Abstract
Recently, the homolog of yeast protein Sec63p was identified in dog pancreas microsomes. This pancreatic DnaJ-like protein was shown to be an abundant protein, interacting with both the Sec61p complex and lumenal DnaK-like proteins, such as BiP. The pancreatic endoplasmic reticulum contains a second DnaJ-like membrane protein, which had been termed Mtj1p in mouse. Mtj1p is present in pancreatic microsomes at a lower concentration than Sec63p but has a higher affinity for BiP. In addition to a lumenal J-domain, Mtj1p contains a single transmembrane domain and a cytosolic domain which is in close contact with translating ribosomes and appears to have the ability to modulate translation. The interaction with ribosomes involves a highly charged region within the cytosolic domain of Mtj1p. We propose that Mtj1p represents a novel type of co-chaperone, mediating transmembrane recruitment of DnaK-like chaperones to ribosomes and, possibly, transmembrane signaling between ribosomes and DnaK-like chaperones of the endoplasmic reticulum.
Keywords: BiP/endoplasmic reticulum/molecular chaperones/Mtj1p/ribosome
Introduction
The initial step in the biogenesis of ∼30% of mammalian proteins is their integration into the membrane or their transport into the lumen of the endoplasmic reticulum (ER). Typically, protein integration and protein transport into the ER require signal peptides at the N-terminus of the precursor proteins and a transport machinery, involving soluble and membrane proteins. Protein integration or transport into the ER can occur co- or post-translationally. Post-translational protein transport into the yeast ER involves the Sec complex in the membrane, comprising the Sec61p subcomplex (Deshaies and Schekman, 1987; Esnault et al., 1993; Panzner et al., 1995), the putative signal peptide receptor subcomplex (Deshaies and Schekman, 1989; Deshaies et al., 1991; Green et al., 1992) and the DnaJ-like subunit, Sec63p (Toyn et al., 1988; Rothblatt et al., 1989; Sadler et al., 1989) plus lumenal DnaK-like proteins, i.e. Kar2p (Vogel et al., 1990; Nguyen et al., 1991; Sanders et al., 1992; Lyman and Schekman, 1995, 1997) and Lhs1p (Baxter et al., 1996; Craven et al., 1996; Hamilton and Flynn, 1996). Furthermore, Sec63p and Kar2p were reported to be involved in co-translational transport into the yeast ER (Brodsky et al., 1995; Young et al., 2001). Co-translational protein transport into dog pancreas microsomes involves a similar Sec61p complex (Görlich et al., 1992; Görlich and Rapoport, 1993; Hartmann et al., 1994). Two lines of evidence suggested that lumenal DnaK-like proteins, i.e. BiP/Grp78 and Grp170, are involved in co-translational protein transport into dog pancreas microsomes (Dierks et al., 1996; Hamman et al., 1998). Recently, a mammalian homolog of yeast protein Sec63p was discovered and shown to be an abundant protein in canine pancreatic microsomes (Skowronek et al., 1999; Meyer et al., 2000; Tyedmers et al., 2000). Futhermore, protein export from the ER into the cytosol, delivering misfolded proteins to the proteasome for degradation, has been shown to involve Sec61αp and Kar2p/BiP in mammals and yeasts (Knittler et al., 1995; Wiertz et al., 1996; Pilon et al., 1997; Plemper et al., 1997) and Sec63p in yeast (Plemper et al., 1997). Undoubtedly, the ER chaperones are also involved in protein folding and assembly in the ER.
In the ER of Saccharomyces cerevisiae, four DnaJ-like proteins have been identified, the three membrane proteins Sec63p (Toyn et al., 1988; Rothblatt et al., 1989; Sadler et al., 1989), Jem1p (Nishikawa and Endo, 1997) and Scj2p (Eki et al., 1996) and the lumenal protein Scj1p (Schlenstedt et al., 1995). All of these proteins have a lumenal J-domain. The cytosol of S.cerevisiae contains zuotin, a protein that was originally described to comprise a J-domain plus a so-called histone H1-like region. Recently, zuotin was characterized as a ribosome- associated co-chaperone of Ssb1p, Ssb2p and Ssz1p, i.e. ribosome-associated DnaK-like proteins, and as a chaperone of nascent polypeptides (Yan et al., 1998; Gautschi et al., 2001). The histone H1-like or highly charged region was shown to be crucial for ribosome interaction.
A putative Sec63p-related protein had been discovered in murine tumor cells and had been termed Mtj1p (murine tumor cell DnaJ-like protein 1) (Brightman et al., 1995; Chevalier et al., 2000). Here we asked if Mtj1p is present in the pancreatic ER and we addressed questions related to both its structure and its function. We observed that Mtj1p is present in pancreatic microsomes but less abundant than Sec63p. The lumenal J-domain of Mtj1p was shown to have a higher affinity for BiP as compared with Sec63p, thereby further characterizing this chaperone–co-chaperone interaction (Chevalier et al., 2000). We found that Mtj1p contains a single transmembrane domain and a cytosolic domain that can interact with ribosomes.
Results
Mtj1p is present in the pancreatic endoplasmic reticulum
In order to determine if Mtj1p is present in the pancreatic ER, anti-peptide antibodies against the murine protein were raised and affinity purified. The purified antibodies that were directed against the C-terminal peptide reacted only with the expected protein in immunoblots, employing total cellular or microsomal protein of rat pancreas cells and microsomal protein of canine pancreas (data not shown). The abundance of Mtj1p in microsomal preparations from rat and dog pancreas suggested that Mtj1p is an ER protein. The purified antibodies that were directed against the C-terminal peptide were used for immunofluorescence and immunoelectron microscopy, respectively, employing rat pancreas cells. Both microscopic analyses confirmed Mtj1p as a protein of the pancreatic ER (data not shown). Furthermore, the fluorescence microscopic analysis, employing digitonin, suggested that the C-terminal domain of Mtj1p faces the cytosol (see below).
In order to determine how abundant Mtj1p is in dog pancreas microsomes, immunoaffinity purification of this protein from microsomal detergent extracts with antibodies that were directed against the C-terminal peptide was employed. Subsequently the purified protein (Figure 2) was subjected to amino acid sequence analysis (Figure 1) and it was used as standard for the determination of its concentration in microsomal suspensions as described for Sec63p (Tyedmers et al., 2000). Dog pancreas microsomes were solubilized in detergent at high salt concentration and the post-ribosomal supernatant was applied to immobilized antibodies that were directed against the C-terminal peptide of Mtj1p. The dominant protein of the peptide eluate, as compared with the washing solution, had an apparent molecular mass in SDS of 66 kDa and was identified as Mtj1p by immunoblot analysis (Figure 2). In addition, the protein of interest was transferred to PVDF membranes and subjected to sequence analysis (Figure 1). The sequence analysis confirmed the identity of the purified protein and established that the protein band corresponds to a single protein, i.e. canine Mtj1p. Based on this information, the amount of protein present in the band of a gel of a similar protein preparation was determined by comparison with protein standards, which were run on the same gel and stained simultaneously (data not shown). Subsequently, an aliquot of the same sample of purified protein was run on the same gel together with increasing amounts of microsomes, and the known amount of purified protein served as a standard for the western blot signals (data not shown). We determined a concentration of 0.36 µM for Mtj1p in the microsomal suspensions. Thus Mtj1p is less abundant than Sec63p (1.98 µM; Tyedmers et al., 2000) in dog pancreas microsomes. Furthermore, the sequence analysis indicated that the first putative transmembrane domain of Mtj1p, as deduced from DNA sequence analysis (Figure 1; Brightman et al., 1995), is a functional signal peptide which is cleaved upon integration of the precursor polypeptide into the ER membrane (see below).
