Abstract
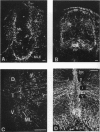
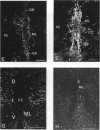
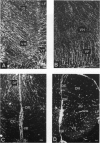
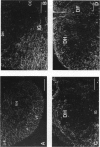
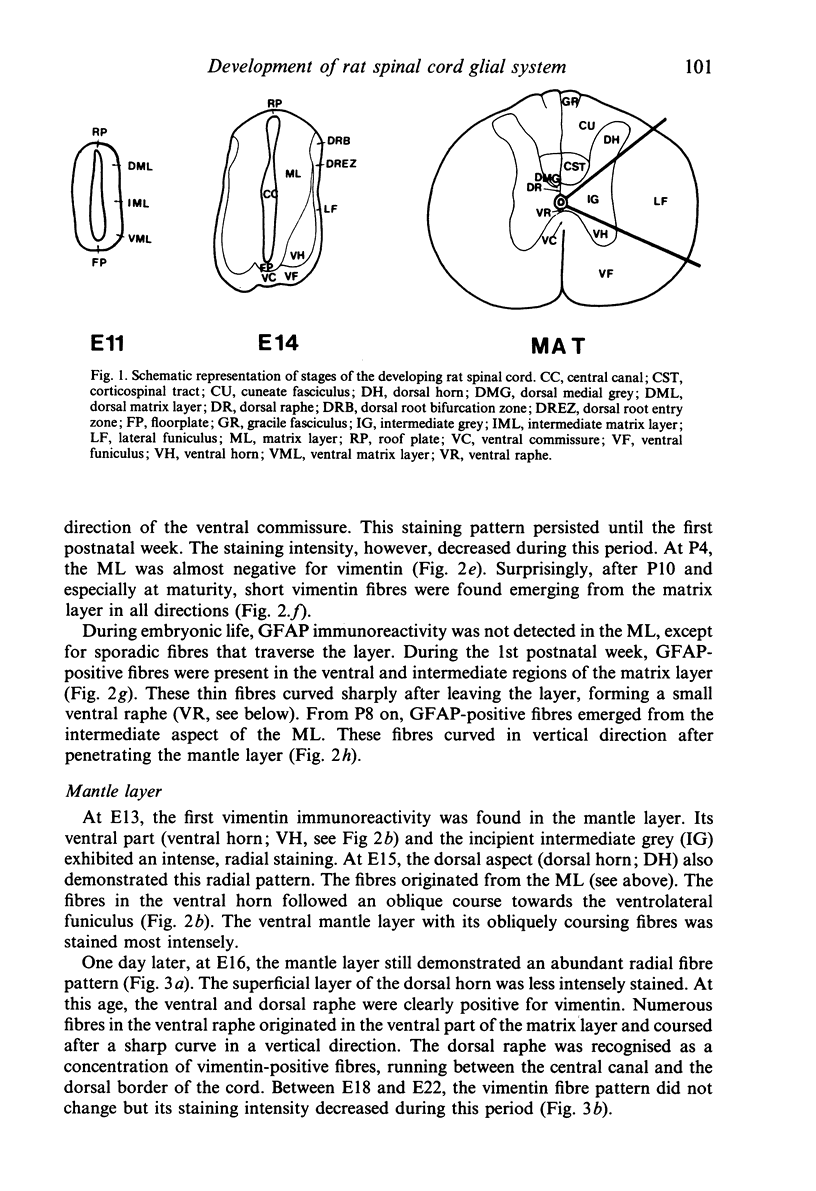
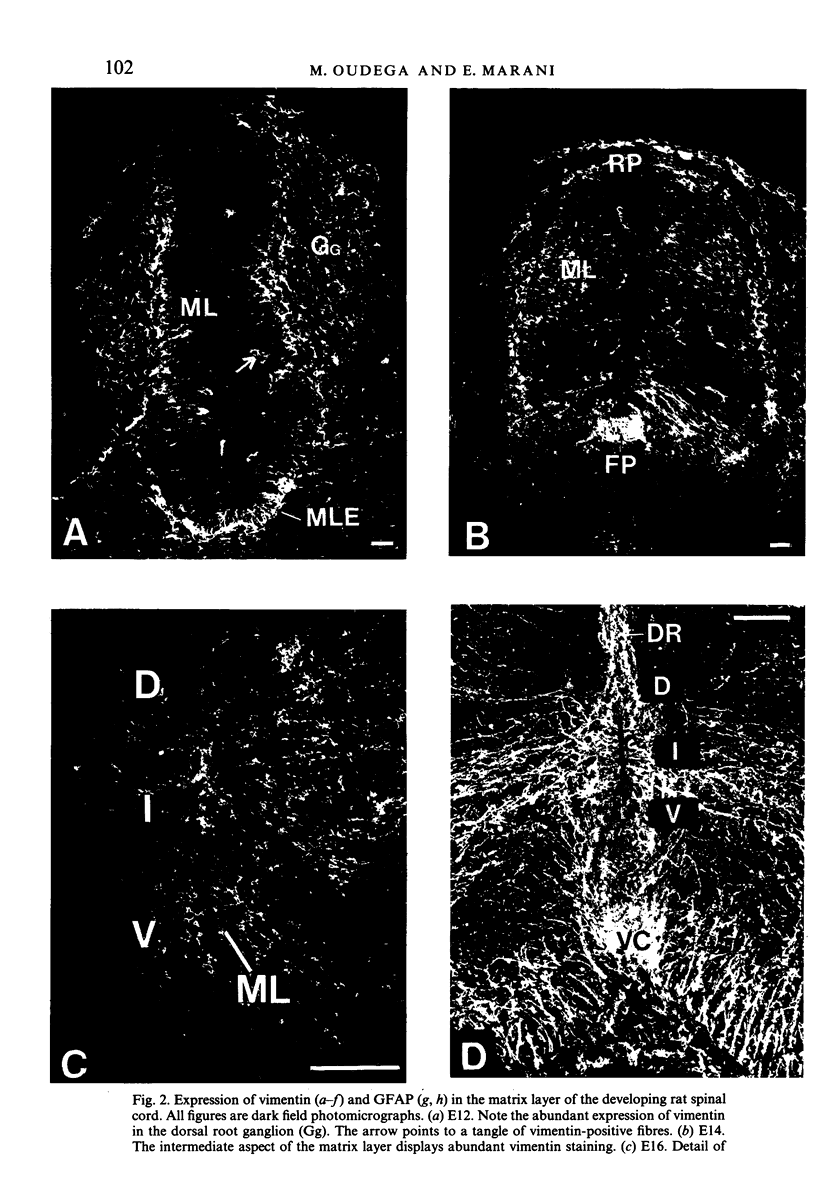
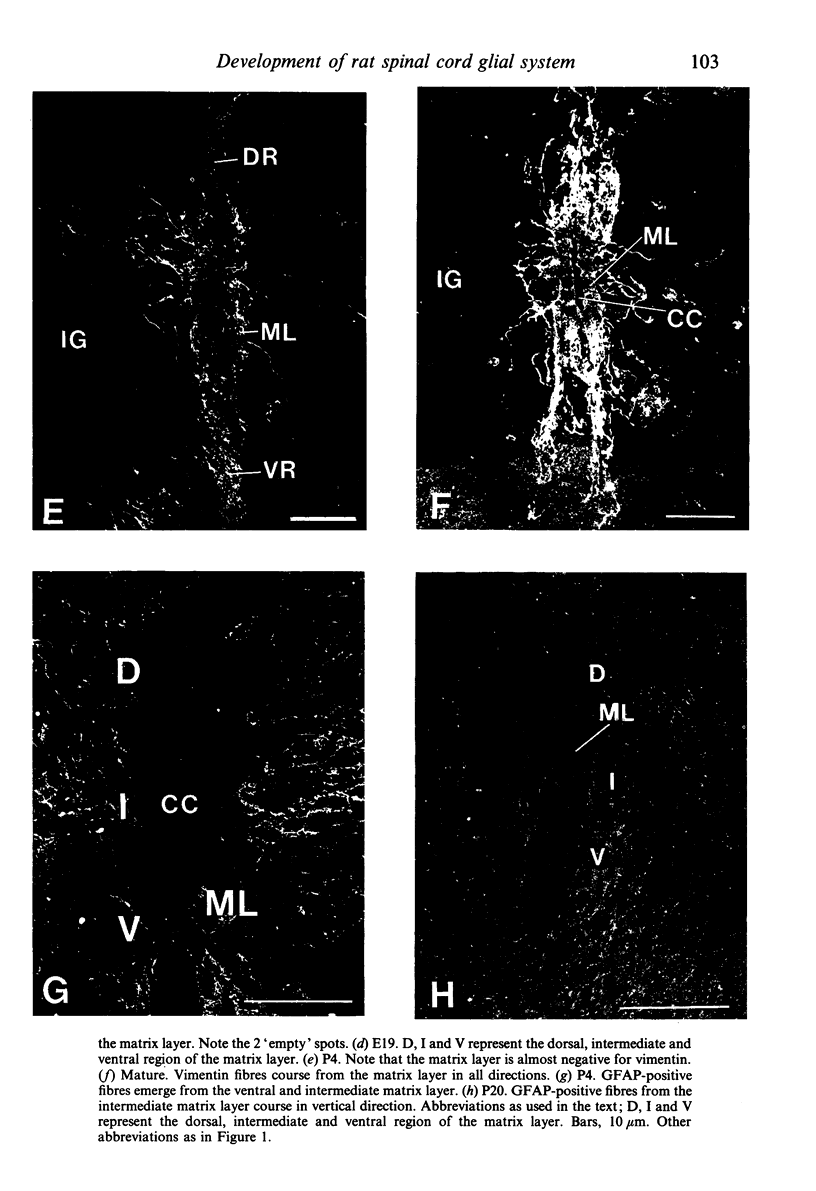
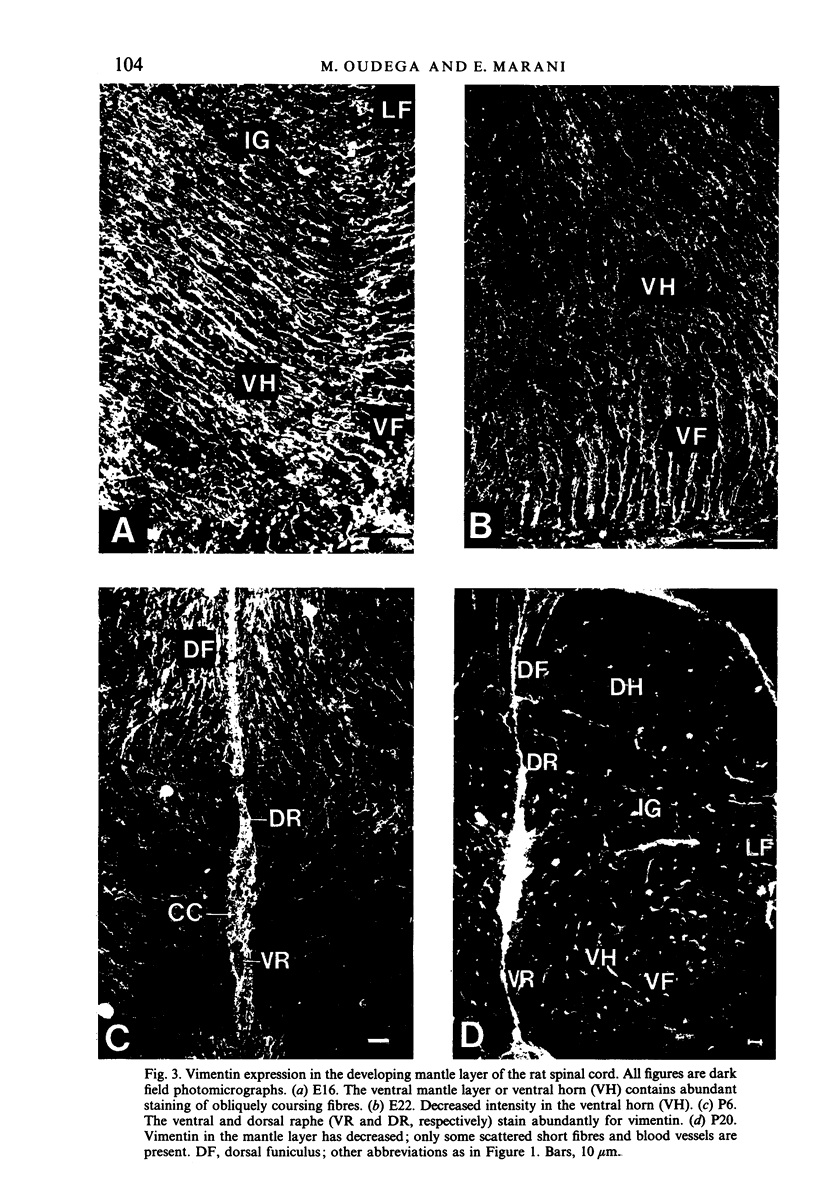
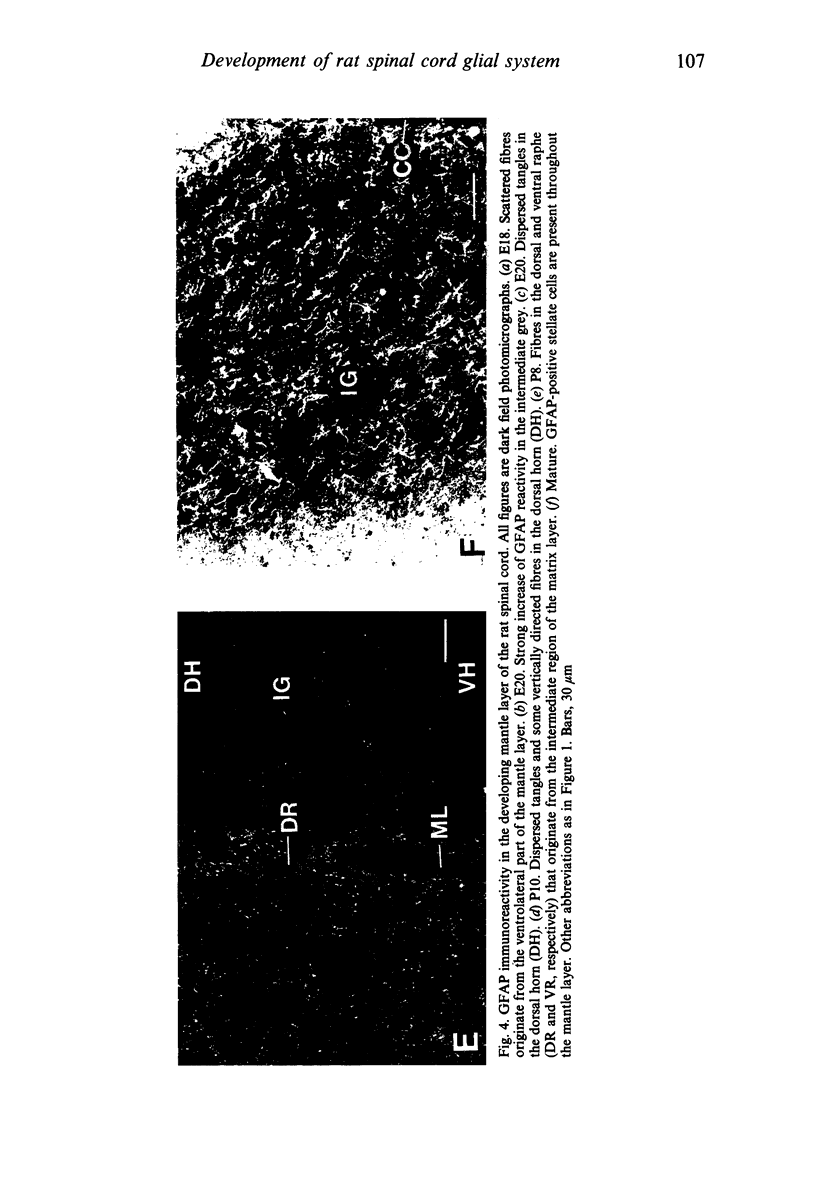
The glial system in the developing rat spinal cord was studied using immunocytochemistry. Antibodies to vimentin and glial fibrillary acidic protein (GFAP) were used. At E11, vimentin was first found in