Abstract
In this study, we investigated the subcellular and molecular mechanisms underlying promyelocytic leu kemia (PML)-induced premature senescence. We demonstrate that intact PML nuclear bodies are not required for the induction of senescence. We have determined further that of seven known PML isoforms, only PML IV is capable of causing premature senescence, providing the first evidence for functional differences among these isoforms. Of interest is the fact that in contrast to PML+/+ fibroblasts, PML–/– cells are resistant to PML IV-induced senescence. This suggests that although PML IV is necessary for this process to occur, it is not sufficient and requires other components for activity. Finally, we provide evidence that PML IV-induced senescence involves stabilization and activation of p53 through phosphorylation at Ser46 and acetylation at Lys382, and that it occurs independently of telomerase and differs from that elicited by oncogenic Ras. Taken together, our data assign a specific pro-senescent activity to an individual PML isoform that involves p53 activation and is independent from PML nuclear bodies.
Keywords: p53/PML/PML nuclear bodies/senescence/telomerase
Introduction
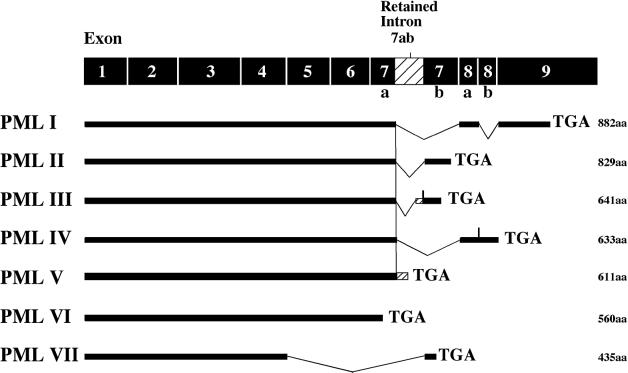
The promyelocytic leukemia (PML) gene was initially identified in patients with acute PML (APL), in which it is fused to the retinoic acid receptor α (RARα) gene as a result of the t(15;17) chromosomal translocation (de The et al., 1991; Goddard et al., 1991; Kakizuka et al., 1991; Kastner et al., 1991). Overexpression of PML induces either growth arrest or apoptosis depending on cell type and/or level of expression (reviewed in Pearson and Pelicci, 2001). Conversely, PML gene inactivation results in increased cell proliferation and tumor susceptibility (Wang et al., 1998). The PML gene consists of nine exons and at least seven spliced transcripts differing in their 3′ ends, giving rise to seven PML isoforms designated PML I–VII according to the unified PML nomenclature by Jensen et al. (2001). The specific functions of the splice variants, which most likely rely on their C-terminal sequences, remain largely unexplored.
All PML isoforms, with the sole exception of cytoplasmic PML isoform VII, concentrate within discrete subnuclear structures, termed PML nuclear bodies (NBs), which are disrupted in a retinoic acid-reversible manner in APL cells (Dyck et al., 1994; Koken et al., 1994; Weis et al., 1994). The integrity of the NBs is also compromised by the herpesvirus protein ICPO and the cytomegalovirus protein IE1, which have been shown to associate with and entirely disrupt PML NBs (Everett, 2001). PML is subject to post-translational modification by SUMO, a small ubiquitin-related modifier (Sternsdorf et al., 1997; Kamitani et al., 1998; Muller et al., 1998) and sumoylation was shown to be required for proper formation of the NBs and recruitment of NB-associated proteins (Ishov et al., 1999; Zhong et al., 2000a; Lallemand-Breitenbach et al., 2001). The plethora of colocalizing proteins with PML NBs, including SP100, Daxx, CBP, pRB and p53 among others, has led to the notion that these bodies either function as nuclear depots/dumps (reviewed in Negorev and Maul, 2001) or as organizing centers where nuclear processes such as transcription or apoptosis are executed and regulated (reviewed in Zhong et al., 2000b).
Cellular senescence was first recognized by Hayflick (1961) as a process that prevents normal fibroblasts from growing indefinitely in culture. This process, now known as replicative senescence, is tightly linked to telomere shortening (reviewed in Stewart and Weinberg, 2000; Mathon and Lloyd, 2001; Shay and Wright, 2001). Stimuli having no or only marginal effects on telomere shortening, such as oxidative stress, DNA damage, chromatin remodeling and intense mitogenic signaling, have also been shown to induce growth-arrest in primary fibroblasts, which is reminiscent of cellular senescence. These stimuli induce senescence only after a few cell divisions, i.e. prematurely (reviewed in Serrano and Blasco, 2001).
Consistent with its role in tumor suppression, cellular senescence is controlled by a number of tumor suppressor genes; the most crucial of these encode the p53 and pRB proteins (reviewed in Bringold and Serrano, 2000; Lundberg et al., 2000; Campisi, 2001). p53 is a transcriptional regulator controlling a wide range of genes that cause cell-cycle arrest, and its transcriptional activity and stability increase when cells senesce (reviewed in Itahana et al., 2001). It was recently shown that mice with increased p53 activity age prematurely, thus providing the first evidence for a link between p53 activation, cellular senescence and organismal ageing (Tyner et al., 2002). p53 can be activated by a variety of signals, some of which produce specific p53 post-translational modifications such as acetylation and phosphorylation (reviewed in Prives and Hall, 1999; Appella and Anderson, 2001). Recently, a link between premature senescence, PML NBs and p53 has been established. Upon PML overexpression, p53 and CBP are recruited to NBs and a ternary complex between p53, CBP and PML is formed leading to increased acetylation of p53 at lysine 382, enhancement of p53 activity and the induction of premature senescence (Ferbeyre et al., 2000; Pearson et al., 2000). However, the connection between PML NBs and activation of p53 therein was circumstantial, and therefore it remained to be seen whether PML NBs are indeed the deciding factor for premature senescence to occur.
In the present work, we show that only constitutive overexpression of PML IV is capable of promoting premature senescence in PML+/+ murine and human primary fibroblasts but not in PML–/– murine cells. PML IV-induced senescence is telomerase-independent, activates p53 and differs from premature senescence elicited by Ras. All these changes are independent from PML sumoylation and intact PML NBs. Together, these findings provide new insights into the functionality of PML isoforms and PML NBs.
Results
PML-induced premature senescence is specific for isoform IV and is telomerase independent in primary human fibroblasts
To date, seven different PML isoforms have been characterized that differ only in their C-termini (Jensen et al., 2001) (Figure 1). Ectopic expression of PML elicits premature senescence in human as well as murine primary fibroblasts (Ferbeyre et al., 2000; Pearson et al., 2000). To determine whether PML-induced senescence is a common feature of all PML isoforms, we compared isoforms I to V for their ability to cause premature senescence. PML VI and VII were not included in this study as they only consist of the conserved core region, with PML VII also being cytoplasmic. Nuclear localization has been shown recently to be indispensable for the growth-suppressive effect of PML (Fagioli et al., 1998; Le et al., 1998; Ferbeyre et al., 2000).
