Abstract
The enhancement of the human estrogen receptor α (hERα, NR3A1) activity by the orphan nuclear receptor COUP-TFI is found to depend on the establishment of a tight hERα–COUP-TFI complex. Formation of this complex seems to involve dynamic mechanisms different from those allowing hERα homodimerization. Although the hERα–COUP-TFI complex is present in all cells tested, the transcriptional cooperation between the two nuclear receptors is restricted to cell lines permissive to hERα activation function 1 (AF-1). In these cells, the physical interaction between COUP-TFI and hERα increases the affinity of hERα for ERK2/p42MAPK, resulting in an enhanced phosphorylation state of the hERα Ser118. hERα thus acquires a strengthened AF-1 activity due to its hyperphosphorylation. These data indicate an alternative interaction process between nuclear receptors and demonstrate a novel protein intercommunication pathway that modulates hERα AF-1.
Keywords: AF-1/ERK2/estrogen receptor/COUP-TFI/physical interaction
Introduction
Estrogens (estradiol, E2) are small lipophilic steroid hormones, predominantly synthesized by ovaries. Although commonly recognized as the pivotal hormone for female reproductive tract physiology, E2 is also involved in homeostasis of other tissues, and also performs important roles in males (Lombardi et al., 2001; Nilsson et al., 2001). Critical roles of E2 involve a control of the cell proliferation/differentiation balance (Feigelson and Henderson, 1996). Most of the physiological functions of E2 are exerted by specific nuclear receptors, termed estrogen receptors (ERα and ERβ; NR3A1 and NR3A2; Nuclear Receptor Committee, 1999). These proteins involved in controlling endocrine and reproductive functions (Couse and Korach, 1999) are evolutionarily widely distributed from chordates to mammals, and belong to the transcription factor superfamily of nuclear receptors (NRs; Laudet, 1997). This superfamily includes ligand-inducible transcription factors, such as the steroid, retinoid and thyroid hormone receptors, as well as orphan receptors for which no ligand has been identified so far (Giguere, 1999). ERs bind as dimers to DNA, on specific estrogen response elements (EREs; Parker, 1995). ERs activate transcription of target genes through an N-terminal activation function (AF-1) in the B domain, and a C-terminal AF-2 region (Parker, 1995; see Figure 1A). These AFs exhibit distinct properties, which depend on both cell and promoter contexts (Metzger et al., 1992). AF-2-mediated activation requires ligand-induced conformational changes, which allow interaction with co-activators that possess histone acetyltransferase activity and multiprotein complexes that interact with the general transcription machinery (Freedman, 1999). Extracellular signals influence ER functions by modulating the receptor phosphorylation state (Weigel, 1996). ER actions are also influenced by other transcription factors (Gaub et al., 1990; Salvatori et al., 2000), and orphan receptors such as SF-1 (NR5A1; Le Dréan et al., 1996).
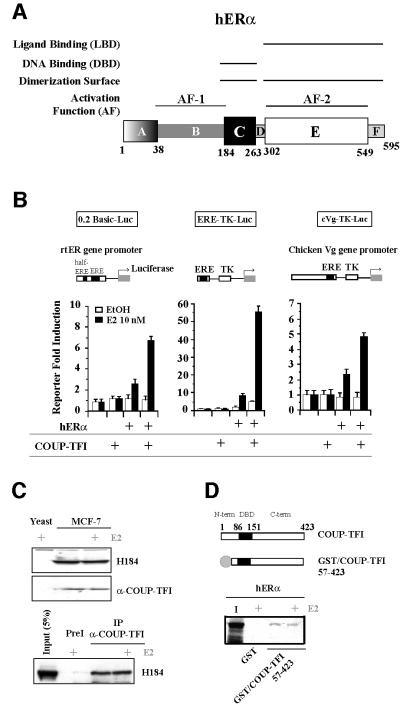
Fig. 1. Physical and transcriptional interrelationships between hERα and COUP-TFI. (A) Schematic illustration of the hERα sequence: domains and associated functions. (B) Transfection experiments in CHO-K1 cells were performed using the 0.2 basic, ERE-TK or chicken vitellogenin (Vg) reporters, pCH110 internal control and 25 ng of each expression vector (pCMV5/hERα and pCDNA/COUP-TFI). Cells were treated with ethanol (EtOH) or 10–8 M estradiol (E2). Values are shown as the fold induction of normalized luciferase reporter activity (mean ± SEM). (C) Co-immunoprecipitation of the endogenous hERα–COUP-TFI complex in MCF-7 cells using anti-COUP-TFI- specific antibody. A goat pre-immune serum (PreI) was the control. The presence of each component of the complex was checked by western blots. (D) GST pull-down experiments performed using 250 ng of the GST–COUP-TFI 57–423 fusion protein or GST as a control, with in vitro labeled hERα. Input is 40% of the labeled protein used in the assay.
COUP-TFs are orphan NRs that are highly expressed in the developing nervous system, where they exert crucial roles in specific cell fates (Qiu et al., 1997). In addition, COUP-TFs are expressed in a wide variety of tissues such as liver, uterus and mammary gland, where they may regulate vital biological functions and correct organogenesis (Pereira et al., 1995). Several of these tissues also express ERs, and cross-talk between both NRs was suggested in the context of genes expressed in estrogen-sensitive tissues such as the uterus. In agreement with a role for COUP-TFs as transcriptional repressors (Cooney et al., 1993), these orphan receptors were shown to repress the estrogen-regulated expression of the oxytocin and hepatocyte growth factor (HGF) genes (Jiang et al., 1997; Chu et al., 1998). On the other hand, a positive transcriptional effect of COUP-TFI on ERα activity was demonstrated for the first time on the rainbow trout ERα (rtERα) promoter (Lazennec et al., 1997). The aim of this current study was to investigate the mechanisms underlying the positive action of COUP-TFI on ERα activity. First, we extend this positive interplay to other promoters and to the human ERα (hERα). Then, we describe a new pathway where the formation of a tight ERα–COUP-TFI intermediate complex leads to an increased recruitment of the ERK2/p42MAPK, phosphorylation of the hERα on Ser118 and enhancement of its transcriptional activity.
Results
Transcriptional cooperation and physical interaction between hERα and COUP-TFI
Although previously described as a repressive factor, COUP-TFI enhances the hERα activity on the rtERα promoter gene, an ERE-TK synthetic promoter, the chicken vitellogenin (Vg) promoter in CHO-K1 cells (Figure 1B) and, hence, presumably on other target genes in vivo. One way that COUP-TFI could influence hERα activity is by a direct interaction between the two proteins, the resulting complex exhibiting properties that differ from those of each NR. The occurrence of an interaction between both endogenous proteins was examined in MCF-7 cells that express these NRs (Figure 1C). A co-immunoprecipitation experiment performed with a specific antibody directed against COUP-TFI revealed that endogenous hERα and COUP-TFI are found in the same protein complex, with or without E2 treatment. To demonstrate a physical interaction between the two NRs, we used a GST fusion protein with the longest COUP-TFI sequence possible [residues 57–423, encompassing half of the N-terminal region, the DNA-binding domain (DBD) and the ligand-binding domain (LBD)]. Subsequent in vitro GST pull-down assays confirm the occurrence of a ligand-independent interaction between COUP-TFI and in vitro labeled hERα (Figure 1D).
Molecular dissection of the physical interaction between hERα and COUP-TFI
To map the domains of both NRs involved in their interaction, we performed two-hybrid assays, whose accuracy was tested by fusing the full-length COUP-TFI and hERα to the activation domain (AD) or DBD of the yeast transcriptional factor Gal4. Subsequent assays shown in Figure 2A confirm the ligand independence of the interaction between the two NRs. A dominant-negative form of COUP-TFI (Adam et al., 2000), harboring a C141S point mutation within its DBD, is unable to interact with hERα (Figure 2A). On the contrary, this mutant maintains its ability to interact with wild-type COUP-TFI. This indicates that the mechanisms underlying the interaction between COUP-TFI and hERα are probably different from those involved in COUP-TFI homodimerization.
