Abstract
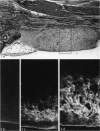
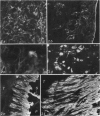
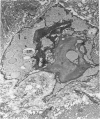
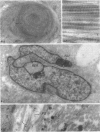
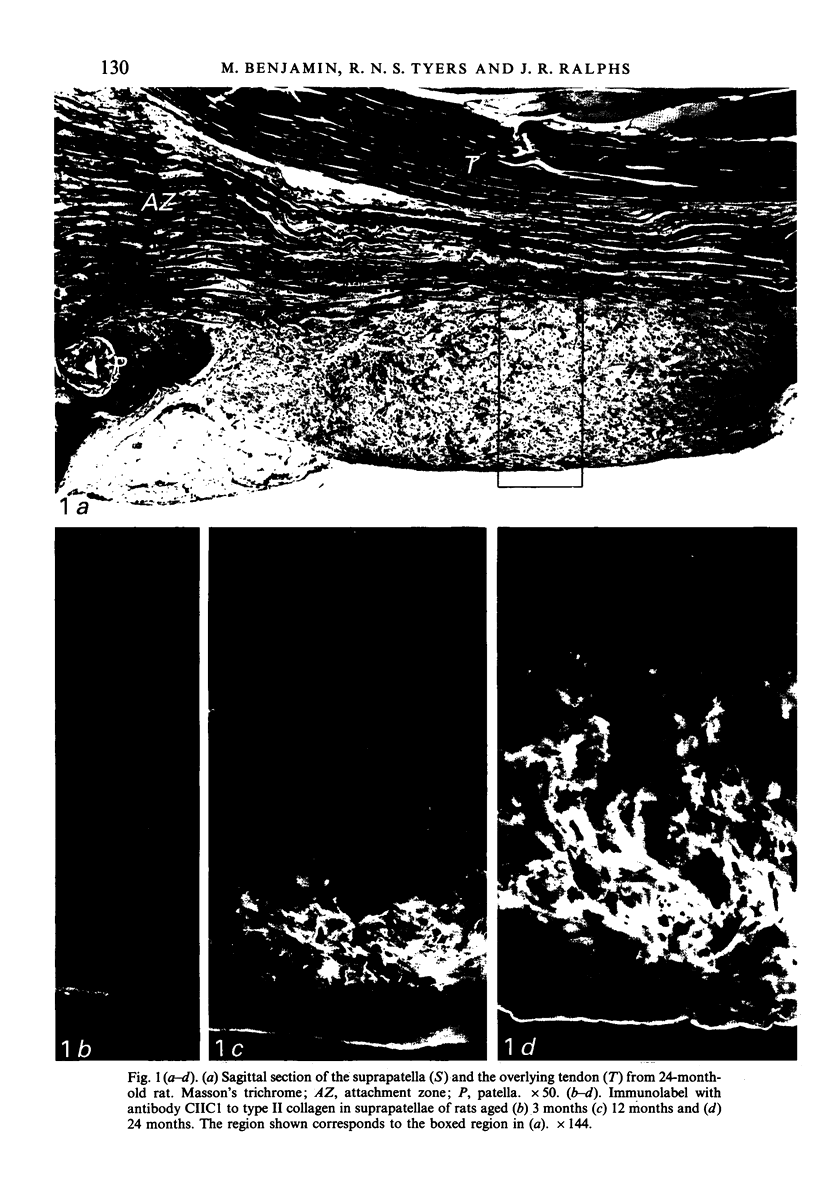
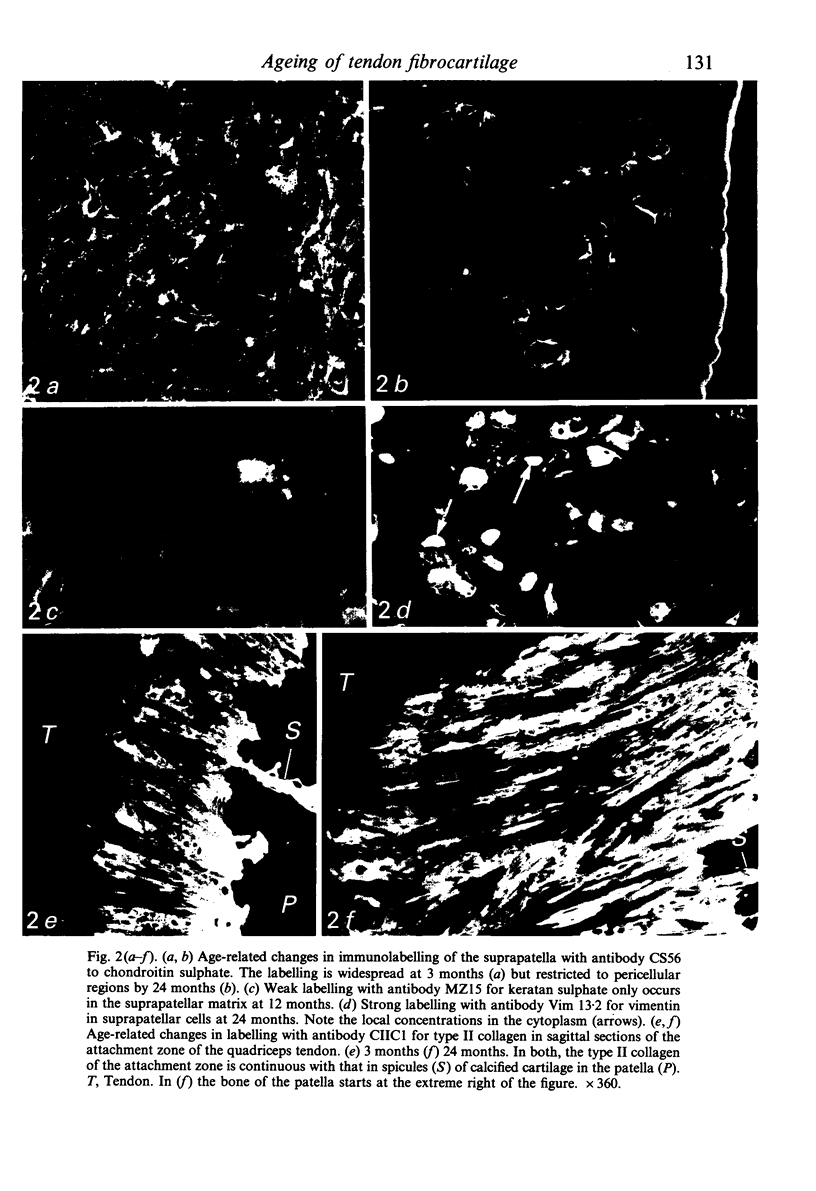
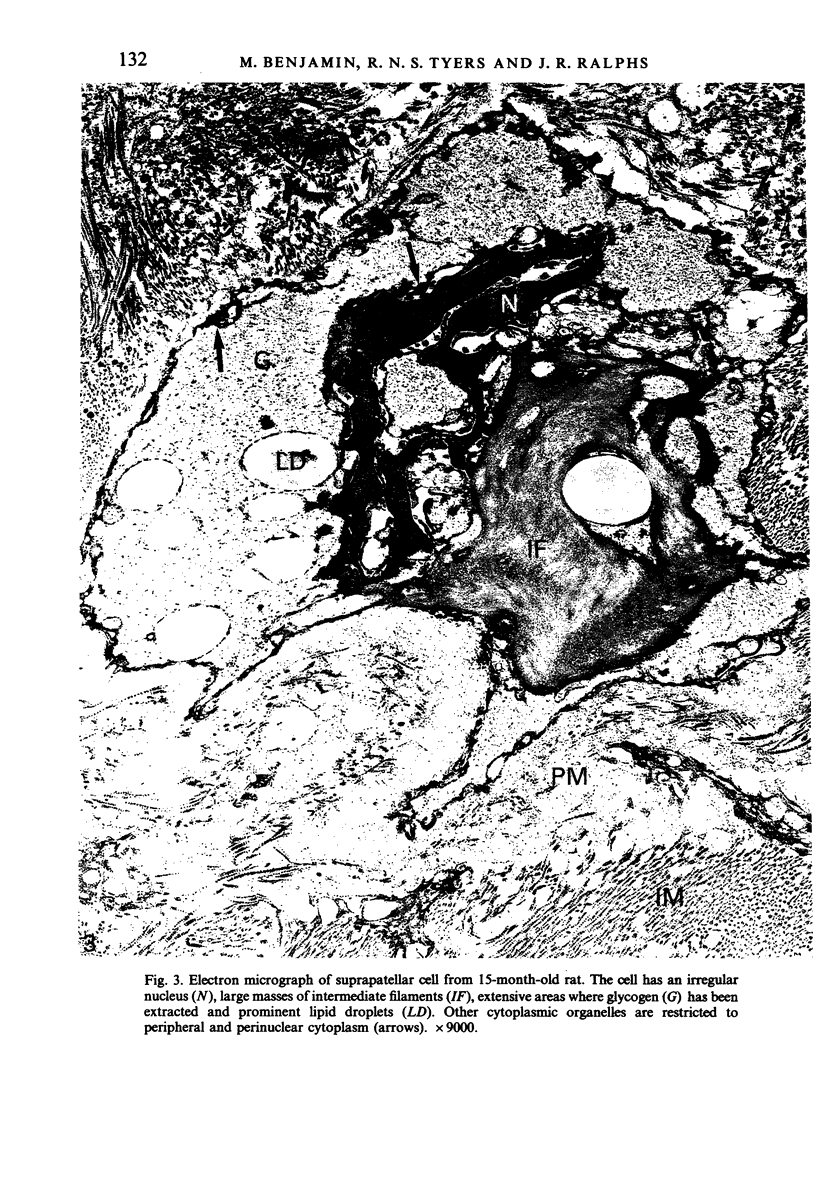
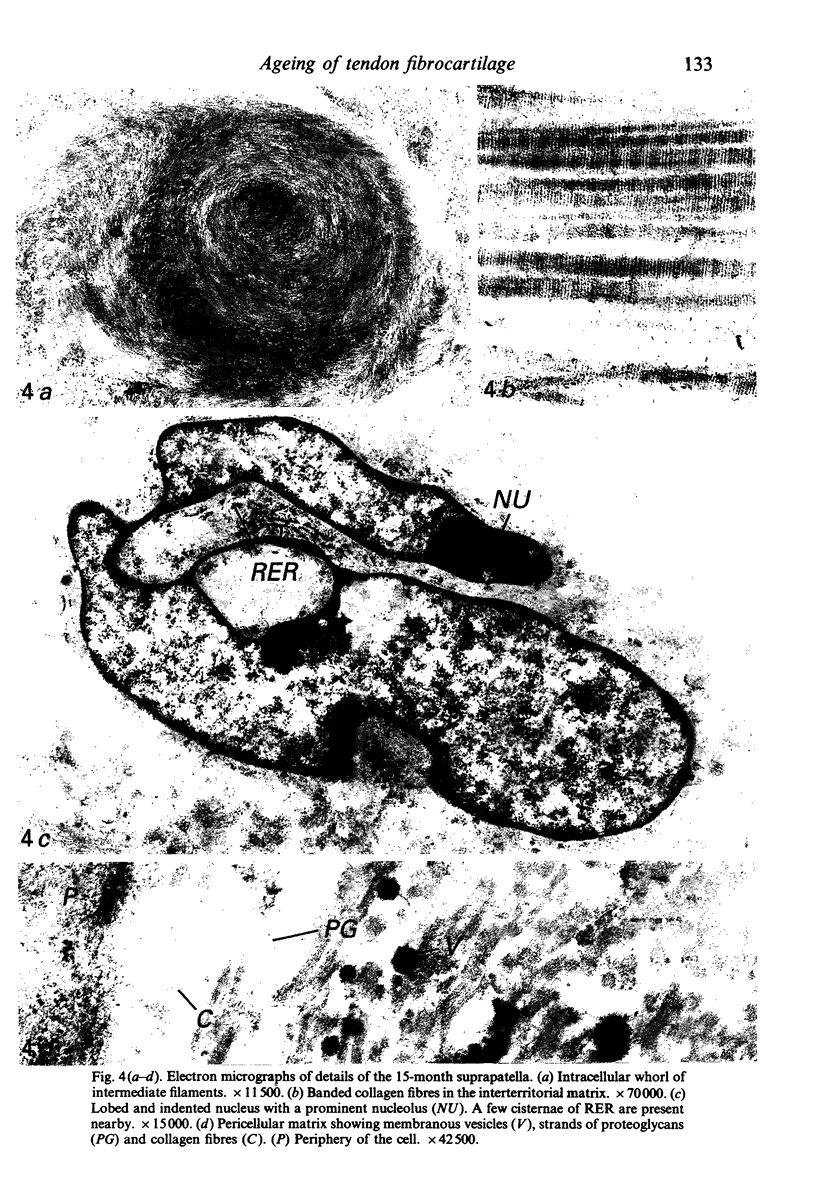
Age-related changes are reported in the rat suprapatella: a fibrocartilage that resists compression of the quadriceps tendon against the femur in the flexed knee. The suprapatella was studied by histology, immunohistochemistry and electron microscopy in rats aged 11-14 weeks, and 12, 15, 18 and 24 months. Type II collagen was absent in the matrix of animals 11-14 weeks old, but appeared by 12 months; immunolabelling increased further with age. Chondroitin sulphate was present in all animals, although immunolabelling decreased with age. Keratan sulphate appeared transiently at 12 months. The structure of the suprapatellar cells also changed with age. In some respects the suprapatellar cells of aged rats are similar to those of younger animals; they contain relatively few organelles and their cytoplasm is packed with intermediate filaments which contain vimentin. However, lipid droplets and glycogen are more prominent in older animals, and the nuclei