the membrana limitans externa. In the matrix layer, short vimentin protrusions were found near the membrana limitans externa at E12. In addition, vimentin was scattered throughout the matrix layer, where it was also present as vimentin-positive tangles. Later in development, vimentin immunoreactivity was distributed in a distinct radial pattern in the matrix layer. During the first postnatal weeks, vimentin was replaced by GFAP which is therefore expressed in a similar radial pattern. This orderly structural organisation of vimentin and GFAP in the matrix layer could indicate the involvement of both proteins in morphogenetic processes such as neuron migration and cell organisation. In the mantle layer, a distinct radial vimentin immunoreactivity was replaced by GFAP immunoreactivity during the first 2 postnatal weeks. In addition, GFAP fibres appeared first, at E18, in the ventral mantle layer associated with the motor neuron columns. These glial fibres originated from a local source. In the dorsal mantle layer, GFAP-positive fibres were oriented tangentially, which is different from the overall radial arrangement. This expression pattern may be related to the ingrowth of primary afferents. In the ventral and dorsal raphe, a major vimentin expression was replaced by a minor presence of GFAP. Within the white matter, a vimentin-positive radial pattern was demonstrated which, after birth, was replaced by GFAP. This palisading pattern suggested an involvement of both proteins in the development and guidance of the ascending and descending spinal cord fibre systems. The general transition from the expression of vimentin to the expression of GFAP in the rat spinal cord takes place during the first 3 postnatal weeks.
