini kit (Qiagen, Valencia, CA), and 1 µg of total RNA was used for cDNA synthesis. The reverse transcription was performed using the ThermoScript RT–PCR system (Gibco BRL, Rockville, MD). The primer sequences and PCR conditions were described previously (Keller et al., 1993; Tanaka et al., 1998; Kuramochi-Miyagawa et al., 2001).
Western blotting and co-immunoprecipitation analysis
The ES cells or the induced blood cells (2 × 105) were suspended in 15 µl of 3% SDS, 10% glycerol, 5% 2-mercaptoethanol and 62.5 mM Tris–HCl pH 6.8. The protein samples were electrophoresed on a 10–20% gradient SDS–Tris-glycine gel (BIO CRAFT, Tokyo, Japan), and transferred to an Immobilon PVDF membrane (Millipore, Bedford, MA). The membranes were probed with the rat monoclonal anti-GATA-1 antibody (N6), rabbit polyclonal anti-GATA-2 antibody (H-116) and rabbit polyclonal anti-PU.1 antibody (T-21), all purchased from Santa Cruz (Santa Cruz, CA). The horseradish peroxidase (HRP)-conjugated anti-rat and rabbit IgG antibodies (Zymed, San Francisco, CA) were used as secondary antibody. The membranes were stained with an enhanced chemiluminescence (ECL) detection kit (Amersham Pharmacia, Buckinghamshire, UK).
293T cells were transfected with pCMV-FLAG-GATA-2 or pCAG-FLAG-GATA-2/ERT, and pAct-c-myb (a kind gift from Dr S.Ishii, the Institute of Physical and Chemical Research, Ibaragi, Japan) or pEF-PU.1 (a kind gift from Dr T.Oikawa, Sasaki Institute, Tokyo, Japan), by the calcium phosphate method. The cell lysates from the transfected 293T cells were incubated with the mouse monoclonal anti-FLAG antibody (M2, Sigma) in the binding buffer as described previously (Zhang et al., 1999). The protein complexes bound to the antibody were precipitated by protein G–Sepharose (Amersham Pharmacia) and analyzed by western blotting using mouse monoclonal anti-c-myb antibody (clone 1-1, Upstate biotechnology, Lake Placid, NY) and the anti-PU.1 antibody, followed by the incubation with HRP-conjugated anti-mouse IgG antibody (Zymed) and the anti-rabbit IgG antibody, respectively.
Reporter gene analysis
The reporter genes, SCL-luc, GATA-1-luc, 6MBS-luc (Takahashi et al., 2000) and PU.1-Luc [pGL3-PU(×3)-promoter, kindly provided by Dr T.Oikawa] (Yamamoto et al., 1999), were transfected into CV-1 cells by the calcium phosphate method as previously described (Takahashi et al., 2000) with the various combinations of the effecter genes, pOPI3-GATA-2, pOPI3-GATA-2-ER, pAct-c-myb and pEF-PU.1. The reporter gene activities were measured by the dual-luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer’s recommendation. The transfection efficiency was corrected by the activities of co-transfected pRL-TK (Promega) which encodes Renilla luciferase under the TK promoter.
Acknowledgments
Acknowledgements
The authors thank Dr G.May for valuable discussions and the kind gift, and Drs T.Kina, J.Fujimoto, M.Yamamoto and T.Oikawa for valuable reagents. We also thank Ms Mizokami for secretarial work. This work was supported in part by grants from the Ministry of Education, Science, Sports and Culture and the Japanese Society for Promotion of Sciences (JSPS-RFTF98L01101).
