Abstract
Drosophila provides a powerful genetic model for studying the in vivo regulation of cell death. In our large-scale gain-of-function screen, we identified Eiger, the first invertebrate tumor necrosis factor (TNF) superfamily ligand that can induce cell death. Eiger is a type II transmembrane protein with a C-terminal TNF homology domain. It is predominantly expressed in the nervous system. Genetic evidence shows that Eiger induces cell death by activating the Drosophila JNK pathway. Although this cell death process is blocked by Drosophila inhibitor-of-apoptosis protein 1 (DIAP1), it does not require caspase activity. We also show genetically that Eiger is a physiological ligand for the Drosophila JNK pathway. Our findings demonstrate that Eiger can initiate cell death through an IAP-sensitive cell death pathway via JNK signaling.
Keywords: cell death/Drosophila/JNK/TNF
Introduction
The intrinsic cell death execution mechanisms, which are regulated by the caspases, Apaf-1 and the Bcl-2 family of proteins, are highly conserved throughout evolution (Meier et al., 2000a; Vernooy et al., 2000; Shi, 2001). In Caenorhabditis elegans, the regulation of cell death is intrinsic and works through a genetic program. In Drosophila and mammals, on the other hand, cell death is largely regulated through extrinsic mechanisms; this is known as social control of cell death (Raff, 1992). Although a number of extrinsic death factors have been identified in vertebrates, until now, none has been reported in invertebrates, despite abundant similarities in the cell death mechanisms between Drosophila and vertebrates.
Drosophila is a powerful genetic model for studying the in vivo role of cell death and its physiological regulation. The intrinsic cell death pathway in flies is stimulated by the Drosophila killer proteins, Reaper, Hid and Grim, or a pro-apoptotic Bcl-2 family member, Drob-1/Debcl/dBorg-1/dBok (Vernooy et al., 2000). Ectopic expression of these cell death trigger proteins in the developing Drosophila eye results in a reduced-eye phenotype (Grether et al., 1995; Chen et al., 1996; White et al., 1996; Colussi et al., 2000; Igaki et al., 2000). On the other hand, the pro-apoptotic adapter molecules such as Drosophila Apaf-1 (Dark) or a Drosophila caspase drICE do not cause the reduced eye size (H.Kanuka and M.Miura, unpublished data), suggesting that the small eye phenotype would be generated by an overexpression of the ‘apical’ cell death triggers, the proteins whose activities are regulated at the transcriptional levels or by the levels of their inhibitory molecules. The reduced-eye phenotype should also be generated by the extrinsic death stimuli such as ligand/receptor-mediated signaling. A Drosophila in vivo expression screen has an advantage for identifying the factors that function through such the cell–cell interactions as well as the cell autonomous factors.
To identify novel cell death-inducing factors in Drosophila, we screened for genes that had a potential to reduce the eye size when misexpressed in the developing eye. Here, we describe the first invertebrate tumor necrosis factor (TNF) superfamily ligand identified in this screen, Eiger. We provide genetic evidence that Eiger can induce inhibitor-of-apoptosis (IAP)-sensitive cell death through the activation of the Drosophila JNK pathway. Genetic analysis of eiger mutant flies reveals that Eiger is a physiological ligand for the JNK pathway in the eye disc. Our findings also provide a model for a caspase-independent cell death pathway through the JNK signaling that can be blocked by IAP.
Results
To discover the cell death triggers encoded in the Drosophila genome, we conducted a misexpression screen using the GAL4/UAS system. The GS vector is a P element-based gene search vector with UAS enhancers (Toba et al., 1999). We crossed a collection of 5000 lines harboring the GS vector (GS lines) with an eye-specific GAL4 (GMR-GAL4) strain to screen for genes that generated the reduced-eye phenotype. Six GS lines resulted in greatly reduced eyes in a GAL4-dependent manner, which we called Regg strains (Regg1–6, for reduced-eye generator with a GMR-GAL4 driver).
Identification of a Drosophila TNF superfamily ligand, Eiger, as a novel cell death trigger
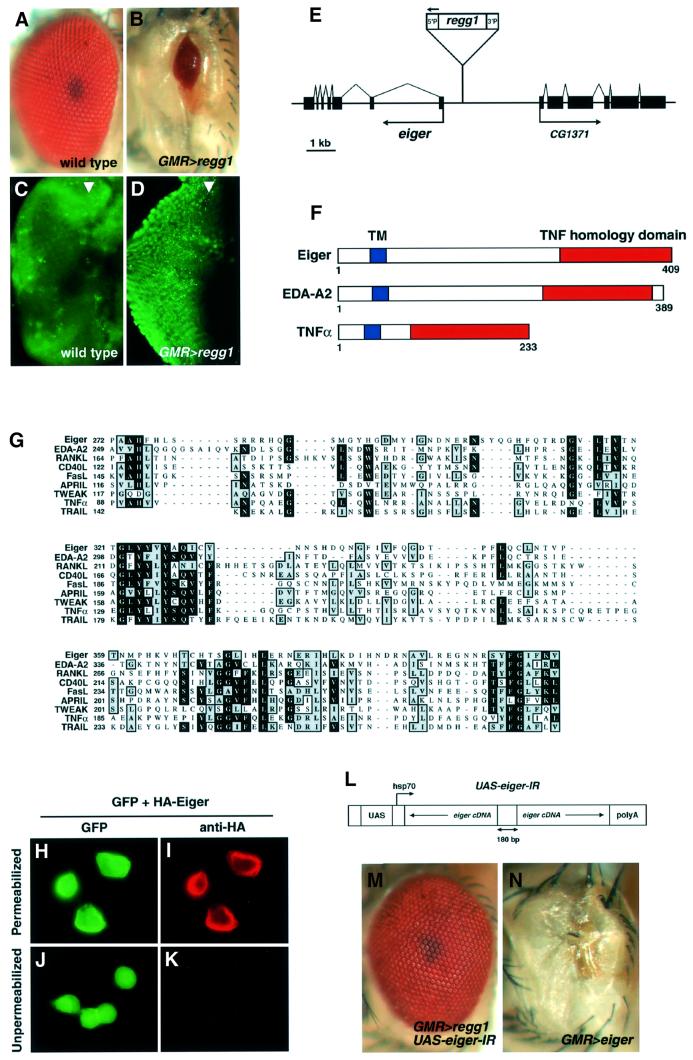
The F1 progeny generated by mating the GMR-GAL4 strain and Regg1 (GS9830) strain (GMR>regg1GS9830) displayed a strong reduced-eye phenotype (Figure 1B) compared with the wild-type eye (Figure 1A). To assess whether the small eye phenotype of GMR>regg1GS9830 flies was generated by the acceleration of cell death, we stained eye discs with acridine orange to detect dying cells (Figure 1C and D). Acridine orange is commonly used as a marker for apoptotic cell death in Drosophila (Abrams et al., 1993). In GMR>regg1GS9830 discs, numerous acridine orange-staining cells were observed, indicating that regg1GS9830 induced massive apoptotic cell death, leading to the reduced-eye phenotype. Using the inverse PCR method, we determined the insertion site of the P element in the Regg1GS9830 strain, and found a predicted gene, CG12919, adjacent to the insertion site. The GS9830 strain was generated by inserting the GS6 vector, which has a green fluorescent protein (GFP) trailer (UAS-GFP-SV40 terminator) near the 3′P end and a UAS enhancer near the 5′P end, so that a misexpression of vector-flanking sequence occurs 5′P side only (T.Aigaki, unpublished data). As expected, CG12919 was the only gene with an elevated expression level in a GAL4-dependent manner (see Supplementary figure S1 available at The EMBO Journal Online). We sequenced a Drosophila EST clone, LP03784, which included the nucleotide sequence of CG12919, and identified the open reading frame (ORF) of a novel gene we named eiger (EDA-like cell death trigger; see below) (Figure 1E). eiger encoded a protein of 409 amino acids with a C-terminal TNF homology domain and a hydrophobic transmembrane domain, indicating that Eiger was the first Drosophila member of the TNF superfamily (Figure 1F). The absence of a signal peptide suggested that Eiger was a type II membrane protein, which is typical of the members of the TNF ligand family. The sequence of the C-terminal TNF domain of Eiger showed highest homology with human EDA-A2 (28% identity), and also showed significant homology with all known TNF superfamily members including RANKL, CD40L, FasL, APRIL, TWEAK, TNF-α and TRAIL (Figure 1G).