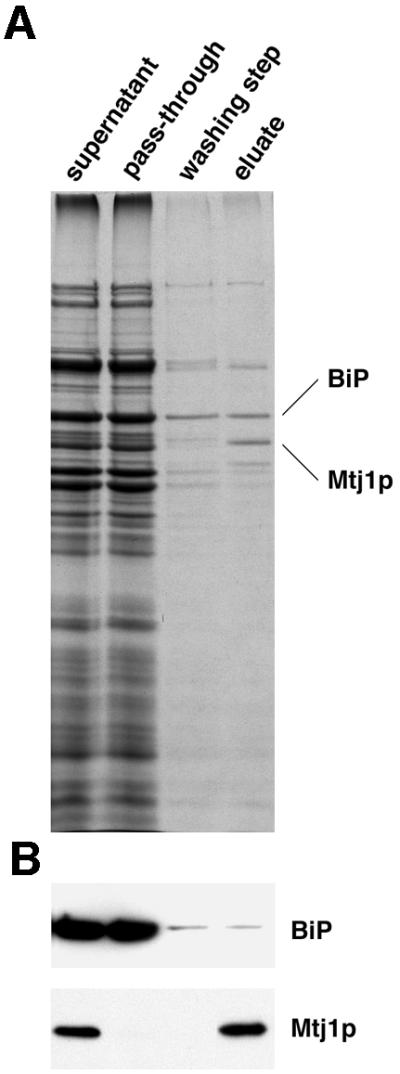
Fig. 2. Immunoaffinity purification of canine Mtj1p from pancreatic microsomes. The post-ribosomal supernatant, derived from a detergent extract of microsomes, was incubated with antibody resin as described in Materials and methods. The specifically bound material was eluted with peptide. Aliquots of the post-ribosomal supernatant and the pass-through as well as 80 times larger aliquots of the last washing step and the eluate were subjected to electrophoresis in polyacrylamide gels and subsequent staining with Coomassie Blue (A) or subsequent electroblotting to PVDF membranes, followed by immunological detection (B). Minor amounts of BiP were detected in washing buffer and eluate, probably due to the known ability of this protein to bind to denatured proteins and peptides.
Fig. 1. Sequence of Mtj1p. Sequence of murine Mtj1p (Brightman et al., 1995) and the N-terminal sequence of canine Mtj1p (shown below the murine sequence). The protein sequence data for canine Mtj1p will appear in the SWISS-PROT Protein Data Bank under accession number P82539. Note that: (i) two dots represent an identical amino acid residue, present in the canine and the murine protein; (ii) x represents an ambiguous result in the amino acid analysis; (iii) the underlined murine sequences represent putative transmembrane domains; (iv) italics indicate the J-domain; and (v) peptide sequences shown above the murine protein sequence represent peptides that were used for immunization.
Mtj1p is a membrane protein, comprising a lumenal J-domain and a large cytosolic domain
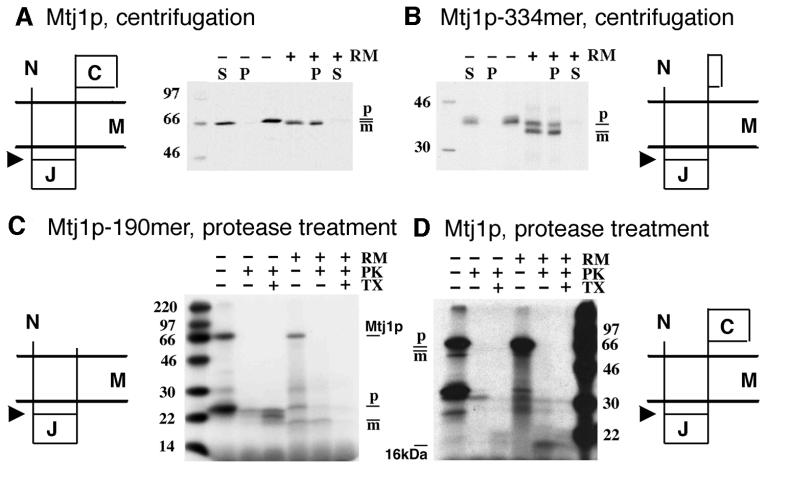
In order to characterize Mtj1p further, canine microsomes were subjected to carbonate extraction (data not shown) and to proteolysis (Figure 3A and B), respectively. Subsequently, protease sensitivity and carbonate sensitivity, respectively, of canine Mtj1p in intact microsomes were evaluated in immunoblots. Furthermore, the murine Mtj1p and two nascent Mtj1p polypeptide chains were synthesized in rabbit reticulocyte lysates in the presence of dog pancreas microsomes. Subsequently, the translation reactions were subjected to centrifugation and sequestration analysis, respectively, and the various samples were analyzed by SDS–PAGE and fluorography (Figure 4). Canine Mtj1p was found predominantly in the carbonate-resistant pellet. Thus carbonate extraction of dog pancreas microsomes characterized canine Mtj1p as a membrane protein. When dog pancreas microsomes were treated with proteinase K, canine Mtj1p was degraded (Figure 3A). High levels of protease gave rise to a fragment of ∼16 kDa, which was recognized by the antibodies directed against the J-domain of Mtj1p (Figure 3A). This fragment was observed when protease treatment was carried out in the absence of detergent but not in its presence. An established lumenal marker protein, such as BiP, was also protease resistant in the absence of detergent and protease sensitive in its presence (Figure 3B). Thus, the 16 kDa fragment of canine Mtj1p corresponds to the lumenal J-domain plus its membrane anchor (see below). After synthesis of murine Mtj1p and of a nascent polypeptide chain, corresponding to 334 N-terminal amino acid residues of Mtj1p, in reticulocyte lysates in the presence of canine microsomes and subsequent centrifugation, the translation products were observed in the microsomal pellets (Figure 4A and B). Apparently, the majority of these products had been processed by signal peptidase upon membrane integration, i.e. had a lower apparent molecular mass as compared with the primary translation products, seen after synthesis in the absence of microsomes. When in vitro synthesis of Mtj1p in the presence of microsomes was followed by protease treatment, a 16 kDa fragment of Mtj1p was detected after proteolysis in the absence of detergent but not in its presence (Figure 4D). This fragment was not observed when in vitro synthesis was carried out in the absence of microsomes (Figure 4D). Therefore, we concluded that this fragment was derived from the mature form of Mtj1p and corresponds to the lumenal J-domain plus its membrane anchor. In order to obtain direct support for this conclusion, nascent polypeptide chains, corresponding to 190 N-terminal amino acid residues of Mtj1p, were synthesized in reticulocyte lysates in the presence of canine microsomes and [3H]leucine (Figure 4C). Again, a significant proportion of these polypeptide chains was processed by signal peptidase. When in vitro synthesis of the Mtj1p 190mer in the presence of microsomes was followed by protease treatment, the mature polypeptide was found to be protease resistant after proteolysis in the absence of detergent but not in its presence (Figure 4C). This protease-resistant polypeptide was not observed when in vitro synthesis was carried out in the absence of microsomes. Thus, upon membrane integration, Mtj1p 190mer was processed to the corresponding mature polypeptide that was resistant to externally added protease. This mature polypeptide corresponded to the lumenal J-domain plus its single membrane anchor. Furthermore, this mature polypeptide had an electrophoretic mobility similar to that of the protease-resistant fragments, derived from Mtj1p after synthesis in the presence of microsomes and [3H]leucine (Figure 4D) and derived from endogenous canine Mtj1p (Figure 3A), respectively. Therefore, the data from the in vitro translation experiments confirmed that Mtj1p is synthesized as a larger precursor polypeptide whose signal peptide is cleaved upon integration into the ER membrane. In summary, Mtj1p contains a single transmembrane domain, separating an N-terminal and lumenal J-domain from a C-terminal and cytosolic domain.