Full text
PDF




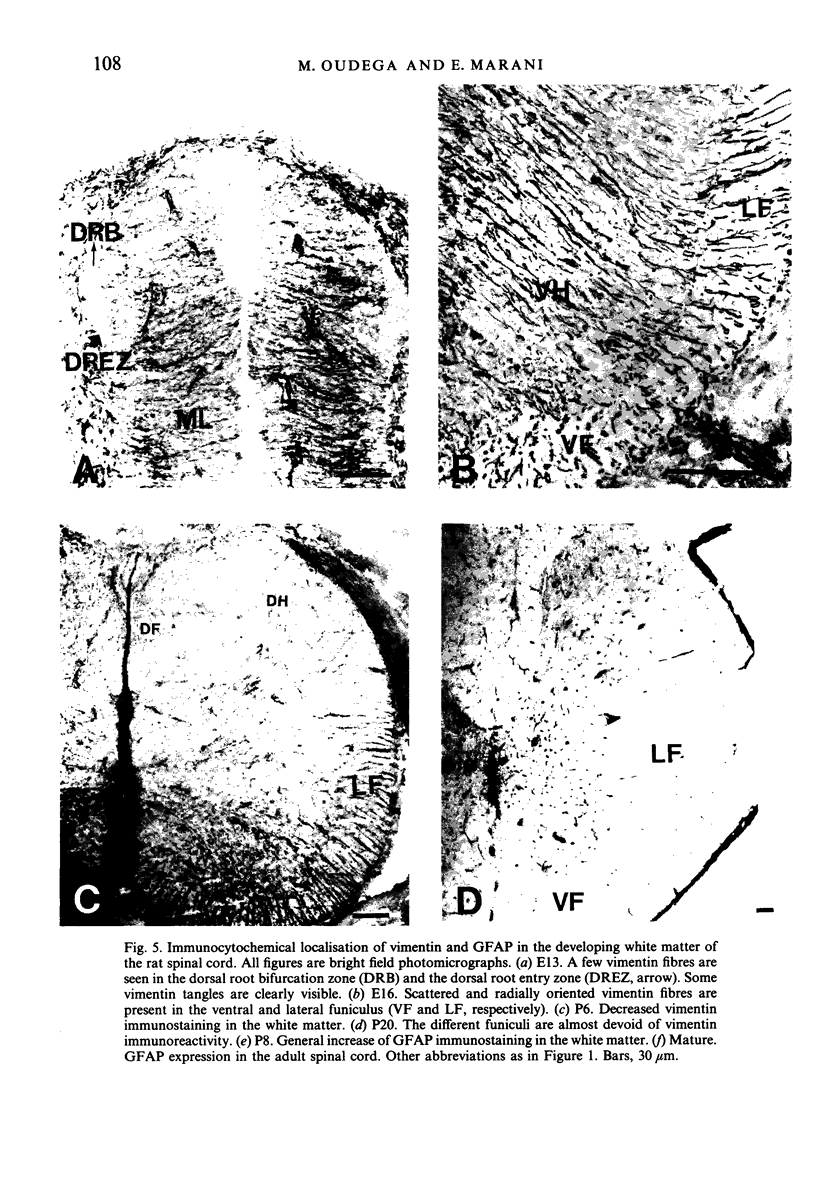
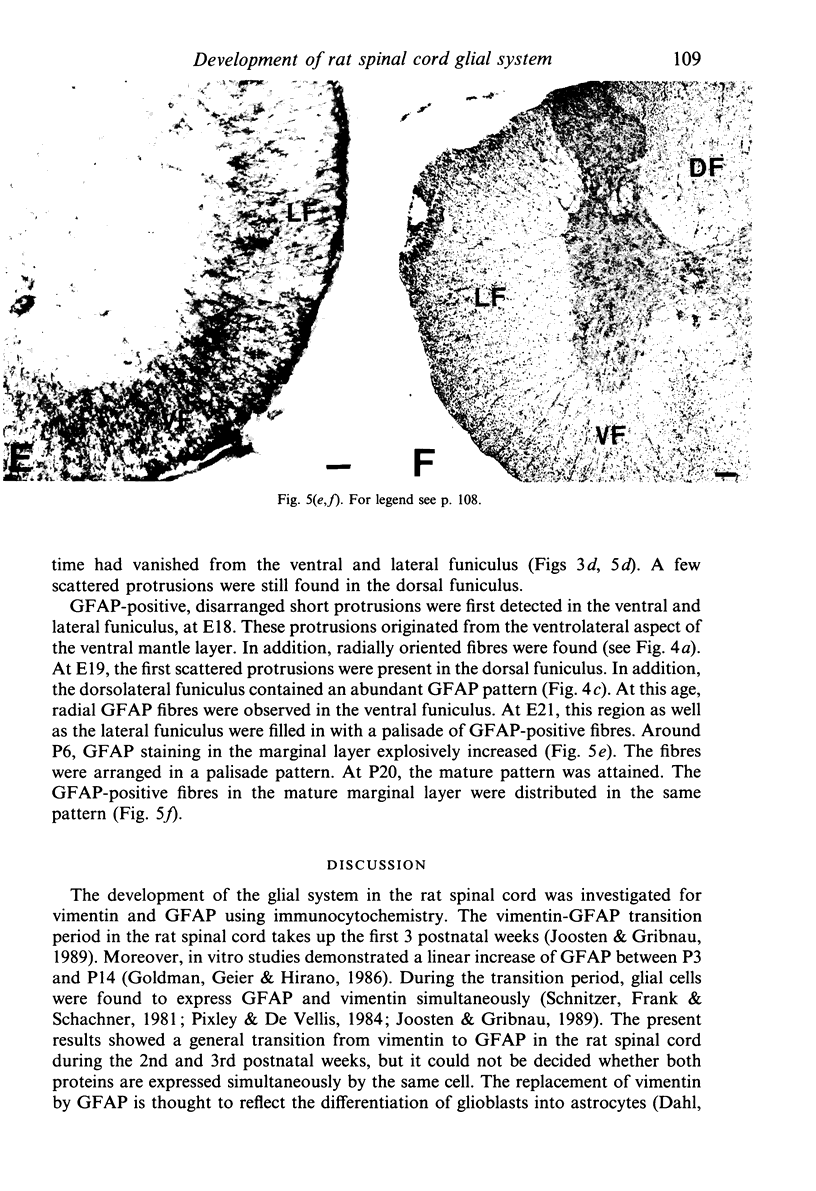





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Bartlett P. P., Raff M. C. Astrocytes, ependymal cells, and oligodendrocytes develop on schedule in dissociated cell cultures of embryonic rat brain. Dev Biol. 1981 Apr 30;83(2):301–310. doi: 10.1016/0012-1606(81)90476-0. [DOI] [PubMed] [Google Scholar]
- Aguayo A. J., David S., Bray G. M. Influences of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J Exp Biol. 1981 Dec;95:231–240. doi: 10.1242/jeb.95.1.231. [DOI] [PubMed] [Google Scholar]
- Baumal R., Kahn H. J., Bailey D., Phillips M. J., Hanna W. The value of immunohistochemistry in increasing diagnostic precision of undifferentiated tumours by the surgical pathologist. Histochem J. 1984 Oct;16(10):1061–1078. doi: 10.1007/BF01002895. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. Astrocyte-specific protein and radial glia in the cerebral cortex of newborn rat. Nature. 1974 Nov 1;252(5478):55–56. doi: 10.1038/252055a0. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. Specificity of the glial fibrillary acidic protein for astroglia. J Histochem Cytochem. 1977 Jun;25(6):466–469. doi: 10.1177/25.6.69656. [DOI] [PubMed] [Google Scholar]
- Bignami A., Raju T., Dahl D. Localization of vimentin, the nonspecific intermediate filament protein, in embryonal glia and in early differentiating neurons. In vivo and in vitro immunofluorescence study of the rat embryo with vimentin and neurofilament antisera. Dev Biol. 1982 Jun;91(2):286–295. doi: 10.1016/0012-1606(82)90035-5. [DOI] [PubMed] [Google Scholar]
- Bitner C., Benjelloun-Touimi S., Dupouey P. Palisading pattern of subpial astroglial processes in the adult rodent brain: relationship between the glial palisading pattern and the axonal and astroglial organization. Brain Res. 1987 Dec 15;465(1-2):167–178. doi: 10.1016/0165-3806(87)90238-0. [DOI] [PubMed] [Google Scholar]
- Bovolenta P., Liem R. K., Mason C. A. Development of cerebellar astroglia: transitions in form and cytoskeletal content. Dev Biol. 1984 Mar;102(1):248–259. doi: 10.1016/0012-1606(84)90189-1. [DOI] [PubMed] [Google Scholar]
- Bovolenta P., Liem R. K., Mason C. A. Glial filament protein expression in astroglia in the mouse visual pathway. Brain Res. 1987 May;430(1):113–126. doi: 10.1016/0165-3806(87)90181-7. [DOI] [PubMed] [Google Scholar]
- Bunge R. P. Glial cells and the central myelin sheath. Physiol Rev. 1968 Jan;48(1):197–251. doi: 10.1152/physrev.1968.48.1.197. [DOI] [PubMed] [Google Scholar]
- Cabral F., Gottesman M. M. Phosphorylation of the 10-nm filament protein from Chinese hamster ovary cells. J Biol Chem. 1979 Jul 25;254(14):6203–6206. [PubMed] [Google Scholar]