Fig. 1. Identification of Eiger, a Drosophila TNF superfamily protein. (A and B) A misexpression screen identified a GS strain, GS9830 (as Regg1), which resulted in a reduced eye when driven by a GMR-GAL4 driver. Eye phenotypes of wild type (A) and regg1GS9830/GMR-GAL4 (B) are shown. (C and D) Acridine orange staining detected numerous dying cells in third-instar larval eye discs of the regg1GS9830/GMR-GAL4 strain (D) compared with the wild-type strain (C). Many acridine orange-positive cells were observed behind the morphogenetic furrow (arrowhead), corresponding to the expression domain of the GAL4 driver. (E) A novel ORF encoding Eiger was identified from an EST clone (LP03784) containing the nucleotide sequence of CG12919, the expression of which was simulated in a GAL4-dependent manner in the Regg1GS9830 strain. (F) Schematic structures of Eiger, EDA-A2 and human TNF-α. (G) The amino acid sequence of the TNF homology domain of Eiger is aligned with the sequences of human EDA-A2, RANKL, CD40L, FasL, APRIL, TWEAK, TNF-α and TRAIL. Identical and conserved residues are denoted with blocks of black or shading, respectively. The Eiger cDNA sequence has been deposited with DDBJ/EMBL/GenBank (accession No. AB073865). (H–K) Eiger is a type II membrane protein with a cytoplasmic N-terminus. S2 cells were transfected with the expression vectors for HA-Eiger (pUAS-HA-eiger) and GFP (pUAS-GFP), together with a driver plasmid, pWAGAL4 (actin-GAL4). Twenty-four hours after transfection, cells were immunostained with an anti-HA monoclonal antibody and a Cy3-labeled secondary antibody after (H and I) or prior to (J and K) fixation and permeabilization. (L) Schematic structure of the IR expression construct of eiger. Two partial sequences of eiger cDNA were cloned as a head-to-head inverted repeat that was separated by a non-palindromic 180 bp linker into the pUAST vector. (M and N) eiger is the responsible gene for Regg1. The reduced-eye phenotype induced by regg1GS9830 (A) was completely suppressed by eiger-IR (M). Overexpression of exogenous Eiger caused a reduced-eye phenotype (N). Genotypes are regg1GS9830/+; GMR-GAL4/UAS-eiger-IR (M) and UAS-eiger/GMR-GAL4 (N).
To examine whether Eiger was indeed a membrane protein, we transfected S2 cells with expression vectors for an N-terminal hemagglutinin (HA)-tagged Eiger and GFP. In permeabilized cells, cell surface staining was observed in GFP-positive cells by anti-HA immunostaining (Figure 1H and I). When immunostained prior to fixation and permeabilization, however, the staining was not detected (Figure 1K), consistent with Eiger being a type II transmembrane protein with an extracellular C-terminus and a cytoplasmic N-terminus.
To confirm that eiger was the gene responsible for the reduced-eye phenotype of GMR>regg1GS9830, we generated transgenic flies possessing an inverted repeat (IR) expression construct of the eiger cDNA (Figure 1L) that would specifically inhibit Eiger expression in a GAL4-dependent manner via a mechanism of RNA interference. This construct worked as a specific inhibitor of Eiger expression in a cell culture system (data not shown). We found that the Regg1 phenotype was completely rescued by the eiger-IR transgene (Figure 1M), demonstrating that eiger was indeed the gene responsible for the reduced-eye phenotype of GMR>regg1GS9830. Furthermore, ectopic expression of exogenous Eiger in the eye caused a reduced-eye phenotype similar to that of Regg1GS9830 (Figure 1N). Because regg1GS9830 activated eiger expression in a GAL4-dependent manner without affecting the expression of adjacent genes (see Supplementary figure S1), we used the Regg1GS9830 strain for further genetic analyses.
Overexpression of Eiger results in severe developmental defects that are distinct from the defects caused by Reaper
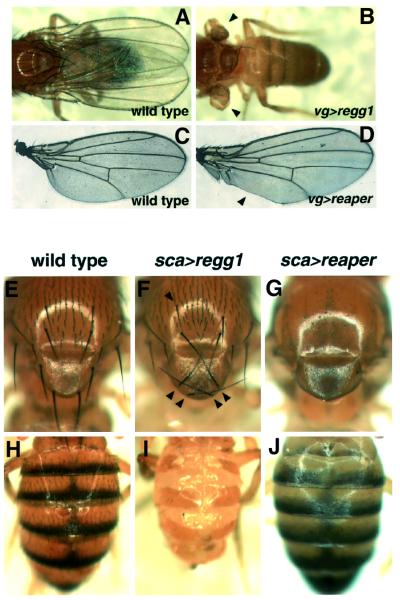
Ectopic expression of Eiger in the eye resulted in a reduced-eye phenotype similar to that of Reaper (White et al., 1996), a Drosophila ‘intrinsic’ cell death trigger (see Figure 3A and E). We further analyzed the effects of Eiger overexpression in other tissues using different GAL4 drivers. When overexpressed in the dorsoventral compartments of the wing discs by a vg-GAL4 driver, Eiger entirely blocked wing formation (Figure 2B), whereas Reaper induced only a regional defect (Figure 2D). Ectopic expression of Eiger in precursor cells for the external sensory organs by a sca-GAL4 driver resulted in disorganized macrochaetae in the notum and scutellum (Figure 2F), whereas Reaper induced a complete loss of bristles in these regions (Figure 2G). In the abdomen, on the other hand, sca>regg1GS9830 resulted in a severe developmental defect (Figure 2I), whereas sca>reaper caused a loss of bristles in the tergum (Figure 2J). Thus, Eiger has the potential to induce developmental defects distinct from the defects caused by the cell-autonomous killer protein Reaper.