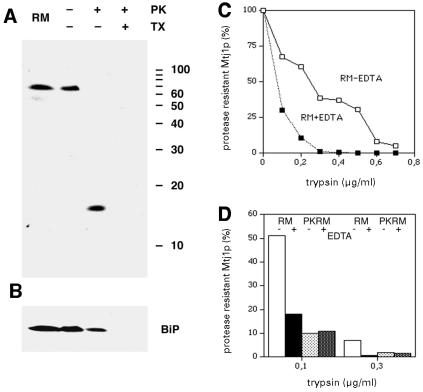
Fig. 3. Protease sensitivity of canine Mtj1p in dog pancreas microsomes. Aliquots of dog pancreas microsomes (RM in A–D) or of ribosome-stripped dog pancreas microsomes (PKRM in D) were left untreated or were supplemented with proteinase K (PK, 170 µg/ml) (A and B) or with trypsin (0.1–0.7 µg/ml) (C and D). (A and B) Digestion was carried out in the absence or presence of Triton X-100 [TX, 0.2% (v/v)] as indicated. (C and D) Digestion was carried out in the absence or presence of EDTA (10 mM) as indicated. After incubation for 60 min at 18 (A and B) or 0°C (C and D), the protease was inhibited and the samples were subjected to SDS–PAGE. The PVDF membranes were incubated with antibodies against the J-domain (A), BiP (B) or the C-terminal peptide of Mtj1p (C and D). The bound antibodies were made visible by incubation with ECL and exposure to X-ray film. The silver precipitation was quantified by densitometry (C and D). The intensity, obtained after protease treatment, is given as a percentage of the intensity derived from the untreated sample. The protein ladder was run on the same gel.
Fig. 4. In vitro synthesis of murine Mtj1p and nascent Mtj1p polypeptide chains. Mtj1p (A and D) and nascent Mtj1p polypeptide chains corresponding to 334 (B) or 190 (C) N-terminal amino acid residues were synthesized in rabbit reticulocyte lysates in the absence or presence of dog pancreas microsomes (RM) and in the presence of [35S]methionine (A and B) or [3H]leucine (C and D) as described in Materials and methods. The precursors of Mtj1p, Mtj1p-334mer and Mtj1p-190mer, respectively, are indicated with their predicted membrane orientation (C, C-terminal and cytosolic domain; J, lumenal J-domain; M, ER membrane; N, N-terminus; arrowhead, predicted cleavage site for signal peptidase). (A and B) The translation reactions were divided into two aliquots. One aliquot was subjected to centrifugation (10 min, 12 000 g, 4°C). The resulting supernatants (S) and pellets (P) as well as the aliquots, corresponding to the complete translation reactions, were subjected to SDS–PAGE and fluorography. (C and D) The translation reactions were divided into three aliquots. One aliquot was left untreated, the second aliquot was supplemented with proteinase K (PK; 170 µg/ml) and the third aliquot was supplemented with proteinase K plus Triton X-100 (TX; 0.2%). After incubation for 60 min at 0°C, the protease was inhibited and the samples were subjected to SDS–PAGE and fluorography. Note that, intentionally, the restriction digestion of the plasmid within the coding region was incomplete, giving rise to two translation products, the full-length protein (Mtj1p) and the nascent polypeptide chain (C). SDS–PAGE included a sample containing a mixture of 14C-labeled molecular mass standard proteins. p, precursor form; m, mature form. Note that 19 kDa, deduced on the basis of 14C-labeled molecular mass standard proteins, corresponds to 16 kDa as compared with the protein ladder (Figure 3).
The J-domain of Mtj1p functionally interacts with BiP
To determine whether Mtj1p contains a functional J-domain, a hybrid protein, comprising GST and amino acid residues 44–140 of murine Mtj1p (Figure 1), was constructed (termed GST–MtjJ-hybrid). Subsequently, we determined the apparent affinity of BiP for the GST–MtjJ-hybrid by surface plasmon resonance spectroscopy (Figure 5). Anti-GST antibodies were immobilized on a sensor chip. Then GST–MtjJ-hybrid was bound to the immobilized antibodies (measuring cell). GST bound to anti-GST antibodies served as a negative control (and was used in the reference cell). Subsequently, solutions containing increasing concentrations of murine BiP, purified as a recombinant protein from Escherichia coli, were passed over the chip in the absence or presence of ATP and in the presence of ATPγS, respectively. We determined an apparent affinity of BiP for the GST–MtjJ-hybrid in the presence of ATP (KD) of 0.12 × 10–6 M (Sec63p, 5 × 10–6 M; Tyedmers et al., 2000). However, we note that this apparent affinity has to be used with caution as the kinetics could not be fitted perfectly to a 1:1 binding model. Furthermore, it has to be kept in mind that the two apparent affinities refer to soluble GST hybrid proteins and not to the native membrane proteins. There was no interaction seen in the absence of ATP or in the presence of ATPγS (Figure 5B). In this respect, Mtj1p behaves identically to Sec63p (Tyedmers et al., 2000).
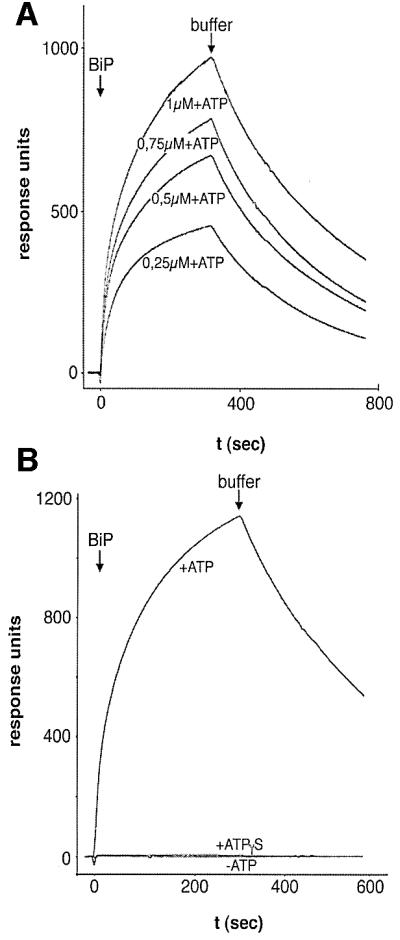
Fig. 5. Apparent affinity of GST–MtjJ-hybrid for BiP as measured by surface plasmon resonance spectroscopy. Monoclonal goat anti-GST antibodies were immobilized on a sensor chip CM5. GST–MtjJ-hybrid was bound to the immobilized antibodies in the measuring cell and GST was bound to the immobilized antibodies in the reference cell. At time zero, defined concentrations of recombinant BiP (2 µM in B) were passed over the chip in the presence of ATP (A) and in the absence or presence of ATP or ATPγS (B), respectively. Each BiP application was followed by application of running buffer. The response units were recorded as the difference between the measuring and reference cell dependent on time. Note that the residuals, representing the difference between measured and fitted data, showed an amplitude of up to 12% of the maximum signal and a non-random distribution.