- Cochard P., Paulin D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J Neurosci. 1984 Aug;4(8):2080–2094. doi: 10.1523/JNEUROSCI.04-08-02080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Immunochemical and immunofluorescence studies of the glial fibrillary acidic protein in vertebrates. Brain Res. 1973 Oct 26;61:279–293. doi: 10.1016/0006-8993(73)90533-7. [DOI] [PubMed] [Google Scholar]
- Dahl D. The vimentin-GFA protein transition in rat neuroglia cytoskeleton occurs at the time of myelination. J Neurosci Res. 1981;6(6):741–748. doi: 10.1002/jnr.490060608. [DOI] [PubMed] [Google Scholar]
- Dupouey P., Benjelloun S., Gomes D. Immunohistochemical demonstration of an organized cytoarchitecture of the radial glia in the CNS of the embryonic mouse. Dev Neurosci. 1985;7(2):81–93. doi: 10.1159/000112279. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Battini R., Kaczmarek L., Rittling S., Calabretta B., de Riel J. K., Philiponis V., Wei J. F., Baserga R. Coding sequence and growth regulation of the human vimentin gene. Mol Cell Biol. 1986 Nov;6(11):3614–3620. doi: 10.1128/mcb.6.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D. L., Lazarides E. Cyclic AMP-modulated phosphorylation of intermediate filament proteins in cultured avian myogenic cells. Mol Cell Biol. 1982 Sep;2(9):1104–1114. doi: 10.1128/mcb.2.9.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. Intermediate filaments. Looking for a function. Nature. 1987 Oct 1;329(6138):392–393. doi: 10.1038/329392a0. [DOI] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. Amino acid sequence characterization of mammalian vimentin, the mesenchymal intermediate filament protein. FEBS Lett. 1983 Oct 31;163(1):22–24. doi: 10.1016/0014-5793(83)81153-3. [DOI] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. Related amino acid sequences in neurofilaments and non-neural intermediate filaments. Nature. 1982 Apr 1;296(5856):448–450. doi: 10.1038/296448a0. [DOI] [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Lamin B constitutes an intermediate filament attachment site at the nuclear envelope. J Cell Biol. 1987 Jul;105(1):117–125. doi: 10.1083/jcb.105.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Two distinct attachment sites for vimentin along the plasma membrane and the nuclear envelope in avian erythrocytes: a basis for a vectorial assembly of intermediate filaments. J Cell Biol. 1987 Jul;105(1):105–115. doi: 10.1083/jcb.105.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Johnson B. Secreted peptides as regulators of neuron-glia and glia-glia interactions in the developing nervous system. J Neurosci Res. 1988 Oct-Dec;21(2-4):487–500. doi: 10.1002/jnr.490210240. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Geier S. S., Hirano M. Differentiation of astrocytes and oligodendrocytes from germinal matrix cells in primary culture. J Neurosci. 1986 Jan;6(1):52–60. doi: 10.1523/JNEUROSCI.06-01-00052.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin M. H., Silver J. Mechanisms of axonal guidance. The problem of intersecting fiber systems. Dev Biol (N Y 1985) 1986;2:565–604. doi: 10.1007/978-1-4613-2141-5_15. [DOI] [PubMed] [Google Scholar]