Fig. 1. Schematic representation of the various PML isoforms. The classification of PML isoforms follows that of Jensen et al. (2001). The total number of amino acids (aa) for each isoform is given.
First, we transduced primary human fibroblasts either with one of the PML isoforms (PML I–V), oncogenic Ras (Ha-rasV12, positive control) or the empty vector (B0, negative control) via retroviral-mediated gene transfer. Following infection, cells were kept under pharmacological selection for 4 days at which point [day = 0, population doubling (PD) = 0] we started monitoring their proliferative properties by growth curves and incorporation of methyl-[3H]thymidine into DNA. All PML isoforms were expressed at similar levels as determined by western blot (Supplementary figure 1A, available at The EMBO Journal Online).
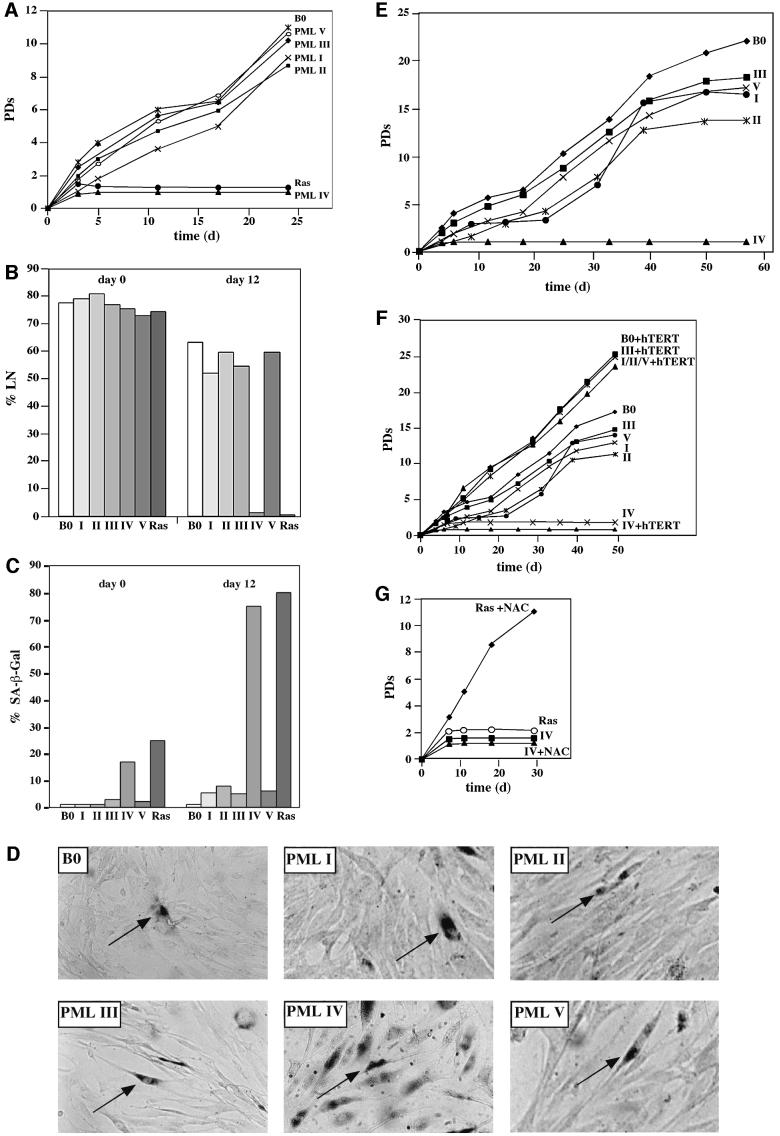
As expected, cells constitutively expressing Ras ceased growth after an initial burst of proliferation. Rapid growth arrest was also observed in response to PML isoform IV (Figure 2A), and in both cases the fraction of cells that synthesized DNA over a 3-day interval dropped from > 75% to < 10% by 10–15 days post-infection (Figure 2B). At the same time, > 70% of the cell population expressed senescence-associated β-galactosidase activity (SA-β-Gal) and assumed a flat morphology (Figure 2C and D, respectively). By contrast, cells expressing the other PML isoforms proliferated in a manner that was initially indistinguishable from that of the empty vector control cells, and expressed no SA-β-Gal (Figure 2A–D). However, these cells also showed a gradual and significant decline in proliferative potential although they displayed no features of apoptosis, and their overall life-span was shortened by 6–10 PDs compared with the vector control (Figure 2E).
Fig. 2. Effect of PML isoforms on cell proliferation, DNA synthesis and induction of premature senescence. (A) Proliferation of normal human cells. WI38 normal human fibroblasts were infected with empty virus as a control (B0), oncogenic Ras- or PML-expressing retroviruses. Cells were selected for 4 days in 3 µg/ml puromycin and the number of population doublings (PDs) was determined over the indicated period of time. Day 0 is the first day after selection. PDs for each time point are the mean value of triplicates. (B and C) DNA synthesis and SA-β-Gal expression in normal human cells. WI38 cells were infected and selected as described in (A). After selection, [3H]thymidine was added for 3 days at the indicated time points (B), and cells were subsequently histochemically stained for SA-β-Gal expression (C) followed by autoradiography as described in Materials and methods. A minimum of 200 cells were counted to determine the percentages of positive SA-β-Gal expression and radiolabeled nuclei (%LN). A cell was only considered SA-β-Gal positive when it was not radiolabeled. PML isoforms are depicted in roman numbers. (D) Morphology and SA-β-Gal expression. Infected WI38 normal human fibroblasts were stained for SA-β-Gal as described and photographed under phase contrast optics. Arrows indicate examples of stained cells. Note the increased number of positive SA-β-Gal cells in PML IV-infected cells. (E) Life-span reduction by PML isoforms I, II, III and V. WI38 normal human fibroblasts were infected as described in (A) and serially passaged until the %LN dropped beyond 5% and the culture did not double for 3 weeks. (F) Telomerase and PML-induced growth arrest. Immortilized hTERT-WI38 fibroblasts were infected as described above and growth followed for the indicated period of time. The growth curves for I, II and V plus hTERT (I/II/V + hTERT) were superimposable. (G) WI38 normal human fibroblasts were infected with the indicated retroviruses as described in (A) and cultured in 2 mM NAC pre- and post-selection for the indicated period of time.
To test whether PML IV-induced senescence could be bypassed by constitutive expression of catalytically active human telomerase (hTERT), and whether the shortened life-span induced by the other PML isoforms was telomere length dependent, we coinfected hTERT-expressing human fibroblasts along with the respective PML isoforms. In the case of PML IV, no differences in the premature senescence phenotypes were observed, whereas for the other PML isoforms growth suppression could be rescued by overexpression of hTERT (Figure 2F). Unlike PML IV, neither SP100 nor Daxx, two other NB-associated proteins, induced premature senescence. However, overall life-span was reduced significantly, a process that could be bypassed by hTERT (Supplementary figure 1B). Together, these findings demonstrate that only one PML isoform, PML IV, is capable of inducing premature senescence when constitutively overexpressed, and that this process is hTERT independent. Furthermore, they hint at a possible role for PML and NBs in maintenance of telomeres.