The cytosolic domain of Mtj1p interacts with ribosomes
To determine the biological activity of the cytosolic domain of Mtj1p, the effect of ribosomes on the protease sensitivity of Mtj1p present in dog pancreas microsomes was studied in a first set of experiments (Figure 3C and D). When rough microsomes (RM) were treated with increasing concentrations of trypsin canine Mtj1p was degraded in a concentration-dependent manner (Figure 3C, –EDTA). At low levels of trypsin, protease sensitivity of Mtj1p was increased by the presence of EDTA (Figure 3C, +EDTA), i.e. under conditions of ribosome dissociation. This EDTA effect was not a general phenomenon since other membrane proteins, such as Sec62p, were not affected (data not shown). When RM were stripped with respect to translating ribosomes by treatment with puromycin plus high salt (i.e. converted to PKRM) and subsequently were subjected to trypsin treatment, Mtj1p showed a higher protease sensitivity as compared to RM (Figure 3D, –EDTA) and the presence of EDTA did not affect protease sensitivity (Figure 3D, +EDTA). Thus, increased protease sensitivity of Mtj1p in RM, as mediated by EDTA, was not a direct effect of EDTA on the protein but an indirect result of ribosome dissociation. Taken together, the effects of puromycin and EDTA suggested that Mtj1p is present in close proximity to or even associated with translating ribosomes on the surface of RM.
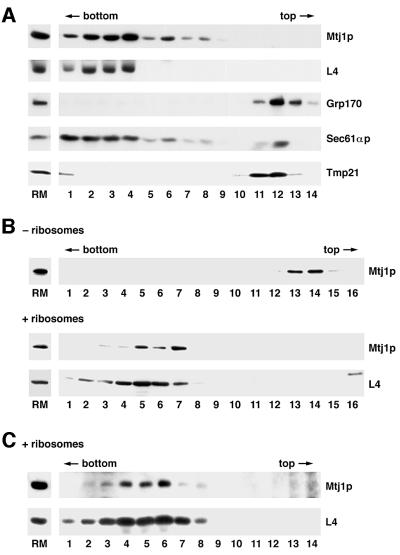
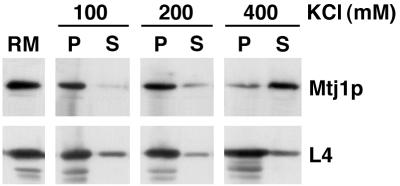
Therefore, in a second set of experiments, the putative ability of Mtj1p present in dog pancreas microsomes to associate with ribosomes was analyzed by detergent solubilization of microsomes and subsequent fractionation of the solubilized proteins by sucrose gradient centrifugation. Subsequently, the fractions were evaluated with respect to Mtj1p as well as to marker proteins in immunoblots (Figure 6A). Ribosomal protein L4 (Dierks et al., 1996) served as a ribosomal marker protein, Grp170 (Dierks et al., 1996) served as a lumenal marker protein, and Sec61αp (Görlich et al., 1992) and the putative cargo receptor protein p23/Tmp21 (Blum et al., 1999) were used as marker proteins of the microsomal membrane. Canine Mtj1p behaved as a ribosome-associated membrane protein, i.e. was found predominantly in the ribosomal fractions. In this respect, Mtj1p behaved similarly to an established ribosome-associated membrane protein (Sec61αp) and differently from other membrane proteins, such as Tmp21, as well from lumenal proteins, such as Grp170. However, in contrast to Sec61αp, Mtj1p was observed in association with ribosomes only at low ionic strength (up to 200 mM KCl; Figure 7) and irrespectively of the presence of nascent polypeptide chains (data not shown). This suggested a different type of interaction between Mtj1p and ribosomes as compared with the ribosome interaction of Sec61αp (Görlich et al., 1992). On the other hand, the puromycin resistance of the Mtj1p–ribosome interaction is reminiscent of what had been reported for the ribosome interactions of yeast zuotin (Yan et al., 1998) and of the mammalian ER membrane chaperone calnexin (Chevet et al., 1999), respectively. When a detergent extract, derived from ribosome-stripped microsomes (PKRM), was subjected to gradient centrifugation after incubation in the absence or presence of ribosomes, Mtj1p co-migrated with ribosomes in the presence of ribosomes but co-migrated with Grp170 in the absence of ribosomes (Figure 6B). Thus, co-migration of Mtj1p with ribosomes was not due to aggregation. In the next experiments, the ability of Mtj1p to associate with ribosomes directly was analyzed by incubation of partially purified canine Mtj1p, as present in the peptide eluate of the immunoaffinity column (Figure 2), with ribosomes and subsequent sucrose gradient centrifugation. Subsequently, the fractions were evaluated with respect to Mtj1p as well as to ribosomal protein L4 in immunoblots (Figure 6C). Mtj1p behaved as a ribosome-associated protein, i.e. was found in the ribosomal fractions. Again, Mtj1p was recovered in the top fractions of the gradient when incubation and centrifugation were carried out in the absence of ribosomes (data not shown).
Fig. 6. Mtj1p interacts with ribosomes. (A) A detergent extract of microsomal proteins, described in Materials and methods, was diluted to a final potassium chloride concentration of 100 mM and subjected to sucrose gradient centrifugation [linear gradient between 10 and 60% (w/v) in low salt extraction buffer without glycerol] for 90 min at 54 000 r.p.m. and 2°C (Beckman SW 55 rotor). The molar ratio between ribosomes and Mtj1p was ∼4:1. (B) A detergent extract, derived from PKRM by solubilization plus centrifugation as described in Materials and methods, was adjusted to a final potassium chloride concentration of 100 mM and incubated with or without ribosomes, derived from dog pancreas, for 15 min at 30°C (± ribosomes). The ratio between ribosomes and Mtj1p was ∼20:1. Subsequently, the mixture was subjected to sucrose gradient centrifugation as described above. (C) A peptide eluate (as shown in Figure 2) was diluted to a final potassium chloride concentration of 100 mM and incubated with ribosomes, derived from dog pancreas, for 15 min at 30°C (+ ribosomes). The ratio between ribosomes and Mtj1p was >20:1. Subsequently, the mixture was subjected to sucrose gradient centrifugation [linear gradient between 10 and 60% (w/v) in low salt extraction buffer without glycerol] for 120 min at 49 000 r.p.m. and 2°C (Beckman SW 55 rotor). After fractionation of the gradients, aliquots of the fractions were precipitated according to Wessel and Flügge (1984) and subjected to SDS–PAGE and subsequent electroblotting to PVDF membranes, followed by immunological detection.
Fig. 7. Mtj1p interacts with ribosomes at low ionic strength. A detergent extract of microsomal proteins, described in Materials and methods, was kept at 400 mM KCl or diluted to final potassium chloride concentrations of 100 or 200 mM. The molar ratio between ribosomes and Mtj1p was ∼4:1. After centrifugation for 30 min at 68 000 r.p.m. and 2°C (Beckman TLA 100.3 rotor), supernatants (S) and pellets (P) were subjected to SDS–PAGE and subsequent electroblotting to PVDF membranes, followed by immunological detection.