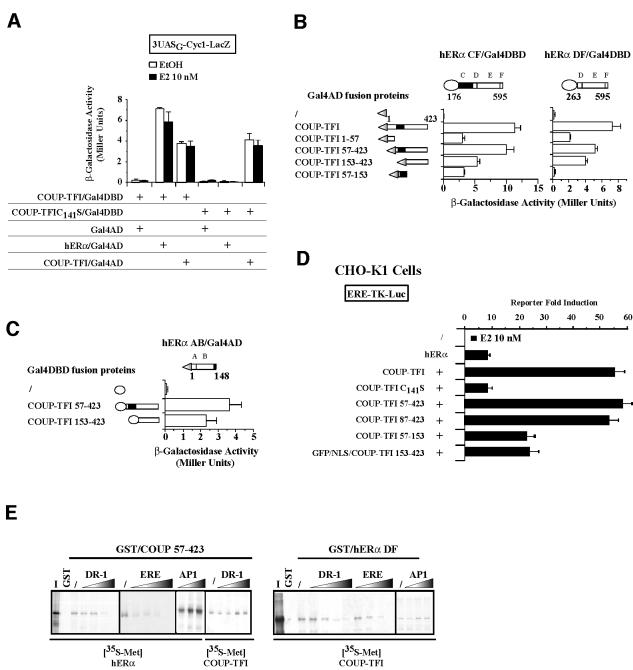
Fig. 2. The hERα–COUP-TFI complex is required for transcriptional cooperation but is destabilized by DNA. (A) Yeast two-hybrid assays using full-length hERα, wild-type and dominant-negative COUP-TFI (C141S point mutation) fused to the Gal4AD or Gal4DBD. Yeast transformants were grown in media including ethanol (EtOH) or 10–8 M estradiol (E2). Liquid β-galactosidase assays were then performed. (B and C) The different domains of COUP-TFI were fused to the Gal4AD or Gal4DBD for two-hybrid assays, which were performed using as bait the complementary fusion proteins indicated at the top of each panel. Results are expressed as the mean ± SD from three independent experiments. (D) Ability of COUP-TFI mutants to enhance hERα activity on the ERE-TK reporter gene, in CHO-K1 cells treated with EtOH or 10–8 E2. Results (mean ± SEM) are expressed as the reporter fold stimulation in the presence of E2. (E) Pull-down experiments were performed with in vitro labeled hERα and COUP-TFI using the GST–COUP-TFI 57–423 or GST–hERα DF proteins. During the incubation, increasing amounts of DR-1, ERE (25–200 ng) and AP-1 as non-specific control (50–200 ng) oligonucleotides were added.
To obtain further information on the specific mechanisms of interaction between the two NRs, we identified their contacting surfaces. The different domains of COUP-TFI were fused to the Gal4AD, while the C–F (CF) or D–F (DF) domains of hERα fused to the Gal4DBD were used as bait proteins (Figure 2B, left). These hERα proteins exhibit no intrinsic transcriptional activity that might have interfered in these assays. This property allowed us to show the ligand independence of all detected interactions (data not shown). The hERα DBD + LBD bait (hERα CF/Gal4DBD) associates with COUP-TFI N-terminal (amino acids 1–57), C-terminal (amino acids 153–423) and DBD (amino acids 57–153) regions. Performing the two-hybrid assays with the hERα LBD alone (hERα DF/Gal4DBD) showed that it associates with COUP-TFI N- and C-terminal regions (Figure 2B, right). The absence of the DBD in this hERα DF/Gal4DBD bait and its failure to bind the COUP-TFI DBD demonstrate that the DBDs of the two NRs form direct contacts (Figure 2B, right). We next wanted to see if the hERα N-terminal domain was able to contact COUP-TFI. When fused to the Gal4DBD, the hERα N-terminal domain activates transcription in yeast through its AF-1 (Métivier et al., 2001). Since such a protein is not informative in two-hybrid assays, we therefore conducted inverse assays. hERα AB domains were fused to the Gal4AD, and COUP-TFI domains to the Gal4DBD. These assays demonstrate that the hERα N-terminal region associates with the C-terminal part of COUP-TFI (Figure 2C). In conclusion, three surfaces of interaction are identified within the two NRs: one mediated by their DBD, one by their C-terminal region and one involving their N- and C-terminal regions. In vitro pull-down assays confirmed the yeast two-hybrid data (see below), indicating that the interaction surfaces used are specific.
Correlation between physical interaction and transcriptional cooperation between hERα and COUP-TFI
We next identified the domains of COUP-TFI involved in the enhancement of hERα transcriptional activity to look for a relationship between physical interaction and transcriptional cooperation. This direct relationship is suggested first by the fact that the COUP-TFI C141S mutant is not able to enhance hERα activity (Figure 2D). The partial or total deletion of the COUP-TFI N-terminal region (57–423 and 87–423 constructs) does not affect the increase in hERα activity (Figure 2D). In contrast, deleting the COUP-TFI C-terminal region (57–153 protein) reduces the transcriptional cooperation by 60%. This points to the involvement of the COUP-TFI DBD and LBD in the transcriptional cooperation with hERα. This is demonstrated further by the hERα activity enhancement by the COUP-TFI DBD alone (residues 57–153) with hERα. In the case of the COUP-TFI LBD, we had to fuse the COUP-TFI 153–423 region to the SV40 nuclear localization signal (NLS; Figure 2D) to obtain a correct localization of the protein, as revealed by green fluorescent protein (GFP; not shown). Expression of this GFP–NLS/COUP-TFI 153–423 protein increases hERα-mediated transcription on the ERE-TK-Luc reporter by 2-fold. In conclusion, both an intact DBD and LBD are required for COUP-TFI to interact physically with hERα and to enhance its activity.
Binding to DNA response elements specific for both NRs destabilizes the hERα–COUP-TFI complex
The above results indicate that the transcriptional cooperation between hERα and COUP-TFI reflects their physical interaction. COUP-TFI can bind EREs (Klinge et al., 1997). One can thus postulate that the hERα–COUP-TFI complex might possess a higher affinity for EREs, compared with hERα homodimers, hence explaining the transcriptional cooperation. Electro phoretic mobility shift assays (EMSAs) were performed using various types of response elements, but in all cases we failed to detect a complex other than each of the NRs homodimers on DNA (data not shown). On the other hand, adding DNA-binding sites for COUP-TFI (DR-1) or ER (ERE), but not unspecific (AP1) sequence, destabilizes the hERα–COUP-TFI complex. As a control, the COUP-TFI–COUP-TFI 57–423 heterodimer remains unaffected by these DNA sequences (Figure 2E). These results suggest that the mechanisms that allow COUP-TFI to enhance hERα activity occur before hERα interacts with its target genes.
Physical interaction with COUP-TFI increases hERα AF-1 activity and phosphorylation of the hERα N-terminal region
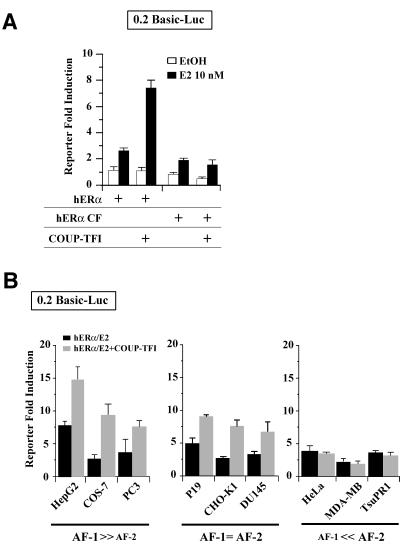
In yeast cells, the hERα N-terminal AF-1 is required for the enhancement of hERα transcriptional activity by COUP-TFI (Petit et al., 1999). Moreover, deleting the hERα N-terminal region (hERα CF) abrogates the transcriptional cooperation between the two NRs in CHO-K1 cells (Figure 3A). We thus hypothesized that the actions of COUP-TFI might target specifically hERα AF-1. To test this assumption, the effect of COUP-TFI on hERα activity was checked in different cell lines, as it has been shown that the relative involvement of AF-1 and AF-2 in hERα transcriptional activity depends upon the cell type (Metzger et al., 1992). Cell contexts can thus be defined as AF-1 or AF-2 permissive, depending upon which AF is involved principally in hERα activity. The relative sensitivity of different cell lines to AF-1 and AF-2 was evaluated by comparing the transcriptional activity of full-length hERα with that of a mutant lacking AF-1 (hERα CF). A similar transcriptional activity of the two proteins would indicate AF-2-permissive cell contexts; no activity of the mutant would indicate AF-1-permissive contexts; and a reduced activity would indicate a mixed context permissive to both AF-1 and AF-2. Based on this classification, data obtained demonstrate that COUP-TFI increases hERα activity in AF-1-permissive cells (mixed and exclusive; Figure 3B). This was not due to cell-specific differences in COUP-TFI or hERα expression, as detected by western blots (see below).