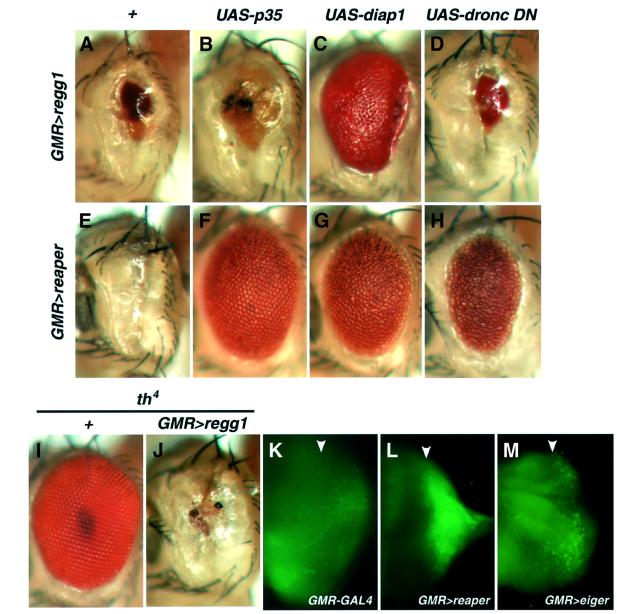
Fig. 3. Eiger activates an IAP-sensitive cell death pathway that does not require caspase activity. (A–H) Genetic interactions of Eiger or Reaper with caspase inhibitory proteins. Genotypes are as follows: regg1GS9830/+; GMR-GAL4/+ (A), regg1GS9830/GMR-GAL4; UAS-p35/+ (B), UAS-diap1/+; regg1GS9830/GMR-GAL4 (C), regg1GS9830/UAS-dronc DN; GMR-GAL4/+ (D), UAS-reaper/GMR-GAL4 (E), UAS-reaper/GMR-GAL4; UAS-p35/+ (F), UAS-diap1/+; UAS-reaper/GMR-GAL4 (G) and UAS-reaper/UAS-dronc DN; GMR-GAL4/+ (H). (I and J) Heterozygosity at the diap1 locus (th4) enhances the Eiger-induced eye phenotype. Whereas flies with a half dosage of the diap1 gene show a perfectly normal eye (I), the reduced-eye phenotype caused by Eiger overexpression (A) is strongly enhanced by the heterozygosity of th4 (J), indicating that endogenous DIAP1 negatively regulates the Eiger-stimulated death signal. Genotypes are th4/+ (I) and regg1GS9830/+; GMR-GAL4/th4 (J). (K–M) (DMe)2R staining of the eye discs shows that Eiger weakly but significantly causes caspase activation. Caspase activity was detected with rhodamine-110 fluorescence released from (DMe)2R. Genotypes are as follows: GMR-GAL4/+ (K), UAS-reaper/+; GMR-GAL4/+ (L) and UAS-eiger/+; GMR-GAL4/+ (M). Arrowheads indicate the position of the morphogenetic furrow.
Fig. 2. Eiger has the potential to induce severe developmental defects that are distinct from the defects caused by Reaper. Eiger or Reaper was ectopically expressed within the dorsoventral boundaries of the wing disc by a vg-GAL4 driver (B and D). Note that Eiger entirely blocked wing formation (B, arrowheads), whereas Reaper induced only a regional defect (D, arrowhead), compared with wild-type wings (A and B). Ectopic expression of Eiger in precursor cells for the external sensory organs driven by sca-GAL4 resulted in disorganization of the macrochaetae in the notum and scutellum (F, arrowheads), and a severe developmental defect of the abdomen (I), whereas Reaper induced a complete loss of bristles in these regions (G and J), compared with wild-type fly (E and H). sca>GAL4 and UAS-reaper flies were mated at 18°C, because their progeny, bearing both transgenes, died as pupae when generated at 25°C.
Eiger activates an IAP-sensitive cell death pathway that does not require caspase activity
In mammals, the binding of death ligands such as TNF-α and FasL to their receptors triggers the activation of caspase-8, leading to the subsequent caspase-dependent cell death cascade (Ashkenazi and Dixit, 1998). To assess whether Eiger stimulates similar death signaling in Drosophila, we co-expressed Eiger and caspase-inhibitory proteins such as baculovirus P35, Drosophila inhibitor-of-apoptosis protein 1 (DIAP1) or a dominant-negative form of DRONC (DRONC-DN), a P35-resistant Drosophila apical caspase (Meier et al., 2000b). P35 and DRONC-DN exhibited only slight suppressive effects on the Eiger-induced eye phenotype (Figure 3B and D), although they strongly suppressed the Reaper-induced eye ablation, as reported previously (White et al., 1996) (Figure 3F and H). These results indicate that Eiger-induced cell death does not require caspase activity differing from the mammalian ‘extrinsic’ cell death system. In contrast, the co-expression of DIAP1 strongly suppressed the Eiger-induced eye phenotype (Figure 3C), suggesting that DIAP1 could block Eiger-activated death signaling through a mechanism that was independent of caspase inhibition. We also assessed genetically whether endogenous DIAP1 negatively regulates the Eiger-induced phenotype. Whereas heterozygous diap1 mutant flies exhibit a normal eye (Figure 3I), GMR>regg1GS9830 flies with a half dosage of the diap1 gene displayed a completely ablated eye phenotype (Figure 3J) compared with the reduced-eye phenotype of GMR>regg1GS9830 flies (Figure 3A), suggesting that DIAP1 is an endogenous inhibitor of Eiger-induced cell death signaling.
To assess whether Eiger does cause caspase activation, the eye disc was stained with (DMe)2R, a caspase sub strate that contains only an aspartate residue linked to rhodamine-110 (Hug et al., 1999). The eye disc from GMR>reaper flies showed a strong rhodamine-110 fluorescence at the region posterior to the morphogenetic furrow (Figure 3L), compared with the eye disc from GMR-GAL4 flies (Figure 3K). In the GMR>regg1GS9830 eye disc, the fluorescence was detected at lower, but significant, levels in many cells posterior to the furrow (Figure 3M). These data suggest that although caspase activation is not essential for cell death execution, Eiger activates both caspase-dependent and -independent signaling pathways.