Premature senescence induced by Ras recently has been shown to be rescued by N-acetylcysteine (NAC), a scavenger of reactive oxygen species (ROS) (Lee et al., 1999); therefore we sought to determine whether PML IV-induced premature senescence could equally be bypassed by NAC treatment. Accordingly, PML IV-infected cells were cultured continually in 2 mM NAC. Unlike Ras, PML IV-induced premature senescence could not be rescued by NAC treatment (Figure 2G). This suggests that PML IV and Ras induce premature senescence by different pathways.
Targeting of p53 and CBP to PML NBs occurs with all PML isoforms
Recruitment of p53 and CBP to the PML NBs was shown to accompany Ras- and PML-induced senescence (Ferbeyre et al., 2000; Pearson et al., 2000), suggesting a causal relationship between the NB localization of these proteins and the senescence process. Given that PML-induced premature senescence is specific for isoform IV, we surmised that this isoform would be more efficient at recruiting p53 and CBP to PML NBs than the other isoforms. We therefore analyzed the effect of the various PML isoforms on the relocalization of endogenous p53 and CBP by immunofluorescence microscopy (Figure 3A and B). In B0 control-infected cells, the coincidence of PML with either p53 or CBP, although weak, was detectable in a number of endogenous PML NBs. Surprisingly, all PML isoforms recruited endogenous p53 and CBP equally well to the PML NBs, and virtually all PML NBs colocalized with p53 and CBP clusters (Figure 3A and B, respectively). It is noteworthy that forced expression of each individual PML isoform gave rise to a distinct NB pattern, ranging from a few subnuclear structures for PML III to numerous ones for PML IV.
Fig. 3. Recruitment of p53 and CBP to PML NBs. (A and B) Representative images of WI38 normal human fibroblasts infected with B0 (control) or the respective PML-isoforms co-stained with either anti-PML (red) and anti-p53 (green) (A) or anti-CBP (green) (B), and analysed by laser scanning microscopy. The merged image of both stainings is shown in yellow. (C) Forced deposition of p53 into PML NBs. Representative images of WI38 normal human fibroblasts infected with a GFP fusion protein consisting of the minimal PML NB-targeting domain of SP100 and the p53 binding domain of MDM2 (SP–MDM2). SP–MDM2 was localized by direct GFP fluorescence (green) and p53 was visualized with an anti-p53 antibody (red). The merge appears in yellow. Note that SP–MDM2 colocalizes 100% with PML NBs (data not shown).
To delineate the requirement for p53 recruitment to the NBs in the senescence process more precisely, we sought to coerce p53 into endogenous PML NBs upon coexpression of a green fluorescent protein (GFP) fusion protein consisting of the minimal NB-targeting domain of SP100 and the p53 binding domain of MDM2 (SP–MDM2 fusion). This chimera was shown recently to efficiently recruit p53 to the PML NBs and to stabilize it (Negorev et al., 2001). When human fibroblasts were infected with this construct, no growth inhibitory effect was observed (data not shown), although p53 was relocalized efficiently to PML NBs (Figure 3C).
Taken together, these results indicate that mere recruitment of p53 and CBP to the PML NBs is not the decisive factor for the premature senescence process elicited by PML IV.
Sumoylation of PML IV and intact PML NBs are dispensable for premature senescence
PML is covalently modified by SUMO at lysines 65, 160 and 490. We showed previously that conjugation to SUMO is a prerequisite for PML to induce proper formation of PML NBs (Zhong et al., 2000a). Therefore, we sought to determine to what extent sumoylation contributes to the pro-senescent property of PML IV. To this end, we infected human fibroblasts with either the sumoylation-deficient triple mutant PML IV-3K, wild-type PML IV or an empty control vector. Surprisingly, PML IV-3K was as potent at arresting cell proliferation and eliciting a senescent-like phenotype as its wild-type counterpart (Figure 4A and B). Using immunofluorescence microscopy, we found that PML IV-3K formed large nuclear aggregates and that it was as competent at recruiting p53 and CBP as was the PML IV wild-type protein (Figure 4C).
Fig. 4. Sumoylation and intact PML NBs are dispensable for premature senescence. WI38 normal human fibroblasts were infected with B0 (control), PML IV or the SUMO-deficient mutant PML IV-3K retroviruses as described in Figure 2, and growth curves (A), percentage of positive SA-β-Gal-stained cells and %LN (B) were determined. (C) Representative images of WI38 normal human fibroblasts infected with PML IV-3K, co-stained with either anti-PML (red) and anti-p53 (green) or anti-CBP (green), and analysed by laser scanning microscopy. (D–F) WI38 normal human fibroblasts were infected with IE1wt or IE1mt retroviruses and selected for 14 days in 400 µg/ml G418. Cells were then superinfected with either B0 (control), PML IV or oncogenic Ras retroviruses, and selected for 4 days in 3 µg/ml puromycin. (D) Representative images of WI38 normal human fibroblasts double-infected with either IE1wt+PML IV (a), IE1mt+PML IV (b) or IE1wt+Ras and IE1mt+Ras (c) retroviruses. Cells were co-stained either with anti-PML (red) and anti-p53 (green) or anti-CBP (green), and analysed by laser scanning microscopy. (E) Growth curves of WI38 fibroblasts co-infected with the indicated IE1mt+B0, IE1wt+B0, IE1wt+IV or IE1mt+IV, IE1wt+Ras or IE1mt+Ras retroviral constructs. IE1wt/mt+IV, cells transduced first with IE1wt or IE1mt followed by transduction with PML IV. (F) %LN and percentage of positive SA-β-Gal-stained cells from (E) at the indicated time points.
Next, we wished to investigate the role of the PML NBs in the PML IV- and Ras-induced senescence process. To address this question we turned to the CMV viral protein IE1, which is known to disrupt the PML NBs entirely, without affecting the overall level of the various NB components (Muller and Dejean, 1999). First, we infected human fibroblasts with wild-type IE1 (IE1wt) or an IE1 point mutant (IE1mt) that leaves the PML NBs intact (Muller and Dejean, 1999) and then, after drug selection, superinfected them with either PML IV or Ras. Although the PML NBs were completely disrupted in the IE1wt-infected cells as verified by immunofluorescence microscopy using anti-PML antibodies (Figure 4D, a and c), induction of the premature senescence phenotype by either PML IV or Ras was identical to that observed in IE1mt-infected cells (Figure 4E and F). In a parallel control experiment, expression of IE1wt or IE1mt alone failed to promote premature senescence. Furthermore, although p53 and CBP were normally recruited into the PML NBs in IE1mt-infected cells (Figure 4D, b), the two proteins were distributed in a microspeckled pattern throughout the nucleoplasm in the presence of IE1wt (Figure 4D, a). In conclusion, this line of experiments clearly demonstrates that sumoylation is not required for PML IV-induced premature senescence. Furthermore, it reinforces the notion that relocalization of p53 and CBP to intact PML NBs is not necessary for PML IV- and Ras-induced senescence.