Fig. 3. Cell-specific enhancement of hERα AF-1 by COUP-TFI. (A) Transfection of CHO-K1 cells with 0.2 basic reporter, internal control pCH110 and expression plasmids for COUP-TFI, full-length or N-terminally truncated hERα (hERα CF). (B) Nine cell lines exhibiting a differential sensitivity to ERα AFs were transfected with vectors encoding hERα alone or with COUP-TFI. Cells were treated with ethanol (EtOH) or 10–8 M estradiol (E2). Values are the fold induction of normalized luciferase reporter activity (mean ± SEM).
A cell-specific formation of the hERα–COUP-TFI complex might be one basis for the cell-dependent action of COUP-TFI on hERα activity. However, co-immunoprecipitation experiments detect a protein complex containing the two NRs even in cells restricted to AF-2 activation (see below). Thus, physical interactions alone do not account for the effect of COUP-TFI on hERα activity in certain cells.
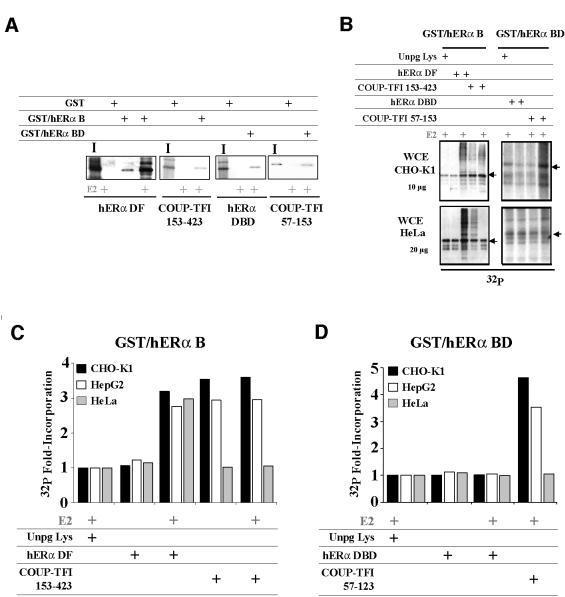
AF-1-mediated transcription stimulation is strongly modulated by cell-specific phosphorylation events (Ali et al., 1993; Le Goff et al., 1994). We therefore postulated that phosphorylation could mediate the cell-specific effect of COUP-TFI on hERα AF-1. To obtain information about a direct impact of COUP-TFI interaction on the hERα phosphorylation state, we developed an in vitro assay in which proteins are first subjected to a GST pull-down procedure. This step is followed by in vitro phosphorylation using whole cell extracts (WCEs) as a donor of kinases. As shown above, two regions of COUP-TFI allow a transcriptional cooperation with hERα: the LBD (amino acids 153–423) and the DBD (amino acids 57–153). These regions were used in the phosphorylation assays, in conjunction with either GST–hERα B or GST–hERα BD domain fusion proteins, with which they can interact (Figure 4A). Data shown in Figure 4B illustrate a typical result obtained after in vitro phosphorylation of the protein complexes. We have shown previously that one of the consequences of the direct interaction between the hERα N- and C-terminal domains is an increase in hERα B domain phosphorylation (Métivier et al., 2001). This provided a positive control for COUP-TFI actions. The quantification of these assays performed with several WCEs as donors of kinases are shown in Figure 4C and D, and demonstrate that (i) COUP-TFI LBD and DBD enhance the phosphorylation of the hERα B or BD domains by 2- to 3.5-fold in a ligand-independent manner; and (ii) this increase occurs with WCEs from CHO-K1 and HepG2 cells (AF-1-permissive) but not from HeLa cells (AF-2-permissive). These actions of COUP-TFI on hERα B domain phosphorylation are specific, since (i) as previously reported, the interaction of hERα LBD with its own B domain requires E2 to affect AF-1 phosphorylation (Métivier et al., 2001); and (ii) hERα DBD interaction with the hERα BD domains does not reproduce the phosphorylation enhancement observed with COUP-TFI DBD.
Fig. 4. Cell-specific enhancement of hERα phosphorylation upon interaction with COUP-TFI. (A) GST pull-down experiments performed with 250 ng of the GST–hERα B or 150 ng of the GST–hERα BD with in vitro labeled COUP-TFI and hERα regions. Controls were made using 250 ng of GST alone, and inputs (I) are 20% of the labeled protein used. (B) In vitro phosphorylation assays were performed using similar amounts of the fusion proteins as in (A), with retained D–F (DF; 153–423) or C–D (DBD; 57–153) domains of hERα or COUP-TFI, in the presence of 5 × 10–6 M estradiol (E2) or ethanol (EtOH). Controls were made using an in vitro translation performed with empty pSG5 vector (Unpg Lys). Phosphorylation was ensured using 10 µCi of [γ-32P]ATP and 10 or 20 µg of CHO-K1 and HepG2 or HeLa WCEs, respectively. (C and D) Phosphoimager quantification of the in vitro phosphorylation assays performed using the same conditions as above. The values shown, normalized by the basal 32P incorporation detected with Unpg Lys, were reproducible (± 1%).
COUP-TFI targets hERα Ser118 phosphorylation by MAPK
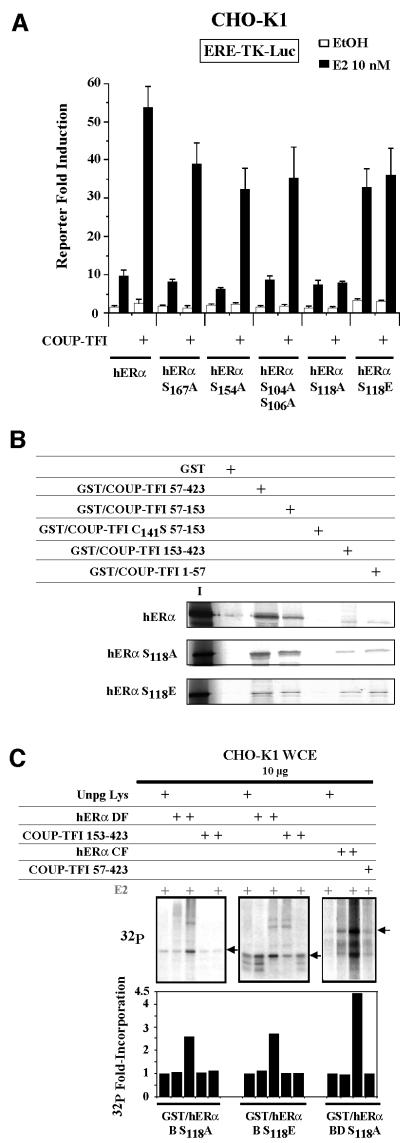
To identify the amino acids within the hERα B domain targeted by this phosphorylation process, we conducted transfection experiments with hERα mutated at the known phosphorylated serines (Ali et al., 1993; Arnold et al., 1994; Le Goff et al., 1994; Rogatsky et al., 1999). COUP-TFI enhanced the activity of the receptors mutated at residues S167, S154, S104 and S106, but not that of S118A. Further, placing a negative charge at the 118 position (S118E) abrogated COUP-TFI action, but also enhanced the activity of the receptor to the same extent that COUP-TFI does on wild-type hERα (Figure 5A). These differences in transactivation are not due to an altered interaction of COUP-TFI with the two S118 mutants (Figure 5B). COUP-TFI is also unable to enhance the phosphorylation state of hERα domains mutated at residue S118 in vitro (Figure 5C). These results suggest that the basis of the transcriptional cooperation mechanism between hERα and COUP-TFI is the provision of a negative charge at the hERα S118 position, which is an essential residue for the hERα AF-1 activity.