become elaborately infolded and multilobed. Type II collagen was present in rats aged 11-14 weeks in fibrocartilage of the attachment of quadriceps femoris to the patella, but with increasing age it spread proximally, further into the tendon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avnur Z., Geiger B. Immunocytochemical localization of native chondroitin-sulfate in tissues and cultured cells using specific monoclonal antibody. Cell. 1984 Oct;38(3):811–822. doi: 10.1016/0092-8674(84)90276-9. [DOI] [PubMed] [Google Scholar]
- Benjamin M., Evans E. J., Copp L. The histology of tendon attachments to bone in man. J Anat. 1986 Dec;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M., Evans E. J. Fibrocartilage. J Anat. 1990 Aug;171:1–15. [PMC free article] [PubMed] [Google Scholar]
- Boskey A. L. Current concepts of the physiology and biochemistry of calcification. Clin Orthop Relat Res. 1981 Jun;(157):225–257. [PubMed] [Google Scholar]
- Craig F. M., Ralphs J. R., Bentley G., Archer C. W. MZ15, a monoclonal antibody recognizing keratan sulphate, stains chick tendon. Histochem J. 1987 Dec;19(12):651–657. doi: 10.1007/BF01676171. [DOI] [PubMed] [Google Scholar]
- Dziewiatkowski D. D., LaValley J., Beaudoin A. G. Age-related changes in the composition of proteoglycans in sheep cartilages. Connect Tissue Res. 1989;19(2-4):103–120. doi: 10.3109/03008208909043892. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Taylor T. K. The knee joint meniscus. A fibrocartilage of some distinction. Clin Orthop Relat Res. 1987 Nov;(224):52–63. [PubMed] [Google Scholar]
- Gillard G. C., Reilly H. C., Bell-Booth P. G., Flint M. H. The influence of mechanical forces on the glycosaminoglycan content of the rabbit flexor digitorum profundus tendon. Connect Tissue Res. 1979;7(1):37–46. doi: 10.3109/03008207909152351. [DOI] [PubMed] [Google Scholar]
- Gyarmati J., Földes I., Kern M., Kiss I. Morphological studies on the articular cartilage of old rats. Acta Morphol Hung. 1987;35(3-4):111–124. [PubMed] [Google Scholar]
- Holmdahl R., Rubin K., Klareskog L., Larsson E., Wigzell H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986 Mar;29(3):400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- Labandeira-Garcia J. L., Guerra-Seijas M. J., Suarez-Nuñez J. M. Quantitative histochemical study of changes with age in intracellular lipids of xiphisternal cartilage in rat and rabbit. Anat Anz. 1987;164(4):255–264. [PubMed] [Google Scholar]
- Littlejohn G. O. More emphasis on the enthesis. J Rheumatol. 1989 Aug;16(8):1020–1022. [PubMed] [Google Scholar]