Stabilization, post-translational modification and transcriptional activation of p53 are specific for PML IV, but independent of PML NB integrity
It has been shown previously that p53 becomes upregulated and post-translationally modified on Ser15 by phosphorylation and Lys382 by acetylation during PML- and Ras-induced senescence (Ferbeyre et al., 2000; Pearson et al., 2000). Acetylation of p53 at Lys382 is most likely carried out by CBP, and appears to stabilize as well as activate p53. PML, p53 and CBP were found to form a stable complex as a result of oncogenic Ras-induced senescence (Pearson et al., 2000). First, we compared the stability of p53 upon constitutive overexpression of PML III and IV. B0- or PML III-infected cells as well as PML IV-arrested cells were incubated with cycloheximide for different periods of time and cell lysates were prepared. We found that the half-life of p53 in PML IV-arrested cells was significantly extended compared with B0- or PML III-infected cells (Figure 5A). Given that p53 was stabilized in PML IV-arrested cells, we examined whether post-translational modification and transcriptional activation were also affected. Further more, we investigated whether the integrity of the PML NBs had any influence on these processes. Accordingly, we compared the status of p53 acetylation and phosphorylation during PML IV-induced senescence with that of other PML isoforms, as well as in an IE1wt background. The levels of immunoprecipitated p53 were normalized by western blotting and acetylation as well as phosphorylation, and were assessed using antibodies specific for acetylated Lys382 and phosphorylated Ser9, 15, 46 and 392. Overexpression of PML IV, but not of PML I and III isoforms, induced a reproducible increase in acetylation of p53 at Lys382, which was not affected by the disruption of the PML NBs as judged by an undiminished acetyla tion of p53 in the presence of IE1wt (Figure 5B). However, we could not detect any change in phosphorylation at Ser9, 15 or 392 (data not shown). Instead, we found phosphorylation of Ser46 to be greatly induced in PML IV-infected cells (Figure 5C). Next, we investigated whether the observed stabilization and post-translational modifications would translate into transcriptional activation of p53 regardless of the structural integrity of PML NBs. To this end, we transiently transfected PML IV-arrested cells and PML I- or III-infected cells with a synthetic p53-response element of the p21 promotor. PML IV activated the promotor ∼5-fold compared with control cells and again this activation was not impaired by coexpression of IE1 wt (Figure 5D).
Fig. 5. PML IV stabilizes and activates p53. (A) WI38 normal human fibroblasts were infected with either B0 (control), PML III or PML IV retroviruses, as described in the legend to Figure 2. Cells were treated with 10 µg/ml cycloheximide for the indicated times when the PML IV-infected culture was terminally arrested, and whole-cell lysates were prepared and probed for p53. (B) WI38 normal human fibroblasts were infected with either B0 (control), PML I, III, IV, IE1wt or IE1wt+PML IV as described in the legend to Figure 2. Cell lysates were prepared when the PML IV-infected culture was terminally arrested, immunoprecipitated with goat anti-p53 antibody and analysed by western blot using anti-p53-acetylLys382 antibody. (C) Total lysates were prepared from WI38 normal human fibroblasts infected with B0 (control) or the indicated PML retroviruses as in Figure 2, and analysed by western blot using anti-p53-phosphoSer46 antibody and anti-QM antibody for normalization. (D) p53 transactivation activity in quiescent, senescent and PML-infected cells. WI38 fibroblasts were infected with the indicated PML and/or IE1wt retroviruses, and selected and transiently co-transfected with a pCMV-β-galactosidase normalization vector and the p21-Luc reporter when the PML IV-infected culture was completely growth arrested. Cells were assayed for β-galactosidase and luciferase reporters. Normalized p21 reporter activity is luciferase/ β-galactosidase activity, with the activity of p21-Luc in proliferating cells set at 1. Shown are the averages of three independent experiments. Within each experiment, transfections were performed in triplicate. Pre-senescent or senescent cells were cultured in 10% serum, in which case pre-senescent cells were growing exponentially, or incubated in 0.2% serum for 3 days, in which case pre senescent cells were quiescent.
In conclusion, this line of results indicates that p53 becomes stabilized and acetylated on Lys382, as well as phosphorylated on Ser46, in a PML IV-restricted manner. These modifications may be required for the optimal activation of p53 by this specific isoform and the ensuing induction of premature senescence. Furthermore, the data presented provide ample evidence that functional complexes between PML IV, p53 and CBP form outside of the PML NBs, given that modification and activation of p53 are independent of the integrity of these structures.
Murine PML–/– primary fibroblasts are refractory to PML IV-induced premature senescence
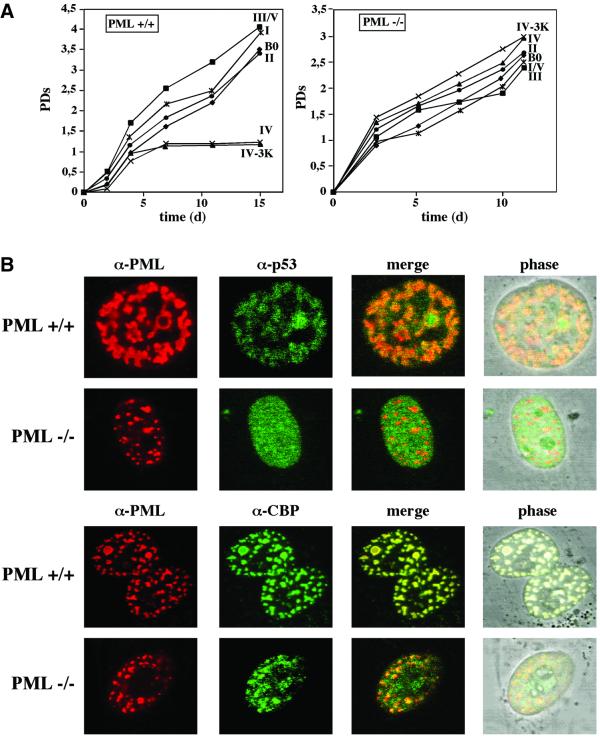
We next sought to determine whether constitutive expression of PML IV would elicit premature senescence in PML–/– murine embryo fibroblasts (MEFs). Accordingly, PML+/+ and PML–/– MEFs were infected with the various PML isoforms and analyzed as described for human fibroblasts. PML+/+ MEFs acquired the same senescent-like phenotype as human fibroblasts exclusively upon constitutive overexpression of PML IV or the sumoylation-deficient PML IV-3K mutant protein (Figure 6A). Unexpectedly, in PML–/– MEFs, PML IV and PLM IV-3K rather enhanced than inhibited cell proliferation and evoked no features of premature senescence (Figure 6A). To exclude the possibility that PML–/– cells sustained secondary mutations in the p53 pathway that would naturally obliterate the premature senescence process, we sequenced the two most critical genes of this pathway, p53 itself and p19ARF. Both genes were free of mutations (data not shown). These results imply that PML IV, while being necessary to induce premature senescence in a PML+/+ background, is not sufficient to do so in PML–/– MEFs.