Fig. 5. COUP-TFI interaction with hERα targets Ser118 phosphorylation. (A) CHO-K1 cells were transfected with the ERE-TK reporter, pCH110 and plasmids expressing hERα point mutated on each of the residues indicated. (B) GST pull-down assays using in vitro labeled wild-type or point-mutated hERα with 250 ng of each of the COUP-TFI fusion proteins or GST alone. Inputs are 20% of the labeled protein used. (C) Coupled GST pull-down/phosphorylation assays performed with GST fused to point-mutated hERα domains. Experiments used 10 µg of CHO-K1 WCE and in vitro translated hERα or COUP-TFI domains. Quantification of the radioactivity incorporation is shown as in Figure 4.
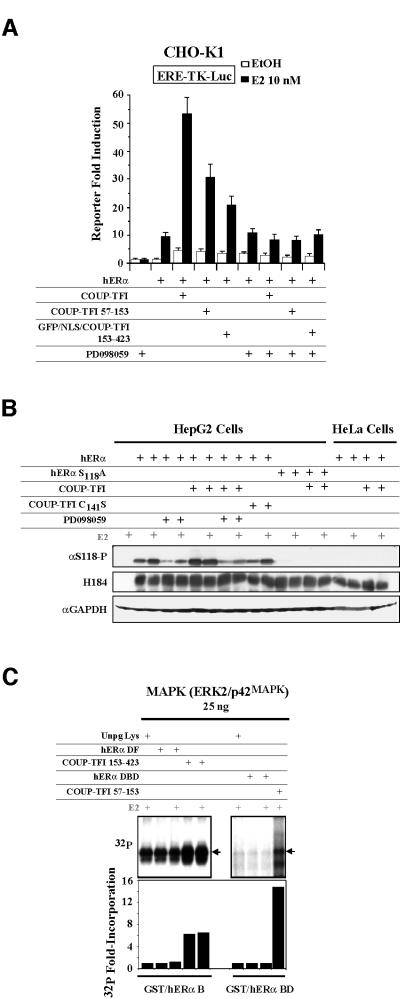
S118 is a target of the mitogen-activated protein kinase (MAPK) pathway (Kato et al., 1995). We thus performed transfection experiments in the presence or absence of an inhibitor of this pathway, PD098059. Inhibiting the MAPK pathway markedly decreases COUP-TFI DBD- and LBD-mediated enhancement of hERα activity (Figure 6A). Western blots using an antibody specifically targeting the phosphorylated S118 were conducted to test the in vivo relevance of the phosphorylation processes (Figure 6B). In transfected HepG2 cells (AF-1-permissive), the amounts of hERα phosphorylated at S118 are increased after E2 treatment, as expected (Kato et al., 1995). Co-expressing COUP-TFI also induces this process, but in a ligand-independent manner. This COUP- TFI-mediated effect needs the formation of the hERα– COUP-TFI complex. Indeed, the COUP-TFI C141S mutant, which cannot interact with hERα (Figure 2), is unable to increase in vivo the S118 phosphorylation above the level found for ER in the absence of COUP-TFI. Treatment with PD098059 abolishes this COUP- TFI-mediated effect, as does S118 point mutation. In AF-2-permissive HeLa cells, the hERα S118 is not phosphorylated, and COUP-TFI has no effect. The results obtained using the PD098059 inhibitor indicated that the MAPKs are involved, either directly or indirectly, in the COUP-TFI-mediated processes that affect hERα phosphorylation and activity. This was analyzed further using a purified constitutively active ERK2/p42MAPK (Sigma) instead of WCE in the pull-down/in vitro phosphorylation assay. Phosphorylation of the hERα N-terminal region by ERK2 is not enhanced after interaction with any hERα domains in the presence of estradiol (Figure 6C). In contrast, COUP-TFI 153–423 and 57–153 regions increase the phosphorylation state of the hERα B and BD domains by 6- or 14-fold, respectively, in a ligand-independent manner. Together with previous results indicating a requirement for an AF-1-permissive cell context, these data demonstrate that a phosphorylation process involving MAPK pathways is the key for the cell-specific enhancement of hERα transcriptional activity by COUP-TFI.
Fig. 6. In vivo enhancement of hERα activity by COUP-TFI involves the MAPK pathway. (A) hERα was expressed alone or in combination with COUP-TFI constructs in CHO-K1 cells, which were treated subsequently with 10 µM of the ERK inhibitor PD098059. Normalized luciferase activities are shown as the mean ± SEM. (B) WCEs from HepG2 or HeLa transfected cells were subjected to western blots using the antibodies indicated on the left. (C) A 25 ng aliquot of purified MAPK was used in the GST pull-down/in vitro phosphorylation assays using 250 or 150 ng of the GST–hERα B or BD fusion proteins, respectively, with either the DF or CD domains of hERα or COUP-TFI. 32P incorporation was measured and expressed as in Figure 4.
hERα hyperphosphorylation induced by COUP-TFI involves an enhanced recruitment of MAPK
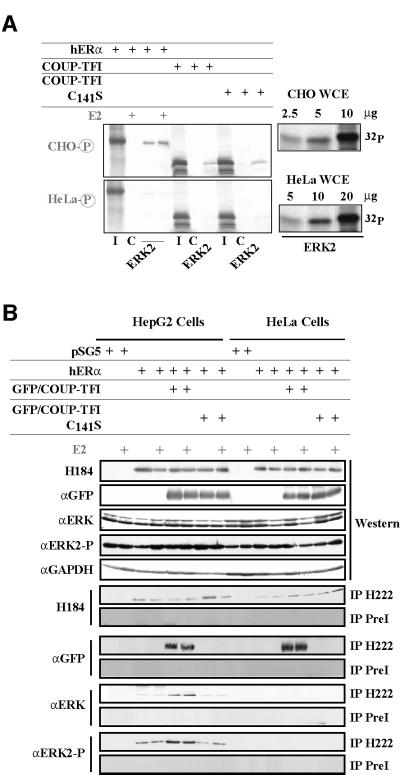
The connection between the enhanced phosphorylation state and an increased targeting of ERK2 to hERα through interaction with COUP-TFI subsequently was tested. As illustrated in Figure 7A, a His-tagged ERK2 protein binds to hERα and COUP-TFI in a ligand-independent manner. This interaction requires the in vitro phosphorylation of the bacterially expressed ERK2 by CHO-K1 WCE. In contrast, phosphorylation by HeLa WCE allows a 10- to 20-fold less efficient association of ERK2 with both NRs, although ERK2 has been phosphorylated to the same extent by both WCEs (Figure 7A, right). Co-immunoprecipitation experiments (Figure 7B) demonstrate this enhanced recruitment in vivo. COUP-TFI does not enhance the level of phosphorylated ERK2, as revealed by western blot using a specific antibody against the phosphorylated form of ERK2. COUP-TFI increases the amounts of activated ERK2 present in the hERα immunoprecipitated complex from HepG2 but not HeLa cells. This requires the physical interaction between the two NRs, since the COUP-TFI C141S mutant does not allow this increase in ERK2 recruitment. Altogether, these data demonstrate that the molecular basis of the positive hERα–COUP-TFI interplay is an enhanced recruitment of ERK2.