Eiger triggers the Drosophila JNK pathway
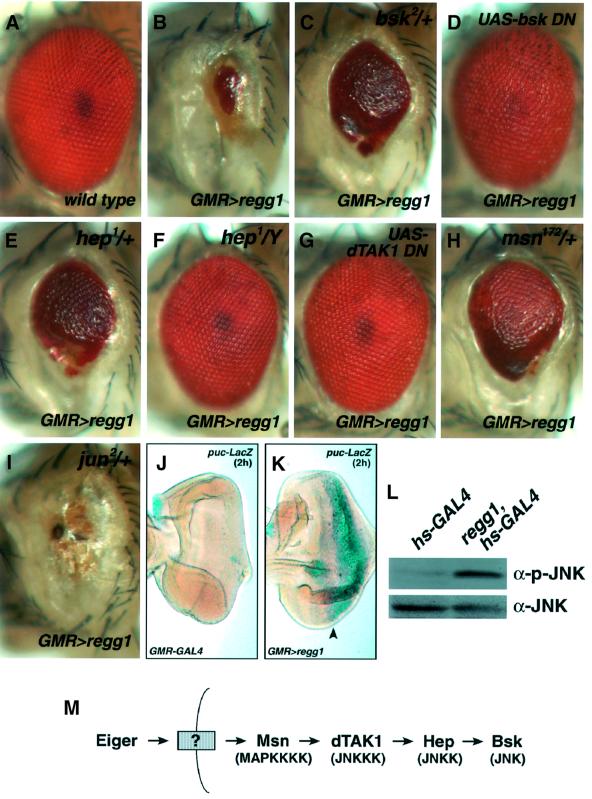
Many mammalian TNF superfamily proteins activate both the NF-κB and the JNK pathway, and activation of the latter pathway facilitates cell death (Davis, 2000). To examine whether Eiger activates the JNK pathway, we tested the genetic interactions of Eiger with the components of the Drosophila JNK cascade (Figure 4A–I). The reduced-eye phenotype induced by Eiger (Figure 4B) was strongly suppressed in a heterozygous mutant of Drosophila JNK, basket (bsk) (Figure 4C). In addition, overexpression of a dominant-negative form of Bsk almost completely suppressed the eye phenotype (Figure 4D). Moreover, heterozygosity at the hemipterous (hep) locus, which encodes Drosophila JNKK, suppressed the reduced-eye phenotype much like bsk did (Figure 4E), and its hemizygosity (null background) rescued the phenotype almost completely (Figure 4F). Furthermore, the co-expression of a dominant-negative form of dTAK1 (Drosophila JNKKK) (Mihaly et al., 2001) also rescued the Eiger-induced phenotype completely (Figure 4G). Misshapen (Msn) is a MAPKKKK that is genetically upstream of the JNK pathway in Drosophila (Su et al., 1998). We found that a half dosage of the msn gene strongly suppressed the Eiger-induced phenotype (Figure 4H). Heterozygosity of Drosophila jun, a target of the JNK pathway, did not show any genetic interaction with Eiger (Figure 4I), raising the possibility that the Eiger-stimulated death-inducing JNK pathway may not require new transcripts that are controlled by Jun.
Fig. 4. Eiger induces a reduced-eye phenotype through the activation of the JNK pathway. (A–I) regg1GS9830 genetically interacts with components of the Drosophila JNK signaling pathway. The Eiger-induced phenotype is suppressed by heterozygosity at the bsk, hep or msn locus (C, E and H, respectively). Hemizygosity at the hep locus almost completely suppresses the phenotype (F). Co-expression of a dominant-negative form of Bsk or dTAK1 completely inhibits the Eiger-induced eye phenotype (D and G). Heterozygosity at the Drosophila jun locus does not suppress the eye phenotype (I). Genotypes are as follows: wild type (A), regg1GS9830/GMR-GAL4; TM3,Sb/+ (B), regg1GS9830/bsk2; GMR-GAL4/+ (C), regg1GS9830/GMR-GAL4; UAS-bsk DN/+ (D), hep1/+; regg1GS9830/+; GMR-GAL4/+ (E), hep1/Y; regg1GS9830/+; GMR-GAL4/+ (F), regg1GS9830/UAS-dTAK1 DN; GMR-GAL4/+ (G), regg1GS9830/+; GMR-GAL4/msn172 (H) and regg1GS9830/jun2; GMR-GAL4/+ (I). (J and K) Overexpression of Eiger activates the JNK pathway. The JNK activation was monitored in GMR-GAL4/+ (J) and regg1GS9830/+; GMR-GAL4/+ (K) background eye disc by puc-LacZ expression. X-gal staining (2 h) of the eye disc shows dramatic activation of the JNK pathway in the region posterior to the morphogenetic furrow (K, arrowhead), where Eiger is overexpressed. (L) Eiger stimulates the phosphorylation of Bsk in vivo. hs-GAL4/+ or regg1GS9830/+; hs-GAL4/+ larvae were heat shocked at 37°C for 45 min and cultured at 25°C for a further 4 h, and then subjected to western blot analysis with an anti-JNK or an anti-phospho-JNK antibody. (M) A model for the JNK signaling triggered by Eiger.
Puckered (Puc) is a dual-specificity phosphatase, the expression of which is induced by the Drosophila JNK pathway to inactivate Bsk, so that puc expression can be used to monitor the extent of activation of the JNK pathway (Adachi-Yamada et al., 1999). To confirm that the JNK pathway was actually activated by Eiger, puc expression level was assessed in the eye disc of GMR>regg1GS9830 flies using a puc-LacZ enhancer-trap allele. The strong induction of puc-LacZ was observed in the region posterior to the morphogenetic furrow of the eye disc (Figure 4K) compared with the control eye disc (Figure 4J). Furthermore, western blot analysis with an anti-phospho-JNK antibody revealed that Bsk was phosphorylated by Eiger overexpression (Figure 4L). These genetic and biochemical data led to a model in which Eiger activates Msn, thereby triggering the JNK signaling pathway, sequentially stimulating dTAK1, Hep and Bsk (Figure 4M). We also tested, using RT–PCR analysis, whether Eiger could stimulate the NF-κB pathway; however, no obvious upregulation of the antimicrobial peptide genes, the target genes of the Drosophila NF-κB pathway, was detected (data not shown).