Fig. 6. PML–/– MEFs are refractory to PML-induced premature senescence. (A) PML–/– MEFs or PML+/+ MEFs were infected with the respective PML retroviruses (in roman numbers) or empty vector control (B0), and growth curves were determined as described in the legend to Figure 2. (B) PML–/– MEFs do not recruit endogenous p53 to neoformed PML NBs. Representative images of PML–/– MEFs or PML+/+ MEFs infected with PML IV retroviruses and co-stained with either anti-PML (red) and anti-p53 (green) or anti-CBP (green), and analysed by laser scanning microscopy. (C) PML–/– MEFs show no increased acetylation of p53 at Lys382 upon PML overexpression. Immunoprecipitation of p53 from cells infected with the indicated retroviruses, and western blot analysis with anti-p53-acetylLys382 antibody as described in the legend to Figure 5.
Having determined that PML IV is not able to induce premature senescence in PML–/– MEFs, we then compared the localization of endogenous p53 and CBP in PML IV-infected PML+/+ MEFs with the localization of these proteins in PML IV-infected PML–/– MEFs by immunofluorescence microscopy (Figure 6B). Similar to what was observed in human fibroblasts, p53 as well as CBP were efficiently recruited to PML NBs in PML+/+ MEFs. Conversely, in PML–/– MEFs, only CBP was still relocalized to the neoformed PML NBs, but not p53, which stained nuclear diffuse. In addition, we found no increase in acetylation of p53 at Lys392 in PML–/– MEFs upon PML IV overexpression, whereas in PML+/+ MEFs, p53 acetylation was greatly induced (Figure 6C).
In conclusion, PML IV overexpression per se is not sufficient to induce acetylation of p53, to recruit p53 to the PML NBs and to promote premature senescence in PML–/– MEFs. This suggests that multiple PML isoforms may have to cooperate for these processes to occur. Yet, it is also possible that certain factors are not expressed in PML–/– MEFs and, therefore, pro-senescent complexes are malassembled.
Discussion
PML and cell growth control
The PML protein and its associated NBs have been implicated in a wide range of cellular processes including gene regulation, apoptosis and growth suppression (Seeler and Dejean, 1999; Zhong et al., 2000b; Negorev and Maul, 2001). Recently, it was shown that ectopic expression of PML can also promote premature senescence in primary human and murine fibroblasts (Ferbeyre et al., 2000; Pearson et al., 2000). PML is a ubiquitously expressed protein exhibiting tumor suppressor function (Mu et al., 1994; Wang et al., 1998). Alternative splicing of mRNAs results in the generation of at least seven different isoforms that differ in their C-termini (Jensen et al., 2001). Although numerous studies have been conducted to link PML and NBs to specific nuclear functions, these studies have been hampered by the fact that generally only one isoform has been investigated, and hence it remained to be seen if the observed effects are specific to a particular isoform or are of a more general nature.
In addressing the role of PML isoforms in the induction of premature senescence, we found that only isoform IV is capable of eliciting a premature senescence-like phenotype in human fibroblasts and PML+/+ MEFs. By contrast, neither PML IV nor any other PML isoform induced growth arrest in PML–/– MEFs. This in turn suggests that PML IV is necessary but not sufficient to elicit a premature senescence phenotype in a PML–/– background.
Human cells have evolved at least two different mechanisms to curb proliferation of cells that are at the brink of oncogenic transformation: (i) apoptosis and (ii) cellular senescence, of which premature senescence caused by oncogenic stress is an example. Another example of cellular senescence in human cells is replicative senescence, which is driven by telomere shortening (Campisi, 2001; Shay and Wright, 2001). Replicative senescence can be bypassed by ectopic expression of hTERT, the catalytic subunit of telomerase (Bodnar et al., 1998; Vaziri and Benchimol, 1998). Our finding that premature senescence induced by PML IV cannot be rescued by overexpression of hTERT is reminiscent of what has also been shown for oncogenic Ras (Wei and Sedivy, 1999). How oncogenic stress induces premature senescence in detail remains largely unknown. In the case of Ras, however, it has been proposed that an increase in intracellular ROS triggers an oxidative damage response that promotes premature senescence rather than apoptosis (Sundaresan et al., 1996). This idea is supported by the finding that Ras-induced arrest can be rescued by culturing human fibroblasts in NAC, a known ROS scavenger (Lee et al., 1999). In contrast, PML IV-induced premature senescence cannot be bypassed by NAC. Together, our data suggest the existence of a senescence pathway that is distinct from replicative senescence as a result of telomere shortening and from that elicited by active Ras and/or oxidative stress.
Unlike PML IV-induced premature senescence, reduction of life-span owing to ectopic expression of the other PML isoforms, SP100 or Daxx can be bypassed by overexpression of hTERT, suggesting that the composition and equilibrium of PML-associated protein complexes can have an impact on telomere biology. This idea is corroborated by the fact that PML is indeed found at telomeric DNA in human cell lines of the ALT (maintenance of telomere length by an alternative, telomerase-independent mechanism) phenotype and also, though less frequently, in telomerase-positive cell lines and normal human fibroblasts (Yeager et al., 1999; Wu et al., 2000).
PML NBs and senescence
In human cells, cellular senescence is controlled by several tumor suppressor genes. The most relevant of these encode the p53 and pRB proteins, which are essential for cells to establish and maintain the senescence growth arrest induced by a variety of stimuli (Lundberg et al., 2000; Stewart and Weinberg, 2000; Campisi, 2001; Mathon and Lloyd, 2001). Both the pRB and p53 pathways were shown to be activated by mitogenic oncogenes such as Ras, RAF, MEK or PML (Ferbeyre et al., 2000; Pearson et al., 2000; Serrano and Blasco, 2001), all of which promote a premature senescence-like phenotype. It has been further proposed that the integrity of the PML NBs is required for the induction of senescence as a result of oncogenic stress. Notably, recruitment of p53 and CBP to PML NBs and the formation of a ternary complex of PML/p53/CBP in the NBs were found to accompany Ras- and PML-induced premature senescence (Ferbeyre et al., 2000; Pearson et al., 2000). However, the idea that PML NBs function as organizing centers where processes such as transcription, apoptosis or senescence are executed or regulated has been put into question (Negorev and Maul, 2001).