Fig. 7. Interaction with COUP-TFI enhances ERK2/p42MAPK recruitment by hERα. (A) In vitro pull-down experiments were performed using 2 µl of the in vitro labeled proteins indicated and 500 ng of the His-tagged ERK2 previously phosphorylated by 10 or 20 µg of CHO-K1 or HeLa WCE, respectively. Controls (C) were run using ion resin previously incubated with bacterial extract expressing no fusion proteins. Inputs (I) are 25% of the proteins used. A radioactive in vitro phosphorylation assay is shown on the right, revealing similar activation of ERK2 by the two WCEs. (B) The H222 antibody was used to co-immunoprecipitate the hERα–COUP-TFI–ERK2 complex in cells. A rat pre-immune serum (PreI) was used as a control. The presence of each component of the complex and activated ERK2 was checked before and after precipitation.
Discussion
Although COUP-TFI was depicted originally as a repressor for other NR functions, there is growing evidence of cross-talk between COUP-TFI and hERα, some of which results in an increased hERα activity (Ktistaki and Talianidis, 1997; Lazennec et al., 1997). This study was therefore initiated to elucidate the molecular basis underlying the positive transcriptional interplay between COUP-TFI and hERα.
Regions of COUP-TFI required (i) to physically associate with hERα and (ii) to enhance the hERα activity are multiple but identical (DBD and C-terminal regions). This implies that the hERα–COUP-TFI interaction is required for the transcriptional cooperation. Within this ‘heteromer’, the DBD–DBD surface is of importance since the COUP-TFI C141S mutant neither provides a proper hERα interaction surface, nor permits the transcriptional interplay. Moreover, the isolated COUP-TFI DBD is sufficient to enhance hERα activity. The COUP-TFI LBD strengthens the contacts with hERα and the transcriptional cooperation. As in the case of some nuclear receptors or hERα homodimers (He et al., 1999; Métivier et al., 2001), the N-terminal region of hERα interacts with COUP-TFI, and vice versa. These observations strongly support a tight association between COUP-TFI and hERα. This confirms a conclusion previously reached by Klinge (1999), using other approaches. It is recalled that in the cell, the two proteins are, of course, entire. Given that the C141S mutation disrupts the other interaction surfaces, we propose that the formation of the complex would be directed first by the DBD–DBD interface. The other interaction surfaces, which could each act separately, stabilize the complex. This sequence of events is of interest when compared with the way in which NR homodimers are formed on DNA: there, dimerization is initiated by the LBD–LBD interface, and stabilized further by allosterical interactions due to the DNA and to the use of the DBD–DBD interface (Lefstin and Yamamoto, 1998). When considered in conjunction with these results, the fact that specific DNA-binding sequences for both NRs dissociate the hERα–COUP-TFI complex is likely to imply that the formation of the hERα–COUP-TFI complex occurs in solution. Actions leading to the transcriptional cooperation between the two NRs are thus likely to occur downstream of the necessary formation of this ‘heteromer’.
Of significance is the finding that only AF-1-permissive cells (i.e. cells in which hERα activity is driven mainly by this AF) allow the increase of hERα activity by COUP-TFI. This cell dependency gave the first clue about a specific mechanism that allows COUP-TFI to enhance hERα AF-1 activity. Co-immunoprecipitation assays demonstrated that the formation of the hERα–COUP-TFI complex alone is not this key process. Phosphorylation pathways are known to influence AF-1 (Ali et al., 1993; Le Goff et al., 1994). We thus hypothesized a role for the phosphorylation pathways, which allow COUP-TFI to target hERα AF-1. Mutation of the phosphorylation target site S118 impairs the positive effect of COUP-TFI on hERα activity. This COUP-TFI-mediated enhancement of hERα S118 phosphorylation occurs only in hERα AF-1-permissive cells, and requires COUP-TFI LBD and DBD. MAPK pathways can lead to hERα S118 phosphorylation (Kato et al., 1995). We also show that ERK2/p42MAPK is involved in the COUP-TFI-induced phosphorylation of the hERα S118. In vivo assays demonstrate that COUP-TFI enhances the interaction between hERα and ERK2 in AF-1- but not AF-2-permissive cells. Thus, the stabilized recruitment of MAPK within a complex involving hERα and COUP-TFI is the basis for the cell-specific effect of COUP-TFI on hERα AF-1.
COUP-TFI but not hERα enhances the S118 phosphorylation through DBD–DBD interactions. Moreover, ERK2 is not involved in the S118 phosphorylation that takes place following the hERα N-terminal region ligand-dependent association with the C-terminal part of the protein (Métivier et al., 2001). This illustrates the specificity of COUP-TFI in the hERα S118 modification. Both COUP-TFI DBD and LBD were able to enhance the phosphorylation of hERα S118. This suggests that the interactions between these two regions of COUP-TFI and hERα induce specific and identical conformational changes within the hERα N-terminal region. Such structural adaptations through dimerization and/or DNA binding were demonstrated for the hERα, glucocorticoid (GR, NR3C1) and progesterone receptors (Kumar et al., 1999; Bain et al., 2000; Greenfield et al., 2001). Furthermore, limited proteolysis experiments evidenced some differences in the overall three-dimensional structure of the hERα–COUP-TFI complex, compared with a hERα homodimer (not shown).
The mechanism reported here is important to integrate the actions of E2 in the whole organism. As is well known, E2 exhibits pleiotropic and opposite effects depending upon the tissues, leading to a cell-specific modulation of the transcription of a repertoire of genes (Nilsson et al., 2001). Cell-specific controls of the expression of the ER and its isoforms, due to a differential use of the multiple promoters of the hERα gene together with alternative splicing, are one of the elements that contribute to explaining these pleiotropic effects (Koš et al., 2001). In addition, the ER activity in itself can be regulated in a cell-specific manner, for instance through the preferential use of AF-1 over AF-2, to activate the transcription of target genes. The cell-specific transcriptional interplay between ER and other NRs such as COUP-TFI is another way to diversify estrogenic responses. Indeed, the two NRs co-exist in certain cell types, such as uterine epithelial cells, ovaries, neonatal reproductive tract, kidney, prostate and liver (Pereira et al., 1995; Shigeta et al., 1996; Qiu et al., 1997; Chu et al., 1998). The relative levels of COUP-TFI might be decisive in discriminating between the positive and negative actions of COUP-TFI on ER functions. These opposite actions are known to depend on two variables: promoter and cell context (Ktistaki and Talianidis, 1997; Klinge, 1999). The repressive actions of COUP-TFI are thought to be due to competition for either the occupancy of DNA-binding sites or for a common co-regulator (Cooney et al., 1993). In the same cell, one way for COUP-TFI to discriminate between opposite actions on other NR activities relies on their relative affinity for a shared response element (Sugiyama et al., 2000). We demonstrate here that the additional element of cell-dependent interplay with kinase pathways plays a role in orienting the balance between repression and activation of hERα activity by COUP-TFs.
Our results also give rise to another question: what are the mechanisms that restrict ERK2 interaction with COUP-TFI and hERα only to certain cells? A specific conformation or alteration within the hERα and COUP-TFI proteins in AF-2-permissive cells due to variations in post-translational modifications might prevent the recruitment of the kinase. Conversely, a specific conformation adopted by the hERα–COUP-TFI complex in AF-1-permissive cells would also be responsible for ERK2 recruitment. Similar amounts of activated ERK2 were present in the in vitro phosphorylation assays (Figures 4 and 5). This implies that in the WCEs from AF-2-permissive cells, other proteins prevent the recruitment of ERK2 to the hERα–COUP-TFI complex. We observed in the in vitro assays that the profile of endogenous phosphorylated proteins was different for each WCE (Figure 4). Principally, two bands were specific to HepG2 WCEs: one at 90 kDa, presumably the pp90rsk (Joel et al., 1998), and one at 140 kDa. Conversely, other proteins (85 and 100 kDa) were visualized specifically using HeLa WCEs. When the identity of these proteins is known, their potential role in the interrelationships between COUP-TFI and hERα might be tested. It might be plausible that the designation of cells as AF-1 or AF-2 permissive might be due to the expression of these proteins. Another explanation for the cell-specific recruitment of ERK2 could be that pathways leading to an activation of hERα by ERK2 are impaired in AF-2-permissive cells.