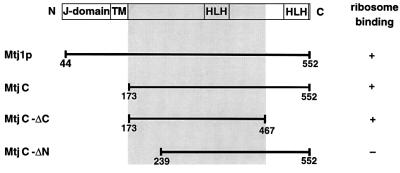
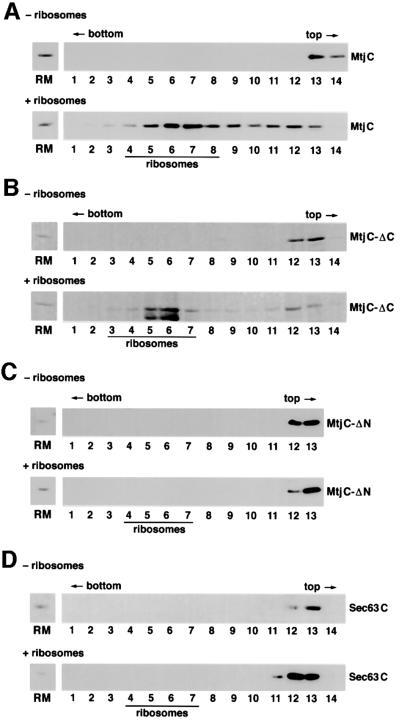
In order to address the specificity of the observed Mtj1p–ribosome interaction, the complete cytosolic domain of murine Mtj1p (termed MtjC), an N-terminally truncated cytosolic domain of murine Mtj1p (termed MtjC-ΔN) and a C-terminally truncated cytosolic domain of murine Mtj1p (termed MtjC-ΔC) were produced as recombinant proteins and analyzed with respect to their ribosome-binding abilities (Figures 8 and 9). The complete cytosolic domain of human Sec63p (termed Sec63C) served as a control. The ability of the recombinant proteins to associate with ribosomes was analyzed by incubation of the four proteins in the absence or presence of ribosomes and subsequent sucrose gradient centrifugation. Subsequently, the fractions were evaluated with respect to recombinant protein as well as to ribosomal proteins in gels and immunoblots, respectively (Figure 9). MtjC and MtjC-ΔC associated with ribosomes, i.e. were found in the ribosomal fractions. In contrast, MtjC-ΔN and Sec63C did not associate with ribosomes under these conditions. All three recombinant Mtj1p derivatives and Sec63C were recovered in the top fractions of the gradient when incubation and centrifugation were carried out in the absence of ribosomes (Figure 9). In summary, these ribosome-binding data suggested that ribosome binding of Mtj1p is specific and involves a charged region, which is located in the cytosolic domain, i.e. between amino acid residues 173 and 239 (Figure 8).
Fig. 8. Recombinant Mtj1p derivatives. The results of the ribosome-binding assays, shown in Figures 6 and 9, are indicated. HLH, helix– loop–helix or myb domain.
Fig. 9. Certain recombinant Mtj1p derivatives interact with ribosomes. (A–D) The indicated recombinant proteins were adjusted to potassium chloride and bovine serum albumin concentrations of 100 mM and 150 µg/ml, respectively, and incubated with or without ribosomes, derived from dog pancreas, for 15 min at 30°C (± ribosomes). The molar ratios between ribosomes and Mtj1p were ∼2:1. Subsequently, the mixtures were subjected to sucrose gradient centrifugation [linear gradient between 10 and 60% (w/v) in low salt extraction buffer without glycerol, adjusted to 33 µg/ml albumin] for 90 min at 54 000 r.p.m. and 2°C (Beckman SW 55 rotor). Note that in the analysis of MtjC-ΔC, lysozyme was used instead of albumin (B). After fractionation of the gradients, aliquots of the fractions were precipitated according to Wessel and Flügge (1984) and subjected to SDS–PAGE and subsequent protein staining with Coomassie Blue (B) or to SDS–PAGE and subsequent electroblotting to PVDF membranes, followed by immunological detection (A, C and D). The position of ribosomes in the gradients, as deduced from the presence of stained ribosomal proteins, is indicated.
The cytosolic domain of Mtj1p can modulate translation
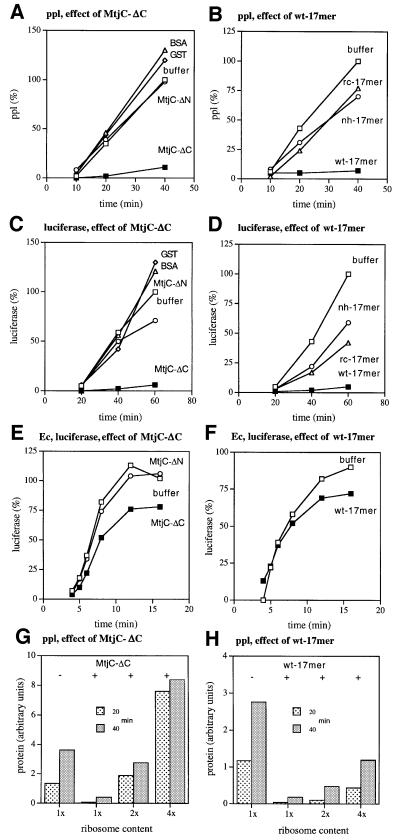
To analyze independently the ribosome interaction of Mtj1p, MtjC-ΔN and MtjC-ΔC were analyzed with respect to their abilities to modulate protein synthesis in vitro. Furthermore, a highly charged peptide (wt-17mer) which is located between amino acid residues 176 and 194 (Figure 8) and two mutant derivatives of this peptide were also synthesized and analyzed. The two mutant derivatives differed from the predominantly α-helical wild-type peptide with respect to helix formation propensity (no helix/nh-17mer) and charge distribution (reverse charge/rc-17mer), respectively. Cell-free protein synthesis was analyzed with respect to pre-secretory versus non-secretory proteins and with respect to mammalian versus bacterial cell-free translation systems (Figure 10). Synthesis of the model pre-secretory protein pre-prolactin (Figure 10A) as well as of the model non-secretory protein luciferase (Figure 10C) was sensitive towards MtjC-ΔC and resistant towards MtjC-ΔN in rabbit reticulocyte lysate. Furthermore, the wt-17mer had a similar inhibitory effect to that of MtjC-ΔC under these conditions, while the nh- and rc-17mers had a lower effect than the wild-type peptide (Figure 10B and D). The inhibitory effects were specific since their extents correlated reciprocally with the ribosome content of the reticulocyte lysate (Figure 10G and H). Bacterial ribosomes were less sensitive towards the recombinant protein and the peptide, respectively, as compared with mammalian ribosomes (Figure 10E and F). These data supported the conclusion that Mtj1p has the ability to interact with ribosomes and pointed to a highly charged region within this cytosolic domain that is responsible for this ability (amino acid residues 177–193). Furthermore, they suggest that the cytosolic domain of Mtj1p may have the ability to modulate protein synthesis.
Fig. 10. In vitro protein synthesis is inhibited by certain Mtj1p derivatives. Bovine pre-prolactin (A and B) and firefly luciferase (C–F) were synthesized in rabbit reticulocyte lysate (A–D, G and H) or E.coli (Ec) lysate (E and F) in the presence of [35S]methionine. (G and H) Prior to translation, the rabbit reticulocyte lysate was fractionated into ribosomes and post-ribosomal supernatant by centrifugation. Subsequently, the post-ribosomal supernatant was combined with the ribosomal pellet that corresponded to the same, the 2- or the 4-fold volume of reticulocyte lysate. The translation reactions were supplemented with buffer [20 mM HEPES–KOH pH 7.5, 500 mM KCl, 2 mM MgCl2, 0.65% CHAPS, 2 mM dithiothreitol (DTT)], purified proteins (final concentration: 0.18 µM) in the same buffer (A, C, E and G), or peptides (final concentration: 50 µM) in the same buffer (B, D, F and H). After various incubation times, aliquots of the complete translation reactions were subjected to SDS–PAGE. The gels were analyzed by phosphoimager analysis. The amount of full-length radiolabeled protein that was produced at the end of the buffer control reaction was set to 100% (A–F). The final concentrations of mammalian lysate and bacterial lysate, respectively, in the translation reactions were 10 (A), 15 (E), 20 (B, C, G and H), 30 (F) and 40% (D) (v/v). Note that the concentration of ribosomes in the rabbit reticulocyte-based translation reaction [40% lysate, (v/v)] is ∼0.3 µM. Furthermore, note that post-translational addition of MtjC-ΔC did not result in a reduced level of translation product as compared with the untreated translation reaction (data not shown). wt-17mer (ELLGRKKRERKKKTGSK); nh-17mer (ELLGRKPRERPKKTGSK); rc-17mer (ELLGREKRERKEKTGSK).