In our studies, we find that all of the PML isoforms tested efficiently recruited endogenous p53 as well as CBP to the NBs both in human fibroblasts and PML+/+ MEFs. We conclude from these results that the mere presence of p53 and CBP in the NBs is not sufficient for establishing the premature senescene phenotype. This notion is further strengthened by the fact that forcing p53 into PML NBs via a fusion protein consisting of the NB protein SP100 and the p53-binding domain of MDM2 has no noticeable effect on growth. In PML–/– MEFs, endogenous p53 remained entirely diffuse, whereas CBP still relocalized to neoformed PML NBs upon ectopic expression of all PML isoforms (Figure 6B; our unpublished data). This implies that PML NBs comprised of only a single isoform are not proficient at recruiting p53 but that additional factors, possibly including other PML isoforms, are necessary for this relocalization to occur.
Sumoylation is important for the formation of intact NBs and the recruitment of specific proteins at these sites (Seeler and Dejean, 2001). We found that neither the lack of SUMO modification of PML nor the ensuing reorganization of the NBs alter the outcome of premature senescence. Moreover, the complete disruption of the NBs by IE1 had no impact on PML IV-induced senescence. This suggests that functional pro-senescent complexes between PML and its partners, p53 and CBP, are assembled and act outside of PML NBs, and that their presence in these structures may be a read-out for the existence of such a complex. By analogy, this could explain the absence of p53 recruitment into neoformed PML NBs in PML–/– MEFs, because no functional pro-senescent PML/p53/CBP complex can be established in these cells. The presence of p53 and CBP in the PML NBs may serve as recycling or supply sites of preformed PML/p53/CBP complexes in PML+/+ cells. It is, however, possible that although these NB-based complexes are not an absolute requirement for the execution of the premature senescence process, they may facilitate it. Together, our data suggest that the functionally active form of PML may not necessarily be associated to NBs, thus challenging the current tenet of PML NBs being the major sites of PML function.
PML and activation of p53
A unique outcome of ectopic expression of PML IV as well as oncogenic Ras, but not of other PML isoforms, in human fibroblasts and PML+/+ MEFs is the stabilization, post-translational modification and transcriptional activation of p53. These events still occur in the complete absence of PML NBs, reinforcing the notion that functional pro-senescent complexes most likely act outside the NBs. Post-translational modification of p53 seems to be a coordinated event, as phosphorylation has been shown to precede acetylation (reviewed in Appella and Anderson, 2001). In our study, we observed that forced expression of PML IV significantly induces phosphorylation at Ser46, acetylation at Lys382 and transcriptional activation of a p21-Luc reporter. Ser46 has recently been shown to be selectively phosphorylated by HipK2, thus facilitating CBP-mediated acetylation of p53 at residue Lys382 and transcriptional activation of p53-dependent gene expression (Hofmann et al., 2001; D’Orazi et al., 2002). In accordance with this work, it was reported that acetylation of Lys382 is required for optimal activation of p53 (Pearson et al., 2000) and that deacetylation of p53 by Sir2 antagonizes PML-induced senescence (Luo et al., 2001; Vaziri et al., 2001; Langley et al., 2002). Moreover, HipK2 was shown to be recruited exclusively by PML IV, but not PML III, to NBs (Hofmann et al., 2001) and to have a growth-suppressive effect when overexpressed in tumor cells (Pierantoni et al., 2001). Preliminary results indicate that PML IV alone is able to interact strongly with HipK2 (our unpublished results). Further experiments have to clarify the exact role of HipK2 in the PML IV-induced senescence process. By contrast, in PML–/– MEFs, we did not observe any modulation in the post-translational modification of p53 upon overexpression of either PML IV or Ras, again reflecting the absence or malassembly of pro-senescent complexes in these cells due to lack of additional PML isoforms and/or cofactors.
In this study, we provide the first comprehensive and comparative analysis of several PML isoforms and their involvement in a specific cellular process, premature senescence. We define PML IV as the isoform that is essential for premature senescence to be initiated and executed. Its direct interaction, especially with p53 (our unpublished results; Fogal et al., 2000) and pRB (Alcalay et al., 1998), the two key players in senescence, make it a very attractive candidate to coordinate this event. In light of the results in PML–/– MEFs, it has become evident that an individual PML isoform is probably not sufficient to execute specific cellular processes, but that there exists an intricate interplay between multiple isoforms and other PML-associated cofactors.
Materials and methods
Vectors, viruses and cell culture
Cloning of retroviral constructs, infection of WI38 human lung fibroblasts as well as PML+/+ and PML–/– MEFs with retroviral constructs, and culturing of transduced cells were performed by standard procedures (for details see Supplementary data available at The EMBO Journal Online).
Senescence analysis
Senescence was assessed using several assays. For growth curves, cells were plated in triplicate at 2.0 × 104 per well in 12-well plates. Relative cell numbers were estimated at various time points using a crystal violet incorporation assay, and the number of PDs (n) was calculated using the following equation:
n = (log10F – log10I) × 3.32
where F is the number of cells at the end of one passage, and I is the number of cells that were seeded at the beginning of one passage. For life-span studies, cells were subcultured when 70–80% confluent at 2 × 104/cm2. Proliferative capacity was assessed by labeling cells for 72 h with [3H]thymidine (10 µCi/ml) followed by autoradiography to determine the percentage of radiolabeled nuclei (%LN) (Bischof et al., 2001). These cells were also co-stained for senescence-associated β-galactosidase (SA-β-Gal), as described previously (Dimri et al., 1995).
Immunoprecipitation, immunoblotting and antibodies
Cells were lysed in buffer [20 mM Tris–HCl pH 7.6, 170 mM NaCl, 1 mM EDTA, 0.5% NP-40, 1 mM dithiothreitol (DTT)] supplemented with 5 µM trichostatin A (Calbiochem, CA), 1 mM nicotinamide (Sigma, MO) and protease inhibitors (Boehringer-Mannheim, Germany). For immunoprecipitation with anti-p53 antibody, equal amounts of lysate (containing 200–300 µg of total cellular protein) were incubated with 1 µg of goat anti-p53 antibody (Santa Cruz, CA) and protein G–Sepharose (Pharmacia, Sweden) for 3 h at 4°C. The use of goat antibody eliminates the heavy chain signal that co-migrates with p53 in subsequent immunoblotting. Immunoblotting was performed according to standard procedures. When immunoprecipitation was not performed, total protein lysates were prepared in 2× SDS–PAGE sample buffer, and 50 µg of protein were separated by 4–15% SDS–PAGE. Antibodies were detected by chemiluminescence using SuperSignal (Pierce, Rockford, IL). The following primary antibodies were used: mouse monoclonal anti-tubulin (Ab1; Oncogene Science, Cambridge, MA), rabbit polyclonal anti-QM (C-17; Santa Cruz), mouse monoclonal anti-p53 (DO-1; Santa Cruz), rabbit polyclonal anti-p53-phosphoSer9, 15 and 392 (Cell Signaling, Beverly, MA), rabbit polyclonal antibody anti-p53-phosphoSer46 (a gift of Yoichi Taya, University of Tokyo, Japan) and rabbit polyclonal anti-p53 acetylLys382 (a gift of Ettore Appella, NIH, Bethesda, MD).