Eiger is predominantly expressed in the nervous system
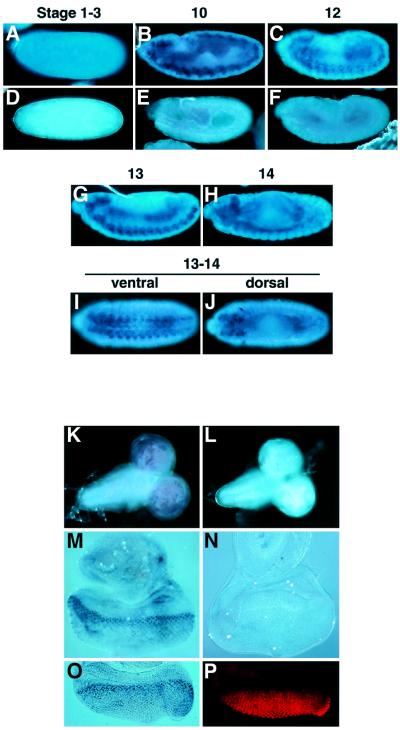
The spatial patterns of eiger expression during embryogenesis and in larval tissues were analyzed by in situ hybridization (Figure 5). Weak signals were detected in pre-blastoderm embryos (stage 1–3), indicating a low level of maternal contribution (Figure 5A). After stage 10, eiger transcripts were predominantly detected in the nervous system of the embryos (Figure 5B, C and G–J). In the third-instar larva, eiger was strongly expressed in the brain hemispheres (Figure 5K) and at the morphogenetic furrow in the eye disc (Figure 5M); it was also expressed at significant levels in many cells posterior to the furrow. Double staining of the eye disc with the eiger RNA probe and an anti-ELAV antibody, which recognizes terminally differentiated neuronal nuclei, revealed that eiger was strongly expressed in the proliferating cells at the furrow (Figure 5O and P). RT–PCR analysis demonstrated that eiger mRNA was expressed at all stages of Drosophila development (data not shown).
Fig. 5. eiger is predominantly expressed in the nervous system. (A–J) Whole-mount in situ hybridization of wild-type embryos at various stages of development using eiger antisense (A–C and G–H) or sense (D–F) RNA probes. A pre-blastoderm embryo showed low levels of eiger expression (A). After germ band extension, strong staining was evident in the nervous system (B, C and G–J). (K–N) In situ analysis of eiger expression in third-instar larval brains (K and L) and eye discs (M and N) using eiger antisense (K and M) or sense (L and M) RNA probes. A high level of eiger expression was evident in the brain hemispheres (K). In the eye disc, stronger staining was detected at the region posterior to the morphogenetic furrow (M). Double staining of the eye discs with the eiger RNA probe (O) and the anti-ELAV antibody (P) revealed that eiger was strongly expressed in the proliferating cells at the furrow.
Eiger is a physiological ligand for the Drosophila JNK pathway
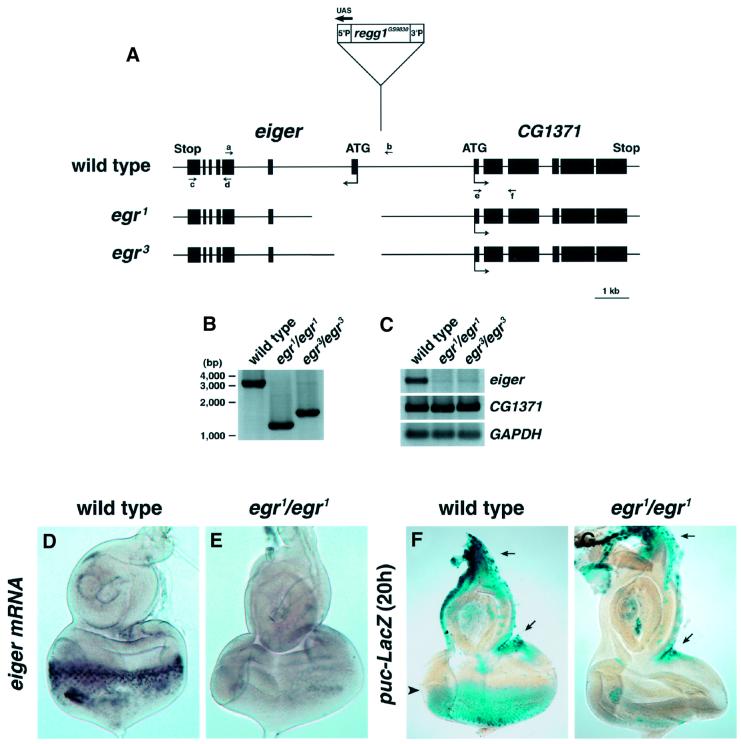
To analyze the physiological role of Eiger, we generated a loss-of-function mutant for eiger by imprecise excision of the P element in the Regg1GS9830 strain. We isolated nine mutants harboring deletions that removed parts of the eiger genomic sequences, but leaving right sequences downstream of the P element intact (Figure 6A and B). egr1 and egr3 mutants were homozygous viable with greatly reduced eiger expressions (Figure 6C and E). In the eye disc of the puc-LacZ enhancer-trap line, endogenous JNK activity could be detected in the region posterior to the morphogenetic furrow by long-time (20 h) X-gal staining (Figure 6F, arrowhead). We found that the puc-LacZ expression was dramatically reduced in the eye disc of the eiger mutant (Figure 6G). Notably, the JNK activities at the marginal region of the eye disc, where endogenous eiger expression was not detected (Figures 5M and 6D), were not affected in the eiger mutant (Figure 6F and G, arrows). These findings indicate that Eiger functions as a physiological ligand for the Drosophila JNK pathway.
Fig. 6. Eiger is a physiological ligand for the JNK pathway. (A) Diagram of the eiger genomic locus and location of the end points of excision mutations in egr1 and egr3 mutant alleles. Exon sequences are shown by closed boxes. (B) PCR analysis of genomic DNA from wild-type and homozygous eiger mutant adult flies using primers a and b [indicated in (A)]. The end points of genomic deletions in eiger mutants were determined by sequencing of the PCR products. (C) RT–PCR analysis of cDNA from wild-type and homozygous eiger mutant adult flies using primers c and d or e and f [indicated in (A)] to determine the expression levels of eiger and CG1371, respectively. GAPDH expression was determined as an internal control. (D and E) In situ analysis of eiger expression in the eye disc from wild-type (D) and egr1/egr1 (E) larvae. (F and G) The JNK activity was monitored in wild-type (F) and egr1/egr1 (G) background eye discs by puc-LacZ expression. Long-time (20 h) X-gal staining was able to detect the endogenous JNK activity in the region posterior to the morphogenetic furrow (F, arrowhead). The JNK activity was dramatically reduced in the eiger mutant (G). Note that the JNK activities in the disc margin, where eiger is not expressed even in the wild-type disc, are not affected in the eiger mutant (F and G).
Discussion
In our in vivo expression screen, we identified Eiger as a novel cell death trigger molecule in Drosophila. The structure and function of Eiger suggest that the extrinsic cell death-inducing mechanism might be evolutionarily conserved in Drosophila. Genetic evidence reveals that caspase activation is not essential to execute Eiger-induced cell death. The Drosophila extrinsic cell death system might predominantly utilize the caspase-independent pathway, in contrast to the intrinsic cell death system, which is regulated by Reaper, Hid and Grim, and depends completely on caspase activation. Although caspases do take part in the apoptotic effects of most of the mammalian TNF ligand/receptor superfamily members studied so far (Ashkenazi and Dixit, 1998; Locksley et al., 2001), there is accumulating evidence that they can also kill the cells in the absence of caspases (Holler et al., 2000; Matsumura et al., 2000; Denecker et al., 2001).