- Hansen S. H., Stagaard M., Møllgård K. Neurofilament-like pattern of reactivity in human foetal PNS and spinal cord following immunostaining with polyclonal anti-glial fibrillary acidic protein antibodies. J Neurocytol. 1989 Aug;18(4):427–436. doi: 10.1007/BF01474540. [DOI] [PubMed] [Google Scholar]
- Herpers M. J., Ramaekers F. C., Aldeweireldt J., Moesker O., Slooff J. Co-expression of glial fibrillary acidic protein- and vimentin-type intermediate filaments in human astrocytomas. Acta Neuropathol. 1986;70(3-4):333–339. doi: 10.1007/BF00686093. [DOI] [PubMed] [Google Scholar]
- Hirano M., Goldman J. E. Gliogenesis in rat spinal cord: evidence for origin of astrocytes and oligodendrocytes from radial precursors. J Neurosci Res. 1988 Oct-Dec;21(2-4):155–167. doi: 10.1002/jnr.490210208. [DOI] [PubMed] [Google Scholar]
- Houle J., Fedoroff S. Temporal relationship between the appearance of vimentin and neural tube development. Brain Res. 1983 Aug;285(2):189–195. doi: 10.1016/0165-3806(83)90051-2. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M., Clarke S., Koppel H. Transitory macrophages in the white matter of the developing visual cortex. II. Development and relations with axonal pathways. Brain Res. 1983 Dec;313(1):55–66. doi: 10.1016/0165-3806(83)90201-8. [DOI] [PubMed] [Google Scholar]
- Joosten E. A., Gribnau A. A. Astrocytes and guidance of outgrowing corticospinal tract axons in the rat. An immunocytochemical study using anti-vimentin and anti-glial fibrillary acidic protein. Neuroscience. 1989;31(2):439–452. doi: 10.1016/0306-4522(89)90386-2. [DOI] [PubMed] [Google Scholar]
- Kalderon N. Differentiating astroglia in nervous tissue histogenesis/regeneration: studies in a model system of regenerating peripheral nerve. J Neurosci Res. 1988 Oct-Dec;21(2-4):501–512. doi: 10.1002/jnr.490210241. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Page C. The dynamics of nerve growth factor-induced neurofilament and vimentin filament expression and organization in PC12 cells. J Neurosci. 1984 Jul;4(7):1705–1714. doi: 10.1523/JNEUROSCI.04-07-01705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin S. K., Kosek J. C., Eng L. F. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol. 1976 Jan 15;165(2):197–207. doi: 10.1002/cne.901650206. [DOI] [PubMed] [Google Scholar]
- Marani E. A method for orienting cryostat sections for three-dimensional reconstructions. Stain Technol. 1978 Sep;53(5):265–268. doi: 10.3109/10520297809111943. [DOI] [PubMed] [Google Scholar]
- Nornes H. O., Das G. D. Temporal pattern of neurogenesis in spinal cord of rat. I. An autoradiographic study--time and sites of origin and migration and settling patterns of neuroblasts. Brain Res. 1974 Jun 14;73(1):121–138. doi: 10.1016/0006-8993(74)91011-7. [DOI] [PubMed] [Google Scholar]