It should be noted that a similar phosphorylation-mediated mechanism was described recently, in which NR interactions with TFIIH were linked to targeting to the cdk7 kinase (Chen et al., 2000). A prolonged phosphorylation state correlated with an increased activity of the STAT5 transcription factor was also observed after physical interaction with GR (Wyszomierski et al., 1999). Modulation of a given NR phosphorylation state by physical interaction with another NR could then be a general process involved in the complex network of pathways orchestrated by these factors. What would be the functional consequence of these events? Upon phosphorylation in the absence of ligand, one could propose that hERα interaction with co-repressors might be reduced, making the receptor more accessible for co-activators. The phosphorylation of specific residues in PPAR (NR3C3), SF-1 and ERβ modulates the recruitment of p160 co-activators (Shao et al., 1998; Hammer et al., 1999; Tremblay et al., 1999). Phosphorylation of hERα S118 by MAPK enhances its physical interaction with the AF-1 co-activator, p68 RNA helicase (Endoh et al., 1999). One can then propose that COUP-TFI modulates the interaction of p68 RNA helicase with hERα. COUP-TFI also interacts with the CBP–p300 complex (Bailey et al., 1998), which is a co-activator for hERα AF-1 that is known to bind SRC-1 and the p68 RNA helicase (Yao et al., 1996; Endoh et al., 1999; Kobayashi et al., 2000). Upon interaction with hERα, COUP-TFI might thus also recruit large protein complexes to modulate hERα actions.
In conclusion, this study of the interplay between COUP-TFI and hERα shows that these NRs are physically interacting, in a ligand-independent manner. This results in an enhancement of hERα transcriptional activity in AF-1-permissive cells, by a mechanism that leads to an increased binding of ERK2. These data add to our understanding of NRs and signal transduction cross-talk, which allow new mechanisms to be envisaged that con tribute to an explanation of the pleiotropic actions of E2.
Materials and methods
Host strains
We used Escherichia coli DH5α, E.coli BL21 (DE3) pLysS (Invitrogen Corp., Carlsbad, CA) or E.coli TG1 bacteria strains, and Y190 yeast strain (Clontech Laboratories, Inc., Palo Alto, CA). Two-hybrid clones were obtained, selected and analyzed in three independent experiments, as described previously (Métivier et al., 2001).
Antibodies
The rabbit polyclonal anti-GFP, anti-Gal4DBD, anti-GAPDH, anti-p42/p44, H184 (directed against the N-terminal part of the hERα) and goat polyclonal anti-COUP-TFI were purchased from TEBU (Le-Perray en Yvelines, France); and the anti-phosphorylated ERK2 from NEB (9106; Frankfurt, Germany). The anti-S118P antibody was a gift from Dr S.Ali (Chen et al., 2000), and the rat monoclonal H222 (directed against the C-terminal part of the hERα) was from Dr B.S.Katzenellenbogen.
Plasmids
Oligonucleotides (Eurogentec, Seraing, Belgium) had the following sequences: EREc, GATCCAGGTCACAGTGACCT; DR1, TCGAGG TCAGAGGTCACGA; and AP-1, TCGACGCTTGATGACTCAGCC GGAA. The SmaI–SmaI, SmaI–EcoRI, SmaI–XmnI, XmnI–EcoRI and EcoRI–EcoRI fragments of the pECE/COUP-TFI or pECE/COUP- TFI C141S vectors (Lazennec et al., 1997) were cloned into pACT2 (Clontech) or pGEX2T (Pharmacia, Buckinghamshire, UK) vectors. COUP-TFI 84/423 and COUP-TFI 153–423/SV40NLS were obtained by PCR, and the latter was cloned in the pEGFP-C3 vector (Clontech). The Gal4DBD fusions with wild-type and deleted COUP-TFI were constructed by excising the corresponding inserts from pACT2 constructs, and ligation into pAS2-1 (Clontech) or pCDNA-3 (Invitrogen). The pCDNA/COUP-TFI and pCDNA/COUP-TFI C141S, and 0.2 basic, chicken Vg and ERE-TK reporter plasmids are described (Lazennec et al., 1997; Flouriot et al., 2000). The hERα/Gal4DBD, hERα/Gal4AD, hERα AB/Gal4DBD, hERα CF/Gal4DBD and hERα DF/Gal4DBD fusions were expressed by pAS2-1 or pACT2 constructs. hERαS118A and hERαS118E included in plasmid pSG5 were provided by Professor P.Chambon, while pCMV-5/hERα, pCMV-5/hERαS167A, pCMV-5/hERαS154A and pCMV-5/hERαS104A,S106A are from Dr B.S.Katzenellenbogen. The GST–hERα B, GST–hERα BD and point mutant versions were expressed using pGEX2T constructs. pRSET/hERK2 was a gift from Dr A.Nebreda.
Cell culture
Cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma, St Quentin Fallavier, France) supplemented with 5% fetal calf serum (FCS; Sigma) at 37°C and 5% CO2. Transient transfections were performed as previously (Métivier et al., 2001) in 6-well plates (HeLa, HepG2, MDA-MB231, PC3, TsuPR1 and DU145 cell lines) or 24-well plates (COS-7, P19 and CHO-K1). Cells were treated for 36 h in DMEM-F12/2.5% charcoal-stripped FCS media supplemented with 10–8 M E2 or ethanol. MCF-7 cells were only treated for 6 h. Treatment with 10 µM of the inhibitor PD098059 (Sigma) or dimethylsulfoxide (DMSO) as a control was performed 4 h before harvesting the cells (Alessi et al., 1995). Luciferase activities were normalized by β-galactosidase activity, and expressed as the mean ± SEM from reporter activity fold induction obtained in four independent transfections.
Protein cell extracts and analysis
Yeast WCEs were obtained as previously described (Petit et al., 1999). Other WCEs were obtained by thawing–freezing cycles of cells in WCE buffer [20 mM HEPES pH 7.9, 400 mM KCl, 2 mM dithiothreitol (DTT) and 20% glycerol]. These conditions were adequate for a maximal ERK2 activation under basal conditions, as checked by western blotting. Analysis of protein expression was performed using the ECL kit (Amersham Pharmacia, Piscataway, NJ), with 50 µg of yeast WCEs or 150 µg of HepG2 or HeLa WCE precipitated by 30% (NH4)2SO4. For immunoprecipitations, 800 µg of WCE in LSB (50 mM Tris pH 7.9, 3 mM MgCl2, 0.1 M KCl, 1 mM DTT, 0.1% NP-40 and 20% glycerol) were cleared for 2 h with 4 µg of rat or goat pre-immune IgG (Sigma), and 50 µl of a protein G–Sepharose beads slurry (Sigma). Supernatants were incubated with 2 µg of H222 or anti-COUP-TFI antibody chemically cross-linked to 50 µl of protein G–Sepharose beads for 6 h at 4°C. After three washes in LSB buffer, retained IgG–protein complexes were denatured before SDS–PAGE, and blotted on a PVDF membrane. Blots were incubated overnight with primary antibodies, washed and incubated for 1 h with corresponding horseradish peroxidase-conjugated antibodies (TEBU).