The genetic data presented here clearly show that the Eiger-induced small eye phenotype depends strongly on the JNK signaling pathway. In mammals, it has been demonstrated that the JNK pathway is essential for the execution of stress-induced cell death. JNK3, a JNK isoform that is selectively expressed in the nervous system, is required for neuronal cell death caused by excitotoxic stress (Yang et al., 1997). Embryonic fibroblasts from mouse deficient for both JNK1 and JNK2 are resistant to UV-stimulated apoptosis (Tournier et al., 2000). Whitfield et al. (2001) have shown that Bim acts downstream of the JNK pathway in NGF-deprivation-induced neuronal cell death. One possible downstream mechanism of the JNK pathway to induce cell death may be transcriptional upregulation of Bim. However, our results suggest the possibility that Eiger-induced cell death signaling may be independent of downstream jun expression, similar to the observation that the effect of UV to cause cell death does not require new gene expression (Tournier et al., 2000). The JNK signaling also mediates heat shock-induced cell death, the execution of which is caspase independent (Gabai et al., 2000). Furthermore, overexpression of the EDA receptor or TAJ/TROY, a member of the TNF receptor superfamily that exhibits extensive homology to the EDA receptor, results in the activation of the JNK pathway and caspase-independent cell death (Eby et al., 2000; Kumar et al., 2001). In some cases, JNK-induced cell death is mediated by the release of mitochondrial apoptogenic factors (Tournier et al., 2000). Recently, it has been shown that cancer cell death induced by TRAIL, a mammalian TNF superfamily ligand, requires mitochondrial release of Smac (Deng et al., 2002). One possible mechanism of Eiger-induced cell death may be JNK-mediated release of mitochondrial caspase-independent cell death factors. In fact, the Drosophila genome also encodes homologs of such molecules: AIF, endo G and HtrA2.
One important feature of Eiger-stimulating cell death signaling is that it can be blocked by DIAP1. It is well understood that IAP family proteins suppress cell death through direct inhibition of caspases (Deveraux and Reed, 1999). Our observations suggest a potential mechanism of IAP that can inhibit caspase-independent cell death. It has been reported that Xenopus cell death induced by TAK1 and TAB1, an activator for TAK1, is blocked by X-chromosome-linked IAP (XIAP) (Yamaguchi et al., 1999). More recently, it has been shown that XIAP attenuates TNF-α-mediated JNK activation in HeLa cells and RelA–/– fibroblasts (Tang et al., 2001). These findings and our data led to a model in Drosophila in which DIAP1 regulates caspase-dependent and -independent cell death pathways by blocking both the caspases and the JNK signaling.
Our loss-of-function study demonstrates that Eiger is a physiological trigger for the JNK pathway in the eye disc. We also show by genetic interaction assays that Eiger-stimulating cell death signaling is mediated by Msn, dTAK1, Hep and Bsk. Although dominant-negative dTAK1 completely suppressed the Eiger-induced phenotype in our experiments, it is also possible that many components of MAP kinase pathways expressed as ‘dominant negatives’ can have a gain-of-function inhibitory activity. In fact, the immune response phenotype of dTAK1 mutants seems to be inconsistent with the idea that dTAK1 participates in the Eiger pathway (Vidal et al., 2001). Another possible JNKKK family member to mediate Eiger signaling is Slipper (Stronach and Perrimon, 2002). Previous genetic studies in Drosophila have revealed that the JNK signaling pathway regulates epithelial morphogenesis during the process of embryonic dorsal closure, and also participates in the control of planar polarity in several tissues (Noselli and Agnes, 1999). It has also been reported that the JNK signaling regulates cell death to maintain normal morphogenesis of the wing (Adachi-Yamada et al., 1999). Eiger might function as a JNK-dependent cell death regulator to facilitate normal morphogenesis of the eye. Further analysis of eiger mutant flies would dissect the physiological role of Eiger in neural development.
In mammals, members of the TNF superfamily play crucial roles in the regulation of infections, inflammation, autoimmune diseases and tissue homeostasis (Locksley et al., 2001). The TNF superfamily ligands bind to their respective receptors leading to the activation of diverse signaling pathways, including the caspase cascade, NF-κB, or MAPKs such as JNK or ERK. Thus, TNF- related ligands can trigger either the extrinsic cell death execution, differentiation or proliferation. Although overexpression of Eiger can strongly induce cell death in the Drosophila compound eye, we can not exclude the possibility that Eiger-stimulated signaling may contribute to cellular events other than cell death execution. In fact, the amino acid sequence of Eiger showed the highest homology (19%) with EDA, a human TNF superfamily ligand, the mutation of which causes impaired ectodermal development. eiger is predominantly expressed in the nervous system, whereas most mammalian TNF/TNF receptor superfamily proteins are expressed in the immune system, raising the possibility that Eiger might regulate proliferation of neural progenitor cells such as TNF-α (Arnett et al., 2001) to maintain normal development of the nervous system. The Drosophila genome has a gene encoding a candidate Eiger receptor with a TNF receptor homology domain and a transmembrane domain. In addition, the Drosophila genome also encodes genes for mediating factors such as TNF-receptor-associated factors (TRAFs) (Liu et al., 1999; Zapata et al., 2000; Shen et al., 2001), FADD (Hu and Yang, 2000) and RIP (IMD) (Georgel et al., 2001), all of which may play a role in Eiger/Eiger receptor signaling. Further genetic study of Eiger and its receptor should help elucidate the universal role of TNF/TNF receptor superfamily proteins in normal development, as well as in some pathophysiological conditions.
Materials and methods
Molecular cloning and expression constructs
The genomic DNA surrounding regg1GS9830 was cloned by an inverse PCR method (http//www.fruitfly.org/about/methods/inverse.pcr.html) and sequenced. A Drosophila EST clone LP03784 was sequenced, and eiger cDNA was amplified by PCR, cloned into the EcoRI–XbaI sites of the pUAST vector to generate pUAS-eiger using the following PCR primers: 5′-AAAGAATTCACCATGACTGCCGAGACCCTCAA-3′ and 5′-AAATCTAGATTACACCTTGAAGATGCCAAA-3′. A head-to-head inverted repeat construct, pUAS-eiger-IR, was generated by inserting the eiger cDNA fragment (nucleotides 177–1230) into the EcoRI site of pUAS-eiger. An expression construct for HA-tagged Eiger was generated by PCR.
Histology, in situ hybridization and immunohistochemistry
Acridine orange staining was performed as described by Wolff and Ready (1991). (DMe)2R (Calbiochem) staining of third-instar larval eye disc was performed at final concentration of 50 µM in phosphate-buffered saline for 15 min at 37°C. In situ hybridization with digoxigenin-labeled eiger RNA probe was carried out as described by White et al. (1996). Anti-ELAV staining of the eye disc was performed using a monoclonal antibody, Elav-9F8A9 (1:10), and a Cy3-labeled anti-mouse IgG secondary antibody as described previously (Kanuka et al., 1999). Immunostaining of S2 cells was performed using an anti-HA monoclonal antibody (1:200) (12CA5; Boehringer Mannheim). The immunoreaction was carried out before or after fixation (4% PFA) and permeabilization (0.1% Triton X-100).