Mtj1p can recruit BiP to ribosomes
Ribosome binding of Mtj1p suggested that it may be involved in recruitment of microsomal Hsp70-type molecular chaperones to ribosomes. Therefore, partially purified canine Mtj1p, as present in the peptide eluate of the immunoaffinity column (Figure 2), was incubated with both ribosomes and BiP in the presence of ATP. Parallel incubations of BiP without any addition or with ribosomes served as controls. Subsequently, ribosomes were reisolated by centrifugation and the ribosome-associated proteins were characterized in immunoblots (Figure 11). BiP was observed to any significant extent in the ribosomal pellet only in the presence of Mtj1p. Thus, Mtj1p, as present in the ER membrane, may also be able to recruit BiP to ribosomes.
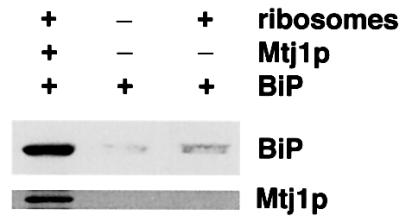
Fig. 11. Mtj1p can recruit BiP to ribosomes in solution. Recombinant BiP (25 pmol) and Mg-ATP (1 mM) were incubated in the absence or presence of peptide eluate (as shown in Figure 2), diluted to a final potassium chloride concentration of 100 mM, and in the simultaneous absence or presence of ribosomes (50 pmol), derived from dog pancreas, for 15 min at 30°C. The molar ratio between ribosomes and Mtj1p was >2:1. Subsequently, the mixture was layered onto a cushion (0.5 M sucrose in 40 mM HEPES–KOH, pH 7.5, 150 mM potassium acetate, 5 mM magnesium acetate, 2 mM DTT) and subjected to centrifugation for 90 min at 100 000 r.p.m. and 2°C (Beckman TLA 100 rotor). The pellets were subjected to SDS–PAGE and subsequent electroblotting to PVDF membranes, followed by immunological detection.
Discussion
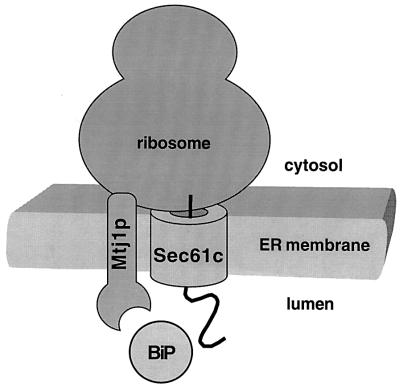
Here we report the identification of the third protein in the canine pancreatic ER with a lumenal J-domain. Previously, we had characterized the mammalian homolog of yeast protein Sec63p (Tyedmers et al., 2000) and identified the putative mammalian homolog of yeast protein Scj1p (termed ERj3p; Bies et al., 1999). We have shown previously that the mammalian Sec63p contains a functional J-domain (Tyedmers et al., 2000). The functional characteristics of this J-domain were similar to those of the corresponding J-domain of yeast Sec63p (Corsi and Schekman, 1997; Misselwitz et al., 1999). Here we observed that Mtj1p also contains a functional J-domain, thus complementing previous work by others (Chevalier et al., 2000). Mtj1p was found in close proximity to translating ribosomes on microsomal surfaces (summarized in Figure 12). This strongly suggests that Mtj1p plays a role in protein biogenesis. The ability of Mtj1p to interact with ribosomes appears to be due to the cytosolic domain, most likely to a highly charged region between amino acid residues 176 and 194. Taken together, this interaction is reminiscent of what had been reported for the ribosome interaction of yeast zuotin (Yan et al., 1998; Gautschi et al., 2001). Therefore, we suggest that Mtj1p plays a role in protein biogenesis similar to yeast zuotin, i.e. recruitment of DnaK-like proteins to ribosomes and thus nascent polypeptide chains (Figure 12). The difference between yeast zuotin and Mtj1p resides in the facts that Mtj1p must act through the ER membrane and that it must affect nascent polypeptide chains which are growing out of the ribosome and the Sec61p complex simultaneously (Figure 12). Therefore, the Hsp70 protein family members of the mammalian ER, BiP/Grp78 and Grp170, can be expected to be recruited to both the ribosome and the Sec61p complex by the membrane-integrated Hsp40 protein family member Mtj1p. Subsequently, BiP/Grp78 and Grp170 could assist in protein transport or in protein folding.
Fig. 12. Observed Mtj1p interactions. Ribosomes with nascent polypeptide chains protect Mtj1p against protease. Protection is abolished by puromycin or EDTA, i.e. ribosome dissociation. Mtj1p binds to ribosomes in detergent solution. The cytosolic domain of Mtj1p binds to ribosomes. Mtj1p recruits BiP to ribosomes in detergent solution. The lumenal J-domain of Mtj1p binds to BiP.
Alternatively, the observed ability of MtjC-ΔC to modulate translation could reflect a regulatory role for Mtj1p. Three possible functions of Mtj1p come to mind which may require transmembrane signaling between DnaK-like chaperones of the ER and ribosomes. The signaling can be envisaged as either modulation of BiP binding to the J-domain as mediated by a ribosome, bound to the cytosolic domain, or ribosome binding as mediated by bound BiP. (i) Lumenal Hsp70 protein family members play a dual role in protein transport into the ER, i.e. are involved in membrane insertion as well as in completion of translocation, and they play these roles in co- as well as in post-translational protein transport. In this context and in analogy to the situation in yeast, Sec63p appears to be the most likely co-chaperone in mammals. However, it is quite possible that in higher eukaryotic cells, the dual role of Sec63p in yeast cells is played by two distinct Hsp40 family members in mammalian cells and that Mtj1p plays this role with respect to co-translational transport. Taking into account the putative tetrameric structure of the Sec61p complex (Hanein et al., 1996), the observed concentration of Mtj1p in microsomal suspensions would be consistent with association of a single Mtj1p with a tetrameric Sec61p complex. In this scenario, the ability of Mtj1p to modulate translation could be involved in allowing signal peptides of nascent precursor polypeptides to be handed over from the signal recognition particle (SRP) to the Sec61p complex while elongation is attenuated. Elongation activity would be restored after BiP binding to Mtj1p. (ii) The membrane integration of newly synthesized polypeptides has been observed to involve BiP, and BiP was proposed to facilitate lumenal gating of the translocon (Liao et al., 1997; Hamman et al., 1998). Furthermore, it has been shown that recognition of the transmembrane segment of a nascent membrane protein occurs inside the ribosome and regulates lumenal gating by BiP. Thus, Mtj1p may transmit the information from the ribosome to BiP and, thereby, regulate lumenal gating. (iii) Since export of misfolded polypeptides from the lumen of the ER to the cytosol appears to involve the same Sec61p complexes and ER chaperones that facilitate import of polypeptides (Knittler et al., 1995; Wiertz et al., 1996; Pilon et al., 1997; Plemper et al., 1997), a regulatory mechanism has to be postulated that signals to the cytosol that protein export is in progress. Along these lines, Mtj1p would be an ideal candidate for such a signal transduction component. It may prevent elongation on ribosomes that are bound to Sec61p complexes while export is going on.
Materials and methods
Materials
The protein ladder (10–200 kDa) was obtained from Life Technologies. Peroxidase conjugate of anti-rabbit IgG goat antibodies and thrombin were from Sigma Chemical Company. [35S]methionine, [3H]leucine, Rainbow [14C]methylated protein molecular weight markers and enhanced chemiluminescence (ECL) were from Amersham Biosciences. PVDF membranes were from Millipore, X-ray films (X-Omat, AR) were from Kodak, and CHAPS was from Calbiochem.