References
- Briegel K., Lim,K.C., Plank,C., Beug,H., Engel,J.D. and Zenke,M. (1993) Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev., 7, 1097–1109. [DOI] [PubMed] [Google Scholar]
- Era T. and Witte,O.N. (2000) Regulated expression of P210 Bcr-Abl during embryonic stem cell differentiation stimulates multipotential progenitor expansion and myeloid cell fate. Proc. Natl Acad. Sci. USA, 97, 1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Era T., Takagi,T., Takahashi,T., Bories,J.C. and Nakano,T. (2000) Characterization of hematopoietic lineage-specific gene expression by ES cell in vitro differentiation induction system. Blood, 95, 870–878. [PubMed] [Google Scholar]
- Evans T. and Felsenfeld,G. (1989) The erythroid-specific transcription factor Eryf1: a new finger protein. Cell, 58, 877–885. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Browne,C.P., Cunniff,K., Goff,S.C. and Orkin,S.H. (1996) Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl Acad. Sci. USA, 93, 12355–12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M. and Bujard,H. (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl Acad. Sci. USA, 89, 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C., Gale,K., Dexter,M., May,G. and Enver,T. (1999) A GATA-2/estrogen receptor chimera functions as a ligand-dependent negative regulator of self-renewal. Genes Dev., 13, 1847–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomi P., Rivera,C.E., Riordan,M., Washington,G., Schechter,A.N. and Noguchi,C.T. (2000) Overexpression of GATA-2 inhibits erythroid and promotes megakaryocyte differentiation. Exp. Hematol., 28, 1423–1431. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Kina,T., MacNeil,I., Uchida,N., Peault,B., Chien,Y.H. and Weissman,I.L. (1990) A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell, 62, 863–874. [DOI] [PubMed] [Google Scholar]
- Keller G., Kennedy,M., Papayannopoulou,T. and Wiles,M.V. (1993) Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol. Cell. Biol., 13, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko L.J., Yamamoto,M., Leonard,M.W., George,K.M., Ting,P. and Engel,J.D. (1991) Murine and human T-lymphocyte GATA-3 factors mediate transcription through a cis-regulatory element within the human T-cell receptor δ gene enhancer. Mol. Cell. Biol., 11, 2778–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi-Miyagawa S., Kimura,T., Yomogida,K., Kuroiwa,A., Tadokoro, Y., Fujita,Y., Sato,M., Matsuda,Y. and Nakano,T. (2001) Two mouse piwi-related genes: miwi and mili. Mech. Dev., 108, 121–133. [DOI] [PubMed] [Google Scholar]
- Landry D.B., Engel,J.D. and Sen,R. (1993) Functional GATA-3 binding sites within murine CD8α upstream regulatory sequences. J. Exp. Med., 178, 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard M., Brice,M., Engel,J.D. and Papayannopoulou,T. (1993) Dynamics of GATA transcription factor expression during erythroid differentiation. Blood, 82, 1071–1079. [PubMed] [Google Scholar]
- Martin D.I., Zon,L.I., Mutter,G. and Orkin,S.H. (1990) Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature, 344, 444–447. [DOI] [PubMed] [Google Scholar]
- Mouthon M.A., Bernard,O., Mitjavila,M.T., Romeo,P.H., Vainchenker,W. and Mathieu-Mahul,D. (1993) Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood, 81, 647–655. [PubMed] [Google Scholar]
- Nakano T. (1995) Lymphohematopoietic development from embryonic stem cells in vitro. Semin. Immunol., 7, 197–203. [DOI] [PubMed] [Google Scholar]
- Nakano T., Kodama,H. and Honjo,T. (1994) Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science, 265, 1098–1101. [DOI] [PubMed] [Google Scholar]
- Nakano T., Kodama,H. and Honjo,T. (1996) In vitro development of primitive and definitive erythrocytes from different precursors. Science, 272, 722–724. [DOI] [PubMed] [Google Scholar]
- Ness S.A. and Engel,J.D. (1994) Vintage reds and whites: combinatorial transcription factor utilization in hematopoietic differentiation. Curr. Opin. Genet. Dev., 4, 718–724. [DOI] [PubMed] [Google Scholar]
- Niwa H., Burdon,T., Chambers,I. and Smith,A. (1998) Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev., 12, 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S.H. (1992) GATA-binding transcription factors in hematopoietic cells. Blood, 80, 575–581. [PubMed] [Google Scholar]
- Orkin S.H. (2001) Transcription factors that regulate lineage decisions. In Stamatoyannopoulos,G., Majerus,P.W., Perlmutter,R.M. and Varmus,H. (eds), The Molecular Basis of Blood Diseases. W.B.Saunders, Philadelphia, PA, pp. 80–102.
- Papayannopoulou T. and Lemischka,I. (2001) Stem cell biology. In Stamatoyannopoulos,G., Majerus,P.W., Perlmutter,R.M. and Varmus,H. (eds), The Molecular Basis of Blood Diseases. W.B.Saunders, Philadelphia, PA, pp. 1–24.