- Mehmet H., Scudder P., Tang P. W., Hounsell E. F., Caterson B., Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 1986 Jun 2;157(2):385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Merrilees M. J., Flint M. H. Ultrastructural study of tension and pressure zones in a rabbit flexor tendon. Am J Anat. 1980 Jan;157(1):87–106. doi: 10.1002/aja.1001570109. [DOI] [PubMed] [Google Scholar]
- Murakami R. Immunohistochemical and immunoblot analyses of collagens in the developing fibrocartilage in the glans penis of the rat. Acta Morphol Neerl Scand. 1987;25(4):279–288. [PubMed] [Google Scholar]
- Okuda Y., Gorski J. P., Amadio P. C. Effect of postnatal age on the ultrastructure of six anatomical areas of canine flexor digitorum profundus tendon. J Orthop Res. 1987;5(2):231–241. doi: 10.1002/jor.1100050209. [DOI] [PubMed] [Google Scholar]
- Okuda Y., Gorski J. P., An K. N., Amadio P. C. Biochemical, histological, and biomechanical analyses of canine tendon. J Orthop Res. 1987;5(1):60–68. doi: 10.1002/jor.1100050109. [DOI] [PubMed] [Google Scholar]
- Postacchini F., Bellocci M., Ricciardi-Pollini P. T., Modesti A. An ultrastructural study of recurrent disc herniation: a preliminary report. Spine (Phila Pa 1976) 1982 Sep-Oct;7(5):492–497. doi: 10.1097/00007632-198209000-00014. [DOI] [PubMed] [Google Scholar]