Purification of Mtj1p and recombinant proteins
Dog pancreas microsomes were prepared as described (Watts et al., 1983). Where indicated, the microsomes were stripped with respect to ribosomes according to published procedures (Görlich and Rapoport, 1993). After re-isolation of microsomes by centrifugation, the pellets were resuspended in extraction buffer (20 mM HEPES–KOH pH 7.5, 400 mM KCl, 1 mM EDTA, 1.5 mM MgCl2, 15% glycerol, 0.65% CHAPS), resulting in a crude extract. Typically, the ribosomes were pelleted by centrifugation for 30 min at 2°C and 68 000 r.p.m. in a Beckman TLA 100.3 rotor. The post-ribosomal supernatant was incubated with affinity-purified and immobilized peptide antibodies for 3 h at 4°C. The suspension subsequently was transferred into a column (Mobitec). After collecting the flowthrough, the column was washed five times with loading buffer before eluting the protein that had bound to the peptide antibodies with the corresponding peptide at 1 mg/ml in loading buffer.
In the case of the GST–MtjJ-hybrid, a PCR product, coding for amino acids 44–140 of murine Mtj1p (Figure 1), was inserted into plasmid pGEX-4T-1, resulting in a plasmid coding for GST and the J-domain of murine Mtj1p. We note that due to the cloning procedure, the hybrid protein contained an additional oligopeptide (GSPEFPGRLERPHRD) at the C-terminus. In the case of the GST–MtjC-hybrids, PCR products coding for amino acids 173–552 and 239–552, respectively, of murine Mtj1p were inserted into plasmid pGEX-4T-1, resulting in plasmids coding for GST plus either the complete cytosolic domain of murine Mtj1p (GST–MtjC) or an N-terminal truncation (GST–MtjC-ΔN). In addition, a PCR product coding for amino acid residues 209–760 of human Sec63p was inserted into plasmid pGEX-4T-1, resulting in a plasmid coding for GST plus the complete cytosolic domain of human Sec63p (GST-Sec63C). Purification of the recombinant proteins was carried out similarly to the purification of GST–Sec63J-hybrid (Tyedmers et al., 2000). In the case of GST–C hybrid proteins, the buffer used for cell lysis was supplemented with CHAPS (0.65%) and KCl (500 mM). The two GST–MtjC-hybrids and the GST–Sec63C-hybrid were subjected to thrombin cleavage. Depending on the conditions, thrombin cleavage of GST–MtjC gave rise to either the complete cytosolic domain of murine Mtj1p (MtjC) or to a C-terminal truncation (MtjC-ΔC) that lacked the 85 C-terminal amino acids as compared with MtjC (i.e. according to N- and C-terminal sequence analysis as well as to apparent molecular mass in SDS). Purification of His-tagged murine BiP was described elsewhere (Bies et al., 1999).
Antibodies
Antibodies against the J-domain of Mtj1p were raised after cleavage of GST–MtjJ-hybrid with thrombin and subsequent purification by glutathione–Sepharose 4B and Mono Q chromatography. Furthermore, antibodies were raised against peptides plus an additional N-terminal cysteine (Figure 1) and immobilized as described (Tyedmers et al., 2000).
In vitro synthesis of Mtj1p
Plasmid pBSF (Brightman et al., 1995), which contains the coding region for murine Mtj1p under control of the T3 promotor, was used to synthesize Mtj1p in a coupled transcription–translation system (Promega) in the presence of [35S]methionine (final concentration: 1.4 mCi/ml) or [3H]leucine. Where indicated, dog pancreas microsomes were present during translation. In some experiments, plasmid pBSF was linearized within the coding region with HincII and BbsI, respectively, prior to in vitro transcription–translation, giving rise to nascent polypeptide chains, comprising the N-terminal 334 or 190 amino acid residues.
Analytical procedures
SDS–gel electrophoresis, electroblotting to PVDF membranes, incubation of PVDF membranes with specific antibodies and peroxidase conjugate of anti-rabbit IgG goat antibodies, visualization of antibodies by incubation of the blots in ECL and subsequent exposure to X-ray film, and amino acid sequencing by Edman degradation were carried out as described previously (Dierks et al., 1996). Surface plasmon resonance spectroscopy was carried out in a BIAlite upgrade system and analyzed as described (Tyedmers et al., 2000).
Acknowledgments
Acknowledgements
Anti-BiP antibodies and anti-p23/Tmp21 antibodies were kindly donated by G.Kreibich (New York) and R.Blum (Homburg), respectively. Purified GST was kindly donated by J.Solsbacher. C.B. was supported by a fellowship from the Graduiertenkolleg ‘Zelluläre Regulation und Wachstum’. This work was supported by the DFG and by the FCI.
References
- Baxter B.K., James,P., Evans,T. and Craig,E. (1996) SSI1 encodes a novel Hsp70 of the Saccharomyces cerevisiae endoplasmic reticulum. Mol. Cell. Biol. 16, 6444–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bies C., Guth,S., Janoschek,K., Nastainczyk,W., Volkmer,J. and Zimmermann,R. (1999) A Scj1p homolog and folding catalysts present in dog pancreas microsomes. Biol. Chem., 380, 1175–1182. [DOI] [PubMed] [Google Scholar]
- Blum R., Pfeiffer,F., Feick,P., Nastainczyk,W., Kohler,B., Schäfer,K.-H. and Schulz,I. (1999) Intracellular localization and in vivo trafficking of p24A and p23. J. Cell Sci., 112, 537–548. [DOI] [PubMed] [Google Scholar]
- Brightman S.E., Blatch,G.L. and Zetter,B.R. (1995) Isolation of a mouse cDNA encoding MTJ1, a new murine member of the DnaJ family of proteins. Gene, 153, 249–254. [DOI] [PubMed] [Google Scholar]
- Brodsky J.L., Goeckeler,J. and Schekman,R. (1995) BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc. Natl Acad. Sci. USA, 92, 9643–9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier M., Rhee,H., Elguindi,E.C. and Blond,S.Y. (2000) Interaction of murine BiP/Grp78 with the DnaJ homologue Mtj1. J. Biol. Chem., 275, 19620–19627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevet E., Wong,H.N., Gerber,D., Cochet,C., Fazel,A., Cameron,P.H., Gushue,J.N., Thomas,D.Y. and Bergeron,J.J.M. (1999) Phosphoryl ation by CK2 and MAPK enhances calnexin association with ribosomes. EMBO J., 18, 3655–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi A.K. and Schekman,R. (1997) Mechanism of polypeptide translocation into the endoplasmic reticulum. J. Cell Biol., 137, 1483–1493. [DOI] [PubMed] [Google Scholar]
- Craven R.A., Egerton,M. and Stirling,C.J. (1996) A novel Hsp70 of the yeast ER lumen is required for the efficient translocation of a number of protein precursors. EMBO J., 15, 2640–2650. [PMC free article] [PubMed] [Google Scholar]
- Deshaies R.J. and Schekman,R. (1987) A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J. Cell Biol., 105, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R.J. and Schekman,R. (1989) SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J. Cell Biol., 109, 2653–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R.J., Sanders,S.L., Feldheim,D.A. and Schekman,R. (1991) Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature, 349, 806–808. [DOI] [PubMed] [Google Scholar]
- Dierks T. et al. (1996) A microsomal ATP-binding protein involved in efficient protein transport into the mammalian endoplasmic reticulum. EMBO J., 15, 6931–6942. [PMC free article] [PubMed] [Google Scholar]
- Eki T. et al. (1996) Analysis of a 36.2 kb DNA sequence including the right telomere of chromosome VI from Saccharomyces cerevisiae. Yeast, 12, 149–167. [DOI] [PubMed] [Google Scholar]