RT–PCR
For RT–PCR analysis of the genes surrounding regg1GS9830, total RNA was prepared from regg1GS9830/+; hs-GAL4/+ flies using Trizol (Gibco-BRL) 3 h after treatment with or without heat shock (twice at 37°C for 30 min with a 30 min interval), reverse transcribed, and subjected to PCR analysis (26 cycles) using the following primers: CG12919 (eiger), 5′-ATGACTGCCGAGACCCTCAAGCCG-3′ and 5′-TTACACCTTGAAGATGCCAAAGTAG-3′; CG1371, 5′-GAAGTCGTCGGCTGCGGTGGATTC-3′ and 5′-GATTTGTCCAACTGGGAGAAGCAG-3′; CG2269, 5′-ATGGCAGCAATGGCCAACCGATTTG-3′ and 5′-CTCCTTGGGCGTAACATTCACCCGC-3′; cdc2rk, 5′-ATGTCCAGCTTAAAGTCAAACGATG-3′ and 5′-GCAGCAGCTCACCAAGAATGC AGC-3′; GAL4, 5′-GCCAATTTTAATCAAAGTGGGAATA-3′ and 5′-GTTTGGTGGGGTATCTTCATCATC-3′; and GAPDH, 5′-CCACTGCCGAGGAGGTCAACTA-3′ and 5′-GCTCAGGGTGATTGCGTATGCA-3′. For antimicrobial peptide genes, cDNA prepared from heat-shocked hs-GAL4/+ or regg1GS9830/+; hs-GAL4/+ flies was subjected to PCR analysis (26 cycles) using primers specific for diptericin, cecropin A, defensin, attacin and drosomycin (Kim et al., 2000).
Cell culture, transfection and western blotting
S2 cells were cultured and transfected as described previously (Igaki et al., 2000). For western blotting, an anti-JNK polyclonal antibody (1:300; Santa Cruz) and an anti-phospho-JNK (p-JNK) polyclonal antibody (1:300; Promega) were used.
Fly stocks
Fly culture and crosses were carried out at 25°C, unless otherwise stated. Canton-S or white1118 was used as a wild-type strain. GMR-GAL4 virgins were crossed to males from the collection of GS strains (Toba et al., 1999). UAS-p35, UAS-diap1, UAS-dronc DN, UAS-bsk DN, UAS-dTAK DN (K46R) strains, th4, bsk2, hep1, msn172 and jun2 mutant strains, and a pucE69 enhancer-trap line were used for genetic interaction assays. We also used hs-GAL4, sca-GAL4 and vg-GAL4 strains. UAS-eiger and UAS-eiger-IR transgenic flies were generated by general P element-mediated transformation.
Generation of eiger mutants
The eiger mutant alleles were isolated in a screen for imprecise excisions of the P element in the Regg1GS9830 strain. The mini-white (w+)-marked P element was mobilized by the Δ2–3 transposase source. Two hundred and four fly lines in which the P element sequences had been excised were identified by their white eye color. Nine imprecise excisions, which removed parts of the eiger genomic sequences without affecting right sequences downstream of the P element, were identified by PCR analysis using primers corresponding to genomic sequences flanking the P element insertion site. Seven homozygous viable mutant lines were obtained with significantly decreased eiger expression.
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We are grateful to Naoko Tokushige, Tetsuo Hiratou and Ryoko Akai for technical support, Ryosuke Takahashi for critical reading of the manuscript, and Hideyuki Okano for invaluable advice. We also thank Jozsef Mihaly, Marek Mlodzik, Bruce Hay, Gerald Rubin, Herman Steller, John Nambu, Takashi Adachi-Yamada, Makoto Nakamura and the Bloomington Stock Center for fly stocks, Yasushi Hiromi for the pWAGAL4 plasmid, and John Gurdon and Ryusuke Niwa for the pUAS-GFP plasmid. We are grateful to Yasuo Uchiyama for encouragement. This work was supported in part by grants from the Japanese Ministry of Education, Science, Sports, Culture and Technology (to M.M.). E.K. is a research fellow of the Junior Research Associate Program, RIKEN. H.K. is a research fellow of the Special Postdoctoral Researchers Program, RIKEN. T.I. and H.K. are research fellows of the Japan Society for the Promotion of Science.
References
- Abrams J.M., White,K., Fessler,L.I. and Steller,H. (1993) Programmed cell death during Drosophila embryogenesis. Development, 117, 29–43. [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T., Fujimura,K.K., Nishida,Y. and Matsumoto,K. (1999) Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature, 400, 166–169. [DOI] [PubMed] [Google Scholar]
- Arnett H.A., Mason,J., Marino,M., Suzuki,K., Matsushima,G.K. and Ting,J.P. (2001) TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nature Neurosci., 4, 1116–1122. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A. and Dixit,V.M. (1998) Death receptors: signaling and modulation. Science, 281, 1305–1308. [DOI] [PubMed] [Google Scholar]
- Chen P., Nordstrom,W., Gish,B. and Abrams,J.M. (1996) grim, a novel cell death gene in Drosophila. Genes Dev., 10, 1773–1782. [DOI] [PubMed] [Google Scholar]
- Colussi P.A., Quinn,L.M., Huang,D.C., Coombe,M., Read,S.H., Richardson,H. and Kumar,S. (2000) Debcl, a proapoptotic Bcl-2 homologue, is a component of the Drosophila melanogaster cell death machinery. J. Cell Biol., 148, 703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Denecker G., Vercammen,D., Declercq,W. and Vandenabeele,P. (2001) Apoptotic and necrotic cell death induced by death domain receptors. Cell. Mol. Life Sci., 58, 356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Lin,Y. and Wu,X. (2002) TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev., 16, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux Q.L. and Reed,J.C. (1999) IAP family proteins—suppressors of apoptosis. Genes Dev., 13, 239–252. [DOI] [PubMed] [Google Scholar]
- Eby M.T., Jasmin,A., Kumar,A., Sharma,K. and Chaudhary,P.M. (2000) TAJ, a novel member of the tumor necrosis factor receptor family, activates the c-Jun N-terminal kinase pathway and mediates caspase-independent cell death. J. Biol. Chem., 275, 15336–15342. [DOI] [PubMed] [Google Scholar]
- Gabai V.L., Yaglom,J.A., Volloch,V., Meriin,A.B., Force,T., Koutroumanis,M., Massie,B., Mosser,D.D. and Sherman,M.Y. (2000) Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol. Cell. Biol., 20, 6826–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgel P. et al. (2001) Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev. Cell, 1, 503–514. [DOI] [PubMed] [Google Scholar]
- Grether M.E., Abrams,J.M., Agapite,J., White,K. and Steller,H. (1995) The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev., 9, 1694–1708. [DOI] [PubMed] [Google Scholar]