Immunofluorescence
Cells in four-well glass slides were cultured for 1–3 days, and fixed and stained with primary and secondary antibodies as described previously (Bischof et al., 2001). For each colocalization experiment, parallel single labelings were performed to guard against the possibility of immunological or optical cross-talk. Primary antibodies used were: mouse monoclonal anti-PML (PG-M3; Santa Cruz), mouse monoclonal anti-p53 (DO-1; Santa Cruz), rabbit polyclonal anti-p53 (FL393; Santa Cruz), mouse monoclonal anti-p53 (DO-7, CM-1; Novocastra, UK), mouse monoclonal anti-CBP (C-1; Santa Cruz), rabbit polyclonal anti-GFP (Clontech, MA) and rabbit polyclonal anti-PML (83), as described previously (Weis et al., 1994). Slides were mounted in VectaShield containing 4′,6-diamidino-2-phenyl-indole (DAPI, 0.4 µg/ml; Vector Laboratories) to visualize nuclear DNA, and viewed by epifluorescence or a single laser confocal section. Images were captured with a CCD camera, and merged using Canvas (Deneba, FL).
Reporter assays
Infected WI38 fibroblasts (5–8 × 103/cm2 on 35 mm dishes) were transfected with 600 ng p21-Luciferase (p21-Luc) (el-Deiry et al., 1993) and 0.1 µg pCMV-β-gal reporters using Lipofectamine Plus (Gibco-BRL). β-galactosidase and luciferase activities were measured using Galacto-Star (TROPIX, CA) and Luciferase Assay Systems (Promega, WI) luminescent assay kits according to the suppliers’ instructions. Luciferase activities were normalized with β-galactosidase activities.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We aknowledge Pier Paolo Pandolfi for providing PML+/+ and PML–/– MEFs. We also thank Ellen Solomon for the PML I expression vector, Ettore Appella for the anti-p53 acetylLys382 antibody, Judy Campisi for the hTERT retrovirus vector, Yoichi Taya for the anti-p53-phosphoSer46 antibody, and Moshe Oren for p21-Luc constructs. Our special thanks go to Enrico Garattini for helpful discussions. We further thank all other members of the laboratory for sharing reagents and advice. This work was supported by grants from the European Economic Community (QLG1), the Association pour la Recherche contre le Cancer, the Ligue Nationale Contre le Cancer, the Fondation de France and the Pasteur– Negri–Weizmann Council. O.B. was supported by the Association for International Cancer Research and O.Kirsh by the Ministère de la Recherche et la Technologie.
References
- Alcalay M., Tomassoni,L., Colombo,E., Stoldt,S., Grignani,F., Fagioli,M., Szekely,L., Helin,K. and Pelicci,P.G. (1998) The promyelocytic leukemia gene product (PML) forms stable complexes with the retinoblastoma protein. Mol. Cell. Biol., 18, 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appella E. and Anderson,C.W. (2001) Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem., 268, 2764–2772. [DOI] [PubMed] [Google Scholar]
- Bischof O., Kim,S.H., Irving,J., Beresten,S., Ellis,N.A. and Campisi,J. (2001) Regulation and localization of the Bloom syndrome protein in response to DNA damage. J. Cell Biol., 153, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar A.G. et al. (1998) Extension of life-span by introduction of telomerase into normal human cells. Science, 279, 349–352. [DOI] [PubMed] [Google Scholar]
- Bringold F. and Serrano,M. (2000) Tumor suppressors and oncogenes in cellular senescence. Exp. Gerontol., 35, 317–329. [DOI] [PubMed] [Google Scholar]
- Campisi J. (2001) Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol., 11, S27–S31. [DOI] [PubMed] [Google Scholar]
- D’Orazi G. et al. (2002) Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat. Cell Biol., 4, 11–19. [DOI] [PubMed] [Google Scholar]
- de The H., Lavau,C., Marchio,A., Chomienne,C., Degos,L. and Dejean,A. (1991) The PML-RAR α fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell, 66, 675–684. [DOI] [PubMed] [Google Scholar]
- Dimri G.P. et al. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA, 92, 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck J.A., Maul,G.G., Miller,W.H.,Jr, Chen,J.D., Kakizuka,A. and Evans,R.M. (1994) A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell, 76, 333–343. [DOI] [PubMed] [Google Scholar]
- el-Deiry W.S. et al. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell, 75, 817–825. [DOI] [PubMed] [Google Scholar]
- Everett R.D. (2001) DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene, 20, 7266–7273. [DOI] [PubMed] [Google Scholar]
- Fagioli M. et al. (1998) Cooperation between the RING + B1-B2 and coiled-coil domains of PML is necessary for its effects on cell survival. Oncogene, 16, 2905–2913. [DOI] [PubMed] [Google Scholar]
- Ferbeyre G., de Stanchina,E., Querido,E., Baptiste,N., Prives,C. and Lowe,S.W. (2000) PML is induced by oncogenic ras and promotes premature senescence. Genes Dev., 14, 2015–2027. [PMC free article] [PubMed] [Google Scholar]
- Fogal V. et al. (2000) Regulation of p53 activity in nuclear bodies by a specific PML isoform. EMBO J., 19, 6185–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard A.D., Borrow,J., Freemont,P.S. and Solomon,E. (1991) Characterization of a zinc finger gene disrupted by the t(15;17) in acute promyelocytic leukemia. Science, 254, 1371–1374. [DOI] [PubMed] [Google Scholar]
- Hayflick L. (1961) The serial cultivation of human diploid cell strains. Exp. Cell Res., 25, 585–621. [DOI] [PubMed] [Google Scholar]
- Hofmann T.G., Moller,A., Sirma,H., Zentgraf,H., Taya,Y., Droge,W., Will,H. and Schmitz,M.L. (2001) Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat. Cell Biol., 10, 10. [DOI] [PubMed] [Google Scholar]
- Ishov A.M. et al. (1999) PML is critical for ND10 formation and recruits the PML-interacting protein Daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol., 147, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahana K., Dimri,G. and Campisi,J. (2001) Regulation of cellular senescence by p53. Eur. J. Biochem., 268, 2784–2791. [DOI] [PubMed] [Google Scholar]
- Jensen K., Shiels,C. and Freemont,P.S. (2001) PML protein isoforms and the RBCC/TRIM motif. Oncogene, 20, 7223–7233. [DOI] [PubMed] [Google Scholar]
- Kakizuka A., Miller,W.H.,Jr, Umesono,K., Warrell,R.P.,Jr, Frankel,S.R., Murty,V.V., Dmitrovsky,E. and Evans,R.M. (1991) Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor, PML. Cell, 66, 663–674. [DOI] [PubMed] [Google Scholar]