- Persons D.A., Allay,J.A., Allay,E.R., Ashmun,R.A., Orlic,D., Jane,S.M., Cunningham,J.M. and Nienhuis,A.W. (1999) Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood, 93, 488–499. [PubMed] [Google Scholar]
- Romeo P.H., Prandini,M.H., Joulin,V., Mignotte,V., Prenant,M., Vainchenker,W., Marguerie,G. and Uzan,G. (1990) Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature, 344, 447–449. [DOI] [PubMed] [Google Scholar]
- Sato N. et al. (2000) Characterization of monoclonal antibodies against mouse and rat platelet glycoprotein V (CD42d). Hybridoma, 19, 455–461. [DOI] [PubMed] [Google Scholar]
- Schuermann M., Hennig,G. and Muller,R. (1993) Transcriptional activation and transformation by chimaeric Fos–estrogen receptor proteins: altered properties as a consequence of gene fusion. Oncogene, 8, 2781–2790. [PubMed] [Google Scholar]
- Shivdasani R.A. and Orkin,S.H. (1996) The transcriptional control of hematopoiesis. Blood, 87, 4025–4039. [PubMed] [Google Scholar]
- Sieweke M.H. and Graf,T. (1998) A transcription factor party during blood cell differentiation. Curr. Opin. Genet. Dev., 8, 545–551. [DOI] [PubMed] [Google Scholar]
- Takada K., Saito,M., Kaneko,H., Iizuka,K., Kokai,Y. and Fujimoto,J. (1995) Novel monoclonal antibody reactive with thrombin-sensitive 74-kDa glycoproteins present on platelets and megakaryocytes both from mouse and rat. Hybridoma, 14, 361–367. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Suwabe,N., Dai,P., Yamamoto,M., Ishii,S. and Nakano,T. (2000) Inhibitory interaction of c-Myb and GATA-1 via transcriptional co-activator CBP. Oncogene, 19, 134–140. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Kunath,T., Hadjantonakis,A.K., Nagy,A. and Rossant,J. (1998) Promotion of trophoblast stem cell proliferation by FGF4. Science, 282, 2072–2075. [DOI] [PubMed] [Google Scholar]
- Tenen D.G., Hromas,R., Licht,J.D. and Zhang,D.E. (1997) Transcription factors, normal myeloid development and leukemia. Blood, 90, 489–519. [PubMed] [Google Scholar]
- Ting C.N., Olson,M.C., Barton,K.P. and Leiden,J.M. (1996) Transcription factor GATA-3 is required for development of the T-cell lineage. Nature, 384, 474–478. [DOI] [PubMed] [Google Scholar]
- Tsai F.Y. and Orkin,S.H. (1997) Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood, 89, 3636–3643. [PubMed] [Google Scholar]
- Tsai F.Y., Keller,G., Kuo,F.C., Weiss,M., Chen,J., Rosenblatt,M., Alt,F.W. and Orkin,S.H. (1994) An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature, 371, 221–226. [DOI] [PubMed] [Google Scholar]
- Tsai S.F., Martin,D.I., Zon,L.I., D’Andrea,A.D., Wong,G.G. and Orkin,S.H. (1989) Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature, 339, 446–451. [DOI] [PubMed] [Google Scholar]
- Visvader J. and Adams,J.M. (1993) Megakaryocytic differentiation induced in 416B myeloid cells by GATA-2 and GATA-3 transgenes or 5-azacytidine is tightly coupled to GATA-1 expression. Blood, 82, 1493–1501. [PubMed] [Google Scholar]
- Visvader J.E., Elefanty,A.G., Strasser,A. and Adams,J.M. (1992) GATA-1 but not SCL induces megakaryocytic differentiation in an early myeloid line. EMBO J., 11, 4557–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader J.E., Crossley,M., Hill,J., Orkin,S.H. and Adams,J.M. (1995) The C-terminal zinc finger of GATA-1 or GATA-2 is sufficient to induce megakaryocytic differentiation of an early myeloid cell line. Mol. Cell. Biol., 15, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M.J., Keller,G. and Orkin,S.H. (1994) Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev., 8, 1184–1197. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Zon,L.I., Ackerman,S.J., Yamamoto,M. and Suda,T. (1998) Forced GATA-1 expression in the murine myeloid cell line M1: induction of c-Mpl expression and megakaryocytic/erythroid differentiation. Blood, 91, 450–457. [PubMed] [Google Scholar]
- Yamamoto H., Kihara-Negishi,F., Yamada,T., Hashimoto,Y. and Oikawa,T. (1999) Physical and functional interactions between the transcription factor PU.1 and the coactivator CBP. Oncogene, 18, 1495–1501. [DOI] [PubMed] [Google Scholar]
- Zhang P., Behre,G., Pan,J., Iwama,A., Wara-Aswapati,N., Radomska,H.S., Auron,P.E., Tenen,D.G. and Sun,Z. (1999) Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc. Natl Acad. Sci. USA, 96, 8705–8710. [DOI] [PMC free article] [PubMed] [Google Scholar]