- Esnault, Y., Blondel,M.-O., Deshaies,R.J., Scheckman,R. and Kepes, F (1993) The yeast SSS1 gene is essential for secretory protein translocation and encodes a conserved protein of the endoplasmic reticulum. EMBO J., 12, 4083–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi M., Lilie,H., Funfschilling,U., Mun,A., Ross,S., Lithgow,T., Rucknagel,P. and Rospert,S. (2001) RAC, a stable ribosome-associated complex in yeast formed by the DnaK–DnaJ homologs Ssz1p and zuotin. Proc. Natl Acad. Sci. USA, 98, 3762–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D. and Rapoport,T.A. (1993) Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell, 75, 615–630. [DOI] [PubMed] [Google Scholar]
- Görlich D., Prehn,S., Hartmann,E., Kalies,K.-U. and Rapoport,T.A. (1992) A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell, 71, 489–503. [DOI] [PubMed] [Google Scholar]
- Green N., Fang,H. and Walter,P. (1992) Mutants in three novel complementation groups inhibit membrane protein insertion into and soluble protein translocation across the endoplasmic reticulum membrane of Saccharomyces cerevisiae. J. Cell Biol., 116, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T.G. and Flynn,G.C. (1996) Cer1p, a novel Hsp70-related protein required for posttranslational endoplasmic reticulum translocation in yeast. J. Biol. Chem., 271, 30610–30613. [DOI] [PubMed] [Google Scholar]
- Hamman B.D., Hendershot,L.M. and Johnson,A.E. (1998) BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation. Cell, 92, 747–758. [DOI] [PubMed] [Google Scholar]
- Hanein D., Matlack,K.E.S., Jungnickel,B., Plath,K., Kalies,K.-U., Miller,K.R., Rapoport,T.A. and Akey,C.W. (1996) Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell, 87, 721–732. [DOI] [PubMed] [Google Scholar]
- Hartmann E., Sommer,T., Prehn,S., Görlich,D., Jentsch,S. and Rapoport,T.A. (1994) Evolutionary conservation of components of the protein translocation complex. Nature, 367, 654–657. [DOI] [PubMed] [Google Scholar]
- Knittler M.R., Dirks,S. and Haas,I.G. (1995) Molecular chaperones involved in protein degradation in the endoplasmic reticulum: quantitative interaction of the heat shock cognate protein BiP with partially folded immunoglobulin light chains that are degraded in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA, 92, 1764–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S., Lin,J., Do,H. and Johnson,A.E. (1997) Both lumenal and cytosolic gating of the aqueous ER translocon pore are regulated from inside the ribosome during membrane protein integration. Cell, 90, 31–41. [DOI] [PubMed] [Google Scholar]
- Lyman S.K. and Schekman,R. (1995) Interaction between BiP and Sec63p is required for the completion of protein translocation into the ER of Saccharomyces cerevisiae. J. Cell Biol., 131, 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman S.K. and Schekman,R. (1997) Binding of secretory precursor polypeptides to a translocon subcomplex is regulated by BiP. Cell, 88, 85–96. [DOI] [PubMed] [Google Scholar]
- Meyer H.-A., Grau,H., Kraft,R., Kostka,S., Prehn,S., Kalies,K.-U. and Hartmann,E. (2000) Mammalian Sec61 is associated with Sec62 and Sec63. J. Biol. Chem., 275, 14550–14557. [DOI] [PubMed] [Google Scholar]
- Misselwitz B., Staeck,O., Matlack,K.E.S. and Rapoport,T.A. (1999) Interaction of BiP with the J-domain of the Sec63p component of the endoplasmic reticulum protein translocation complex. J. Biol. Chem., 274, 20110–20115. [DOI] [PubMed] [Google Scholar]
- Nguyen T.H., Law,D.T. and Williams,D.B. (1991) Binding protein BiP is required for translocation of secretory proteins into the endoplasmic reticulum in Saccharomyces cerevisiae.Proc. Natl Acad. Sci. USA, 88, 1565–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S. and Endo,T. (1997) The yeast Jem1p is a DnaJ-like protein of the endoplasmic reticulum membrane required for nuclear fusion. J. Biol. Chem., 272, 12889–12892. [DOI] [PubMed] [Google Scholar]
- Panzner S., Dreier,L., Hartmann,E., Kostka,S. and Rapoport,T.A. (1995) Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell, 81, 561–570. [DOI] [PubMed] [Google Scholar]
- Pilon M., Schekman,R. and Römisch,K. (1997) Sec61p mediates export of a misfolded secretory protein from the endoplasmic reticulum to the cytosol for degradation. EMBO J., 16, 4540–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemper R.K., Bömler,S., Bordallo,J., Sommer,T. and Wolf,D.H. (1997) Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature, 388, 891–895. [DOI] [PubMed] [Google Scholar]
- Rothblatt J.A., Deshaies,R.J., Sanders,S.L., Daum,G. and Schekman,R. (1989) Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J. Cell Biol., 109, 2641–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler I., Chiang,A., Kurihara,T., Rothblatt,J.A., Way,J. and Silver,P. (1989) A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, an Escherichia coli heat shock protein. J. Cell Biol., 109, 2665–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S.L., Whitfield,K.M., Vogel,J.P., Rose,M.D. and Schekman,R. (1992) Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell, 69, 353–365. [DOI] [PubMed] [Google Scholar]
- Schlenstedt G., Harris,S., Risse,B., Lill,R. and Silver,P.A. (1995) A yeast DnaJ homologue, Scj1p, can function in the endoplasmic reticulum with BiP/Kar2p via a conserved domain that specifies interactions with Hsp70. J. Cell Biol., 129, 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronek M.H., Rotter,M. and Haas,I.G. (1999) Molecular characterization of a novel mammalian DnaJ-like Sec63p homolog. Biol. Chem., 380, 1133–1138. [DOI] [PubMed] [Google Scholar]
- Toyn J., Hibbs,A.R., Sanz,P., Crowe,J. and Meyer,D.I. (1988) In vivo and in vitro analysis of ptl1, a yeast ts mutant with a membrane-associated defect in protein translocation. EMBO J., 7, 4347–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J. et al. (2000) Homologs of the yeast Sec complex subunits Sec62p and Sec63p are abundant proteins in dog pancreas microsomes. Proc. Natl Acad. Sci. USA, 97, 7214–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.P., Misra,L.M. and Rose,M.D. (1990) Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J. Cell Biol., 110, 1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C., Wickner,W. and Zimmermann,R. (1983) M13 procoat and pre-immunoglobulin share processing specificity but use different membrane receptor mechanisms. Proc. Natl Acad. Sci. USA, 80, 2809–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D. and Flügge,U.I. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergent and lipids. Anal. Biochem., 138, 141–143. [DOI] [PubMed] [Google Scholar]
- Wiertz E.J.H., Tortoralla,D., Bogyo,M., Yu,J., Mothes,W., Jones,T.R., Rapoport,T.A. and Ploegh,H.L. (1996) Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature, 384, 432–438. [DOI] [PubMed] [Google Scholar]
- Yan W., Schilke,B., Pfund,C., Walter,W., Kim,S. and Craig,E.A. (1998) Zuotin, a ribosome associated DnaJ molecular chaperone. EMBO J., 17, 4809–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.P., Craven,R.A., Reid,P.J., Willer,M. and Stirling,C.J. (2001) Sec63p and Kar2p are required for the translocation of SRP-dependent precursors into the yeast endoplasmic reticulum in vivo. EMBO J., 20, 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]