- Kamitani T., Kito,K., Nguyen,H.P., Wada,H., Fukuda-Kamitani,T. and Yeh,E.T. (1998) Identification of three major sentrinization sites in PML. J. Biol. Chem., 273, 26675–26682. [DOI] [PubMed] [Google Scholar]
- Kastner P., Perez,A., Lutz,Y., Rochette-Egly,C., Gaub,M.P. and Chambon,P. (1991) Fusion proteins between PML and α-RAR in acute promyelocytic leukemia. C. R. Seances Soc. Biol. Fil., 185, 391–401. [PubMed] [Google Scholar]
- Koken M.H. et al. (1994) The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J., 13, 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V. et al. (2001) Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment and As2O3-induced PML or PML/retinoic acid receptor α degradation. J. Exp. Med., 193, 1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley E., Pearson,M., Faretta,M., Bauer,U.M., Frye,R.A., Minucci,S., Pelicci,P.G. and Kouzarides,T. (2002) Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J., 21, 2383–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le X.F., Vallian,S., Mu,Z.M., Hung,M.C. and Chang,K.S. (1998) Recombinant PML adenovirus suppresses growth and tumorigenicity of human breast cancer cells by inducing G1 cell cycle arrest and apoptosis. Oncogene, 16, 1839–1849. [DOI] [PubMed] [Google Scholar]
- Lee A.C. et al. (1999) Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem., 274, 7936–7940. [DOI] [PubMed] [Google Scholar]
- Lundberg A.S., Hahn,W.C., Gupta,P. and Weinberg,R.A. (2000) Genes involved in senescence and immortalization. Curr. Opin. Cell Biol., 12, 705–709. [DOI] [PubMed] [Google Scholar]
- Luo J., Nikolaev,A.Y., Imai,S., Chen,D., Su,F., Shiloh,A., Guarente,L. and Gu,W. (2001) Negative control of p53 by Sir2α promotes cell survival under stress. Cell, 107, 137–148. [DOI] [PubMed] [Google Scholar]
- Mathon N.F. and Lloyd,A.C. (2001) Cell senescence and cancer. Nat. Rev., 1, 203–213. [DOI] [PubMed] [Google Scholar]
- Mu Z.M., Chin,K.V., Liu,J.H., Lozano,G. and Chang,K.S. (1994) PML, a growth suppressor disrupted in acute promyelocytic leukemia. Mol. Cell. Biol., 14, 6858–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S. and Dejean,A. (1999) Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol., 73, 5137–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S., Matunis,M.J. and Dejean,A. (1998) Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J., 17, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negorev D. and Maul,G.G. (2001) Cellular proteins localized at and interacting within ND10/PML nuclear bodies/PODs suggest functions of a nuclear depot. Oncogene, 20, 7234–7242. [DOI] [PubMed] [Google Scholar]
- Negorev D., Ishov,A.M. and Maul,G.G. (2001) Evidence for separate ND10-binding and homo-oligomerization domains of Sp100. J. Cell Sci., 114, 59–68. [DOI] [PubMed] [Google Scholar]
- Pearson M. and Pelicci,P.G. (2001) PML interaction with p53 and its role in apoptosis and replicative senescence. Oncogene, 20, 7250–7256. [DOI] [PubMed] [Google Scholar]
- Pearson M. et al. (2000) PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature, 406, 207–210. [DOI] [PubMed] [Google Scholar]
- Pierantoni G.M. et al. (2001) High mobility group I (Y) proteins bind HIPK2, a serine-threonine kinase protein which inhibits cell growth. Oncogene, 20, 6132–6141. [DOI] [PubMed] [Google Scholar]
- Prives C. and Hall,P.A. (1999) The p53 pathway. J. Pathol., 187, 112–126. [DOI] [PubMed] [Google Scholar]
- Seeler J.S. and Dejean,A. (1999) The PML nuclear bodies: actors or extras? Curr. Opin. Genet. Dev., 9, 362–367. [DOI] [PubMed] [Google Scholar]
- Seeler J.S. and Dejean,A. (2001) SUMO: of branched proteins and nuclear bodies. Oncogene, 20, 7243–7249. [DOI] [PubMed] [Google Scholar]
- Serrano M. and Blasco,M.A. (2001) Putting the stress on senescence. Curr. Opin. Cell Biol., 13, 748–753. [DOI] [PubMed] [Google Scholar]
- Shay J.W. and Wright,W.E. (2001) Telomeres and telomerase: implications for cancer and aging. Radiat. Res., 155, 188–193. [DOI] [PubMed] [Google Scholar]
- Sternsdorf T., Jensen,K. and Will,H. (1997) Evidence for covalent modification of the nuclear dot-associated proteins PML and Sp100 by PIC1/SUMO-1. J. Cell Biol., 139, 1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S.A. and Weinberg,R.A. (2000) Telomerase and human tumorigenesis. Semin. Cancer Biol., 10, 399–406. [DOI] [PubMed] [Google Scholar]
- Sundaresan M. et al. (1996) Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J., 318, 379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner S.D. et al. (2002) p53 mutant mice that display early ageing-associated phenotypes. Nature, 415, 45–53. [DOI] [PubMed] [Google Scholar]
- Vaziri H. and Benchimol,S. (1998) Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol., 8, 279–282. [DOI] [PubMed] [Google Scholar]
- Vaziri H., Dessain,S.K., Ng Eaton,E., Imai,S.I., Frye,R.A., Pandita,T.K., Guarente,L. and Weinberg,R.A. (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell, 107, 149–159. [DOI] [PubMed] [Google Scholar]
- Wang Z.G., Delva,L., Gaboli,M., Rivi,R., Giorgio,M., Cordon-Cardo,C., Grosveld,F. and Pandolfi,P.P. (1998) Role of PML in cell growth and the retinoic acid pathway. Science, 279, 1547–1551. [DOI] [PubMed] [Google Scholar]
- Wei S. and Sedivy,J.M. (1999) Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res., 59, 1539–1543. [PubMed] [Google Scholar]
- Weis K. et al. (1994) Retinoic acid regulates aberrant nuclear localization of PML-RAR α in acute promyelocytic leukemia cells. Cell, 76, 345–356. [DOI] [PubMed] [Google Scholar]
- Wu G., Lee,W.H. and Chen,P.L. (2000) NBS1 and TRF1 colocalize at promyelocytic leukemia bodies during late S/G2 phases in immortalized telomerase-negative cells. Implication of NBS1 in alternative lengthening of telomeres. J. Biol. Chem., 275, 30618–30622. [DOI] [PubMed] [Google Scholar]
- Yeager T.R., Neumann,A.A., Englezou,A., Huschtscha,L.I., Noble,J.R. and Reddel,R.R. (1999) Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res., 59, 4175–4179. [PubMed] [Google Scholar]
- Zhong S., Muller,S., Ronchetti,S., Freemont,P.S., Dejean,A. and Pandolfi,P.P. (2000a) Role of SUMO-1-modified PML in nuclear body formation. Blood, 95, 2748–2752. [PubMed] [Google Scholar]
- Zhong S., Salomoni,P. and Pandolfi,P.P. (2000b) The transcriptional role of PML and the nuclear body. Nat. Cell Biol., 2, E85–E90. [DOI] [PubMed] [Google Scholar]