- Onteniente B., Kimura H., Maeda T. Comparative study of the glial fibrillary acidic protein in vertebrates by PAP immunohistochemistry. J Comp Neurol. 1983 Apr 20;215(4):427–436. doi: 10.1002/cne.902150407. [DOI] [PubMed] [Google Scholar]
- Pixley S. K., de Vellis J. Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin. Brain Res. 1984 Aug;317(2):201–209. doi: 10.1016/0165-3806(84)90097-x. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972 May;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Roeling T. A., Feirabend H. K. Glial fiber pattern in the developing chicken cerebellum: vimentin and glial fibrillary acidic protein (GFAP) immunostaining. Glia. 1988;1(6):398–402. doi: 10.1002/glia.440010607. [DOI] [PubMed] [Google Scholar]
- Schmechel D. E., Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol (Berl) 1979 Jun 5;156(2):115–152. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Franke W. W., Schachner M. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol. 1981 Aug;90(2):435–447. doi: 10.1083/jcb.90.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman R. L., Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973 Nov 9;62(1):1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Silver J., Lorenz S. E., Wahlsten D., Coughlin J. Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J Comp Neurol. 1982 Sep 1;210(1):10–29. doi: 10.1002/cne.902100103. [DOI] [PubMed] [Google Scholar]
- Singer M., Nordlander R. H., Egar M. Axonal guidance during embryogenesis and regeneration in the spinal cord of the newt: the blueprint hypothesis of neuronal pathway patterning. J Comp Neurol. 1979 May 1;185(1):1–21. doi: 10.1002/cne.901850102. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Tardy M., Fages C., Riol H., LePrince G., Rataboul P., Charriere-Bertrand C., Nunez J. Developmental expression of the glial fibrillary acidic protein mRNA in the central nervous system and in cultured astrocytes. J Neurochem. 1989 Jan;52(1):162–167. doi: 10.1111/j.1471-4159.1989.tb10911.x. [DOI] [PubMed] [Google Scholar]
- Tascos N. A., Parr J., Gonatas N. K. Immunocytochemical study of the glial fibrillary acidic protein in human neoplasms of the central nervous system. Hum Pathol. 1982 May;13(5):454–458. doi: 10.1016/s0046-8177(82)80028-2. [DOI] [PubMed] [Google Scholar]
- Zehner Z. E., Li Y., Roe B. A., Paterson B. M., Sax C. M. The chicken vimentin gene. Nucleotide sequence, regulatory elements, and comparison to the hamster gene. J Biol Chem. 1987 Jun 15;262(17):8112–8120. [PubMed] [Google Scholar]
- van Muijen G. N., Ruiter D. J., van Leeuwen C., Prins F. A., Rietsema K., Warnaar S. O. Cytokeratin and neurofilament in lung carcinomas. Am J Pathol. 1984 Sep;116(3):363–369. [PMC free article] [PubMed] [Google Scholar]