- Holler N. et al. (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature Immunol., 1, 489–495. [DOI] [PubMed] [Google Scholar]
- Hu S. and Yang,X. (2000) dFADD, a novel death domain-containing adapter protein for the Drosophila caspase DREDD. J. Biol. Chem., 275, 30761–30764. [DOI] [PubMed] [Google Scholar]
- Hug H., Los,M., Hirt,W. and Debatin,K.M. (1999) Rhodamine 110-linked amino acids and peptides as substrates to measure caspase activity upon apoptosis induction in intact cells. Biochemistry, 38, 13906–13911. [DOI] [PubMed] [Google Scholar]
- Igaki T., Kanuka,H., Inohara,N., Sawamoto,K., Nunez,G., Okano,H. and Miura,M. (2000) Drob-1, a Drosophila member of the Bcl-2/CED-9 family that promotes cell death. Proc. Natl Acad. Sci. USA, 97, 662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanuka H., Hisahara,S., Sawamoto,K., Shoji,S., Okano,H. and Miura,M. (1999) Proapoptotic activity of Caenorhabditis elegans CED-4 protein in Drosophila: implicated mechanisms for caspase activation. Proc. Natl Acad. Sci. USA, 96, 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.S. et al. (2000) Lipopolysaccharide-activated kinase, an essential component for the induction of the antimicrobial peptide genes in Drosophila melanogaster cells. J. Biol. Chem., 275, 2071–2079. [DOI] [PubMed] [Google Scholar]
- Kumar A., Eby,M.T., Sinha,S., Jasmin,A. and Chaudhary,P.M. (2001) The ectodermal dysplasia receptor activates the nuclear factor-κB, JNK and cell death pathways and binds to ectodysplasin A. J. Biol. Chem., 276, 2668–2677. [DOI] [PubMed] [Google Scholar]
- Liu H., Su,Y.C., Becker,E., Treisman,J. and Skolnik,E.Y. (1999) A Drosophila TNF-receptor-associated factor (TRAF) binds the ste20 kinase Misshapen and activates Jun kinase. Curr. Biol., 9, 101–104. [DOI] [PubMed] [Google Scholar]
- Locksley R.M., Killeen,N. and Lenardo,M.J. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell, 104, 487–501. [DOI] [PubMed] [Google Scholar]
- Matsumura H., Shimizu,Y., Ohsawa,Y., Kawahara,A., Uchiyama,Y. and Nagata,S. (2000) Necrotic death pathway in Fas receptor signaling. J. Cell Biol., 151, 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P., Finch,A. and Evan,G. (2000a) Apoptosis in development. Nature, 407, 796–801. [DOI] [PubMed] [Google Scholar]
- Meier P., Silke,J., Leevers,S.J. and Evan,G.I. (2000b) The Drosophila caspase DRONC is regulated by DIAP1. EMBO J., 19, 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly J., Kockel,L., Gaengel,K., Weber,U., Bohmann,D. and Mlodzik,M. (2001) The role of the Drosophila TAK homologue dTAK during development. Mech. Dev., 102, 67–79. [DOI] [PubMed] [Google Scholar]
- Noselli S. and Agnes,F. (1999) Roles of the JNK signaling pathway in Drosophila morphogenesis. Curr. Opin. Genet. Dev., 9, 466–472. [DOI] [PubMed] [Google Scholar]
- Raff M.C. (1992) Social controls on cell survival and cell death. Nature, 356, 397–400. [DOI] [PubMed] [Google Scholar]
- Shen B., Liu,H., Skolnik,E.Y. and Manley,J.L. (2001) Physical and functional interactions between Drosophila TRAF2 and Pelle kinase contribute to Dorsal activation. Proc. Natl Acad. Sci. USA, 98, 8596–8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. (2001) A structural view of mitochondria-mediated apoptosis. Nature Struct. Biol., 8, 394–401. [DOI] [PubMed] [Google Scholar]
- Stronach B. and Perrimon,N. (2002) Activation of the JNK pathway during dorsal closure in Drosophila requires the mixed lineage kinase, slipper. Genes Dev., 16, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.C., Treisman,J.E. and Skolnik,E.Y. (1998) The Drosophila Ste20-related kinase misshapen is required for embryonic dorsal closure and acts through a JNK MAPK module on an evolutionarily conserved signaling pathway. Genes Dev., 12, 2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., Minemoto,Y., Dibling,B., Purcell,N.H., Li,Z., Karin,M. and Lin,A. (2001) Inhibition of JNK activation through NF-κB target genes. Nature, 414, 313–317. [DOI] [PubMed] [Google Scholar]
- Toba G., Ohsako,T., Miyata,N., Ohtsuka,T., Seong,K.H. and Aigaki,T. (1999) The gene search system. A method for efficient detection and rapid molecular identification of genes in Drosophila melanogaster. Genetics, 151, 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier C. et al. (2000) Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science, 288, 870–874. [DOI] [PubMed] [Google Scholar]
- Vernooy S.Y., Copeland,J., Ghaboosi,N., Griffin,E.E., Yoo,S.J. and Hay,B.A. (2000) Cell death regulation in Drosophila: conservation of mechanism and unique insights. J. Cell Biol., 150, F69–F76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Khush,R.S., Leulier,F., Tzou,P., Nakamura,M. and Lemaitre,B. (2001) Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-κB-dependent innate immune responses. Genes Dev., 15, 1900–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K., Tahaoglu,E. and Steller,H. (1996) Cell killing by the Drosophila gene reaper. Science, 271, 805–807. [DOI] [PubMed] [Google Scholar]
- Whitfield J., Neame,S.J., Paquet,L., Bernard,O. and Ham,J. (2001) Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron, 29, 629–643. [DOI] [PubMed] [Google Scholar]
- Wolff T. and Ready,D.F. (1991) Cell death in normal and rough eye mutants of Drosophila. Development, 113, 825–839. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K. et al. (1999) XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1–TAK1 in the BMP signaling pathway. EMBO J., 18, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.D., Kuan,C.Y., Whitmarsh,A.J., Rincon,M., Zheng,T.S., Davis,R.J., Rakic,P. and Flavell,R.A. (1997) Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature, 389, 865–870. [DOI] [PubMed] [Google Scholar]
- Zapata J.M., Matsuzawa,S., Godzik,A., Leo,E., Wasserman,S.A. and Reed,J.C. (2000) The Drosophila tumor necrosis factor receptor-associated factor-1 (DTRAF1) interacts with Pelle and regulates NFκB activity. J. Biol. Chem., 275, 12102–12107. [DOI] [PubMed] [Google Scholar]