Pull-down and in vitro phosphorylation assays
In vitro translation of proteins using the TNT kit (Promega), ligand treatment, purification of proteins and GST pull-down assays were performed as described previously (Métivier et al., 2001). His6-ERK2 was purified on Probond beads (Invitrogen). After purification, the protein was phosphorylated by incubation with 10 or 20 µg of CHO-K1 or HeLa WCE, respectively, in phosphorylation buffer (PB; 40 mM Tris–HCl pH 7.4, 50 mM MgCl2, 5 mM DTT, 250 µM ATP and phosphatase inhibitors: 1 µM okadaic acid and 200 µM orthovanadate). Similar ERK2 activation was checked by performing the in vitro phosphorylation with [γ-32P]ATP and evaluation of the His6-ERK2 protein labeling. After binding to in vitro labeled proteins, the beads were washed twice in HWB (20 mM Na2HPO4 pH 7.8, 300 mM NaCl, 0.04% Tween-20 and 10 µg/ml concentrations of the protease inhibitors leupeptin, pepstatin and aprotinin), and proteins were eluted in 0.5 ml of HEB (250 mM imidazole, 20 mM Na2HPO4 pH 6.0, 500 mM NaCl and protease inhibitors), precipitated with trichloroacetic acid (TCA) before fractionation by SDS–PAGE. In oligonucleotide competitions, increasing amounts of double-stranded DNA (25–200 ng) were added. A pull-down experiment coupled to an in vitro phosphorylation assay was designed to evaluate the impact of hERα–COUP-TFI association on the phosphorylation of the hERα N-terminal region. For this, 250 ng of GST–hERα B or 150 ng of the GST–hERα BD fusion proteins were incubated in binding buffer [50 mM NaCl, 50 mM Tris pH 8, 0.02% bovine serum albumin (BSA), 0.02% Tween-20, 1 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitors] for 3 h with equal amounts of in vitro translated proteins. These amounts were determined by taking into account the number of methionines and 35S incorporation. Beads were washed four times in WB300 (50 mM Tris pH 8, 300 mM NaCl, 1 mM PMSF, 0.05% Tween-20 and protease inhibitors) and resuspended in binding buffer, before incubation at 30°C for 30 min with 10 µCi of [γ-32P]ATP in PB with 10 or 20 µg of WCEs. Ten, 25 or 50 ng of activated purified ERK2/p42MAPK (double mutant constitutively active; Sigma M3172) were also used instead of the WCE. Proteins bound to the beads were washed twice in WB300, denatured and fractionated by SDS–PAGE. After Coomassie Blue staining and drying, gels were exposed for autoradiography with Kodak Biomax films (Rochester, NY). Amounts of incorporated 32P in phosphorylation assays were quantified using a phosphoimager (Packard). Experiments were carried out at least twice, and were reproducible. The phosphorylations observed in these experiments were due specifically to kinases present in the WCE, and not to those contained in rabbit reticulocyte lysate (Foulkes et al., 1983).
Acknowledgments
Acknowledgements
We are grateful to J.Beaudouin, Dr.M.Kos and Dr G.Reid for their help in writing this manuscript. We greatly acknowledge Dr S.Ali, Dr B.S.Katzenellenbogen, Professor P.Chambon, Dr D.Metzger, Dr A.Nebreda and Dr M.Pfahl for their gifts of antibodies and vectors. This work was supported by fellowships from the ARC and the Ministère de l’Enseignement et de la Recherche (to R.M. and F.A.G.), Ministère des Affaires Etrangères (to R.M.), a fellowship from the EMBL and EMBO (to M.R.H.), and by funds from the CNRS, ARC, the ligue contre le cancer, EMBL and the Fondation Langlois.
References
- Adam F., Sourisseau,T., Métivier,R., Le Page,Y., Desbois,C., Michel,D. and Salbert,G. (2000) COUP-TFI (chicken ovalbumin upstream promoter-transcription factor I) regulates cell migration and axogenesis in differentiating P19 embryonal carcinoma cells. Mol. Endocrinol., 14, 1918–1933. [DOI] [PubMed] [Google Scholar]
- Alessi D.R., Cuenda,A., Cohen,P., Dudley,D.T. and Saltiel,A.R. (1995) PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J. Biol. Chem., 270, 27489–27494. [DOI] [PubMed] [Google Scholar]
- Ali S., Metzger,D., Bornert,J.M. and Chambon,P. (1993) Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO J., 12, 1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S.F., Obourn,J.D., Jaffe,H. and Notides,A.C. (1994) Serine 167 is the major estradiol-induced phosphorylation site on the human estrogen receptor. Mol. Endocrinol., 8, 1208–1214. [DOI] [PubMed] [Google Scholar]
- Bailey P., Sartorelli,V., Hamamori,Y. and Muscat,G.E.O. (1998) The orphan receptor, COUP-TFII, inhibits myogenesis by post-transcriptional regulation of MyoD function: COUP-TFII directly interacts with p300 and MyoD. Nucleic Acids Res., 26, 5501–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain D.L., Franden,M.A., McManaman,J.L., Takimoto,G.S. and Horwitz,K.B. (2000) The N-terminal region of the human progesterone A-receptor. Structural analysis and the influence of the DNA binding domain. J. Biol. Chem., 275, 7313–7320. [DOI] [PubMed] [Google Scholar]
- Chen D., Riedl,T., Washbrook,E., Pace,P.E., Coombes,R.C., Egly,J.M. and Ali,S. (2000) Activation of estrogen receptor α by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Mol. Cell, 6, 127–137. [PubMed] [Google Scholar]
- Chu K., Boutin,J.M., Breton,C. and Zingg,H.H. (1998) Nuclear receptors COUP-TFII and Ear-2: presence in oxytocin-producing uterine cells and functional interaction with the oxytocin gene promoter. Mol. Cell. Endocrinol., 137, 145–154. [DOI] [PubMed] [Google Scholar]
- Cooney A.J., Leng,X.L., Tsai,S.Y., O’Malley,B.W. and Tsai,M.J. (1993) Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor-dependent repression of transactivation by the vitamin D, thyroid hormone and retinoic acid receptors. J. Biol. Chem., 268, 4152–4160. [PubMed] [Google Scholar]
- Couse J.F. and Korach,K.S. (1999) Estrogen receptor null mice: what have we learned and were will they lead us? Endocr. Rev., 20, 358–417. [DOI] [PubMed] [Google Scholar]
- Endoh H. et al. (1999) Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor α. Mol. Cell. Biol., 19, 5363–5372. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Feigelson H.S. and Henderson,B.E. (1996) Estrogens and breast cancer. Carcinogenesis, 17, 2279–2284. [DOI] [PubMed] [Google Scholar]
- Flouriot G., Brand,H., Denger,S., Metivier,R., Kos,M., Reid,G., Sonntag-Buck,V. and Gannon,F (2000) Identification of a new isoform of the human estrogen receptor-α (hER-α) that is encoded by distinct transcripts and that is able to repress hER-α activation function 1. EMBO J., 19, 4688–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes J.G., Ernst,V. and Levin,D.H. (1983) Separation and identification of type 1 and type 2 protein phosphatases from rabbit reticulocyte lysates. J. Biol. Chem., 258, 1439–1443. [PubMed] [Google Scholar]
- Freedman L.P. (1999) Increasing the complexity of coactivation in nuclear receptor signaling. Cell, 97, 5–8. [DOI] [PubMed] [Google Scholar]
- Gaub M.P., Bellard,M., Scheuer,I., Chambon,P. and Sassone-Corsi,P. (1990) Activation of the ovalbumin gene by the estrogen receptor involves the Fos–Jun complex. Cell, 63, 1267–1276. [DOI] [PubMed] [Google Scholar]
- Giguere V. (1999) Orphan nuclear receptors: from gene to function. Endocr. Rev., 20, 689–725. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Vijayanathan,V., Thomas,T.J., Gall,M.A. and Thomas,T. (2001) Increase in the stability and helical content of estrogen receptor α in the presence of the estrogen response element: analysis by circular dichroism spectroscopy. Biochemistry, 40, 6646–6652. [DOI] [PubMed] [Google Scholar]
- Hammer G.D., Krylova,I., Zhang,Y., Darimont,B.D., Simpson,K., Weigel,N.L. and Ingraham,H.A. (1999) Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol. Cell, 3, 521–526. [DOI] [PubMed] [Google Scholar]