- Robson R. M. Intermediate filaments. Curr Opin Cell Biol. 1989 Feb;1(1):36–43. doi: 10.1016/s0955-0674(89)80034-1. [DOI] [PubMed] [Google Scholar]
- Scapinelli R. Morphological and functional changes of the lumbar spinous processes in the elderly. Surg Radiol Anat. 1989;11(2):129–133. doi: 10.1007/BF02096469. [DOI] [PubMed] [Google Scholar]
- Stockwell R. A. The lipid and glycogen content of rabbit articular hyaline and non-articular hyaline cartilage. J Anat. 1967 Nov;102(Pt 1):87–94. [PMC free article] [PubMed] [Google Scholar]
- Townsend F. J., Gibson M. A. A histochemical study of glycogen metabolism in developing cartilage and bone. Can J Zool. 1970 Jan;48(1):87–95. doi: 10.1139/z70-011. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Koob T. J. Structural specialization in tendons under compression. Int Rev Cytol. 1989;115:267–293. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- Yamada M. Ultrastructural and cytochemical studies on the calcification of the tendon-bone joint. Arch Histol Jpn. 1976 Nov;39(5):347–378. doi: 10.1679/aohc1950.39.347. [DOI] [PubMed] [Google Scholar]
- Yamamoto M. An electron microscope study of the distal segment of the os penis of the rat. Arch Histol Cytol. 1989 Dec;52(5):529–541. doi: 10.1679/aohc.52.529. [DOI] [PubMed] [Google Scholar]