- He B., Kemppainen,J.A., Voegel,J.J., Gronemeyer,H. and Wilson,E.M. (1999) Activation function 2 in the human androgen receptor ligand binding domain mediates interdomain communication with the NH2-terminal domain. J. Biol. Chem., 274, 37219–37225. [DOI] [PubMed] [Google Scholar]
- Jiang J.G., Bell,A., Liu,Y.H. and Zarnegar,R. (1997) Transcriptional regulation of the hepatocyte growth factor gene by the nuclear receptors chicken ovalbumin upstream promoter transcription factor and estrogen receptor. J. Biol. Chem., 272, 3928–3934. [DOI] [PubMed] [Google Scholar]
- Joel P.B., Smith,J., Strurgill,T.W., Fisher,T.L., Blenis,J. and Lannigan,D.A. (1998). pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol. Cell. Biol., 18, 1978–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S. et al. (1995) Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science, 270, 1491–1494. [DOI] [PubMed] [Google Scholar]
- Klinge C.M. (1999) Role of estrogen receptor ligand and estrogen response element sequence on interaction with chicken ovalbumin upstream promoter transcription factor (COUP-TF). J. Steroid Biochem. Mol. Biol., 71, 1–19. [DOI] [PubMed] [Google Scholar]
- Klinge C.M., Silver,B.F., Driscoll,M.D., Sathya,G., Bambara,R.A. and Hilf,R. (1997) Chicken ovalbumin upstream promoter-transcription factor interacts with estrogen receptor, binds to estrogen response elements and half-sites and inhibits estrogen-induced gene expression. J. Biol. Chem., 272, 31465–31474. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kitamoto,T., Masuhiro,Y., Watanabe,M., Kase,T., Metzger,D., Yanagisawa,J. and Kato,S. (2000) p300 mediates functional synergism between AF-1 and AF-2 of estrogen receptor α and β by interacting directly with the N-terminal A/B domains. J. Biol. Chem., 275, 15645–15651. [DOI] [PubMed] [Google Scholar]
- Koš M., Reid,G., Denger,S. and Gannon,F. (2001) Genomic organization of the human ERα gene promoter region. Mol. Endocrinol., 15, 2057–2063. [DOI] [PubMed] [Google Scholar]
- Ktistaki E. and Talianidis,I. (1997) Chicken ovalbumin promoter transcription factors act as auxiliary cofactors for hepatocyte nuclear factor 4 and enhance hepatic gene expression. Mol. Cell. Biol., 17, 2790–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Baskakov,I.V., Srinivasan,G., Bolen,D.W., Lee,J.C. and Thompson,E.B. (1999) Interdomain signaling in a two-domain fragment of the human glucocorticoid receptor. J. Biol. Chem., 274, 24737–24741. [DOI] [PubMed] [Google Scholar]
- Laudet V. (1997) Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor. J. Mol. Endocrinol., 19, 207–226. [DOI] [PubMed] [Google Scholar]
- Lazennec G., Kern,L., Valotaire,Y. and Salbert,G. (1997) The nuclear orphan receptors COUP-TF and ARP-1 positively regulate the trout estrogen receptor gene through enhancing autoregulation. Mol. Cell. Biol., 17, 5053–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dréan Y., Liu,D., Wong,A.O.L., Xiong,F. and Hew,C.L. (1996) Steroidogenic factor 1 and estradiol receptor act in synergism to regulate the expression of the salmon gonadotropin IIβ subunit gene. Mol. Endocrinol., 10, 217–229. [DOI] [PubMed] [Google Scholar]
- Lefstin J.A. and Yamamoto,K.R. (1998) Allosteric effects of DNA on transcriptional regulators. Nature, 392, 885–888. [DOI] [PubMed] [Google Scholar]
- Le Goff P., Montano,M.M., Schodinn,D.J. and Katzenellenbogen,B.S. (1994) Phosphorylation of the human estrogen receptor. J. Biol. Chem., 269, 4458–4466. [PubMed] [Google Scholar]
- Lombardi G., Zarilli,S., Colao,A., Paesano,L., Di Somma,C., Rossi,F. and De Rosa,M. (2001) Estrogens and health in males. Mol. Cell. Endocrinol., 178, 51–55. [DOI] [PubMed] [Google Scholar]
- Métivier R., Penot,G., Flouriot,G. and Pakdel,F. (2001) Synergism between estrogen receptor α (ERα) transactivation functions 1 (AF-1) and 2 (AF-2) mediated by SRC-1: requirement for the AF-1 α-helical core and for a direct interaction between the N- and C-terminal domains. Mol. Endocrinol., 15, 1953–1970. [DOI] [PubMed] [Google Scholar]
- Metzger D., Losson,R., Bornert,J.M., Lemoine,Y. and Chambon,P. (1992) Promoter specificity of the two transcriptional activation functions of the human oestrogen receptor in yeast. Nucleic Acids Res., 20, 2813–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. et al. (2001) Mechanisms of estrogen action. Physiol. Rev., 81, 1535–1565. [DOI] [PubMed] [Google Scholar]
- Nuclear Receptor Committee (1999) A unified nomenclature system for the nuclear receptor superfamily. Letter to the editor. Cell, 97, 161–163. [DOI] [PubMed] [Google Scholar]
- Parker M.G. (1995) Structure and function of estrogen receptors. Vitam. Horm., 51, 267–287. [DOI] [PubMed] [Google Scholar]
- Pereira F.A., Qiu,Y., Tsai,M.J. and Tsai,S.Y. (1995) Chicken ovalbumin upstream promoter transcription factor (COUP-TF): expression during mouse embryogenesis. J. Steroid Biochem. Mol. Biol., 53, 503–508. [DOI] [PubMed] [Google Scholar]
- Petit F.G., Métivier,R., Valotaire,Y. and Pakdel,F. (1999) Synergism between a half-site and an imperfect estrogen-responsive element and cooperation with COUP-TFI are required for estrogen receptor (ER) to achieve a maximal estrogen-stimulation of rainbow trout ER gene. Eur. J. Biochem., 259, 385–395. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Pereira,F.A., de Mayo,F.J., Lydon,J.P., Tsai,S.Y. and Tsai,M.J. (1997) Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection and arborization. Genes Dev., 11, 1925–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I., Trowbridge,J.M. and Garabedian,M.J. (1999) Potentiation of human estrogen receptor α transcriptional activation through phosphorylation of serines 104 and 106 by the cyclinA–CDK2 complex. J. Biol. Chem., 274, 22296–22302. [DOI] [PubMed] [Google Scholar]
- Salvatori L., Ravenna,L., Felli,M.P., Cardillo,M.R., Russo,M.A., Frati,L., Gulino,A. and Petrangeli,E. (2000) Identification of an estrogen-mediated deoxyribonucleic acid-binding independent transactivation pathway on the epidermal growth factor receptor gene promoter. Endocrinology, 141, 2266–2274. [DOI] [PubMed] [Google Scholar]
- Shao D., Rangwala,S.M., Bailey,S.T., Krakow,S.L., Reginato,M.J. and Lazar,M.A. (1998) Interdomain communication regulating ligand-binding by PPAR-γ. Nature, 396, 377–380. [DOI] [PubMed] [Google Scholar]
- Shigeta H., Newbold,R.R., McLachlan,J.A. and Teng,C. (1996) Estrogenic effect on the expression of estrogen receptor, COUP-TF and lactoferrin mRNA in developing mouse tissues. Mol. Reprod. Dev., 45, 21–30. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Wang,J.C., Scott,D.K. and Granner,D.K. (2000) Transcription activation by the orphan nuclear receptor, chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI). Definition of the domain involved in the glucocorticoid response of the phosphoenolpyruvate carboxykinase gene. J. Biol. Chem., 275, 3446–3454. [DOI] [PubMed] [Google Scholar]
- Tremblay A., Tremblay,G.B., Labrie,F. and Giguère,V. (1999) Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol. Cell, 3, 513–519. [DOI] [PubMed] [Google Scholar]
- Weigel N.L. (1996) Steroid hormone receptors and their regulation by phosphorylation. Biochem. J., 319, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszomierski S.L., Yeh,J. and Rosen,J.M. (1999) Glucocorticoid receptor/signal transducer and activator of transcription 5 (STAT5) interactions enhance STAT5 activation by prolonging STAT5 DNA binding and tyrosine phosphorylation. Mol. Endocrinol., 13, 330–343. [DOI] [PubMed] [Google Scholar]
- Yao T.P., Ku,G., Zhou,N., Scully,R. and Livingston,D.M. (1996) The nuclear hormone receptor SRC-1 is a specific target of p300. Proc. Natl Acad. Sci. USA, 93, 10626–10631. [DOI] [PMC free article] [PubMed] [Google Scholar]