Abstract
Cyclin-dependent kinase (CDK) Tyr15 phosphorylation plays a major role in regulating G2/M CDKs, but the role of this phosphorylation in regulating G1/S CDKs is less clear. We have studied the regulation and function of Cdc2-Tyr15 phosphorylation in the fission yeast Schizosaccharomyces pombe G1/S CDK Cig2/Cdc2. This complex is subject to high level Cdc2-Tyr15 phosphorylation inhibiting its kinase activity in hydroxyurea-treated cells blocked in S-phase. We show that this Tyr15 phosphorylation is required to maintain efficient mitotic checkpoint arrest, because Cig2 accumulates during the block and this accumulation can advance mitotic onset. This mitotic induction operates, at least in part, through activation of the normal G2/M CDK complex Cdc13/Cdc2. Thus, Tyr15 phosphorylation of G1/S CDK complexes is important in the checkpoint control blocking mitotic onset when DNA replication is inhibited.
Keywords: Cdc2/Cig2/checkpoint/mitosis/Tyr15
Introduction
The cyclin-dependent kinases (CDKs) control the major cell cycle transitions in all eukaryotes. The G2/M CDKs, which act at the onset of mitosis, are generally regulated by phosphorylation of the Tyr15 residue (or its equivalent), located near to the catalytic site of the protein kinase (Norbury and Nurse, 1992). However, potential roles of this phosphorylation in regulating G1/S CDKs have received less attention. It has been proposed in mammalian cells that the Cdc25A phosphatase, which dephosphorylates Tyr15, regulates S-phase entry (Galaktionov et al., 1995; Blomberg and Hoffmann, 1999) and also acts in the S-phase checkpoint, delaying progression through S-phase after DNA damage (Iavarone and Massagué, 1997; Blomberg and Hoffmann, 1999; Jinno et al., 1999; Falck et al., 2001). These observations suggest that G1/S CDK-Tyr15 phosphorylation regulates S-phase and associated checkpoint controls, although the Tyr15 phosphorylation regulation of the relevant G1/S cyclin/CDK complexes (Cdk4,6/CyclinD, Cdk2/CyclinE,A) was not studied. Two studies of Cdk4 Tyr15 phosphorylation have concluded that it regulates progression through G1 when mammalian cells exit quiescence to undergo proliferation (Jinno et al., 1999) and that this phosphorylation is required for G1 arrest induced by UV irradiation (Terada et al., 1995). Another study has shown that Cdk2 Tyr15 also becomes phosphorylated when DNA is damaged with UV light (Mailand et al., 2000; Bartek and Lukas, 2001), resulting in S-phase entry blockage.
To clarify the role of G1/S CDK Tyr15 phosphorylation we have investigated this problem in the fission yeast Schizosaccharomyces pombe. In fission yeast the CDK Cdc2 is required for the onset of both S-phase and mitosis (Stern and Nurse, 1996). Cig2/Cdc2 is the primary G1/S promoting complex (Fisher and Nurse, 1996; Martin-Castellanos et al., 1996; Mondesert et al., 1996), but in cig2-deleted strains the G2/M Cdc13 cyclin can efficiently substitute for the G1/S Cig2 cyclin. The cig2+ gene is periodically expressed during the cell cycle and is controlled by the Cdc10 transcription factor (Mondesert et al., 1996). The Cig2 protein accumulates during G1/S phases, and is degraded in early G2 phase (Yamano et al., 2000). In contrast, the cdc13+ gene is constitutively expressed throughout the cell cycle (Correa-Bordes et al., 1997), and Cdc13/Cdc2 kinase activity is maintained at low level from mitotic exit to the end of G1 by both Cdc13 degradation (Creanor and Mitchison, 1996) and the CDK inhibitor Rum1 (Booher et al., 1989; Correa-Bordes et al., 1997). In vitro and in vivo data show that the Cig2/Cdc2 complex is less sensitive to Rum1 inhibition than Cdc13/Cdc2 (Moreno and Nurse, 1994; Correa-Bordes and Nurse, 1995; Fisher and Nurse, 1996). Thus, Cig2 accumulation in G1 appears to be the main determinant for the timing of S-phase onset.
The G2/M Cdc2 CDK associates with the Cdc13 B-type cyclin and is inhibited by Tyr15 phosphorylation (Gould and Nurse, 1989; Nurse, 1990; Gould et al., 1991) during G2 phase. The Cdc13/Cdc2 complex is also Tyr15 phosphorylated when the DNA replication/DNA damage checkpoint pathway is activated, blocking the onset of mitosis (Francesconi et al., 1997; Rhind et al., 1997; Rhind and Russell, 1998). This phosphorylation is carried out by the mitotic inhibitory kinases Wee1 and Mik1, and is removed by the phosphatase Cdc25 (Moser and Russell, 2000).
Little is known about G1/S CDK Tyr15 phosphorylation and its role in cell cycle control in fission yeast. Previous work has shown that Cdc2 is Tyr15 phosphorylated in S-phase-arrested cells [cdc20ts and cdc22ts mutants, hydroxy urea (HU) block], while no Tyr15 phosphorylation was detected in a G1 block (cdc10ts) (Hayles and Nurse, 1995). These results suggest that Cdc2 becomes Tyr15 phosphorylated when cells enter S-phase. Here we show that Cig2/Cdc2 complexes are entirely inhibited by Tyr15 phosphorylation when cells become blocked in S-phase and, unexpectedly, that this plays a role in preventing mitosis in cells that fail to replicate their DNA fully.
Results
Cig2/Cdc2 is inhibited by Tyr15 phosphorylation in replication block
To investigate the role of Cdc2-Tyr15 phosphorylation during G1- and S-phases in fission yeast we produced a synchronized population of cells by using a cdc25-22 nmt85::cdc13+ thermosensitive strain (Z636) in which the cdc13+ gene is controlled by the low expressing thiamine-repressible nmt1 promoter. This strain allows the removal of Cdc13/Cdc2 complexes from S-phase cells. Without thiamine (promoter ON), the cdc13+ expression level is similar to that of the endogenous cdc13+ promoter. These cells were synchronized in late G2 by incubating cdc25-22 at 36°C for 4 h, and after 3 h the culture was split, and cdc13+ expression was switched OFF by thiamine addition in one of the two cultures, with cdc13+ expression being maintained in the other culture control. The cells were shifted to 25°C and, in the control (Figure 1B), Cdc13 persisted until cells entered anaphase (Yamano et al., 1998), was then partially degraded, and increased again as cells proceeded through the next cell cycle. In contrast, in the presence of thiamine (promoter OFF), Cdc13 was not detected after anaphase (Figure 1C). DNA content analysed by fluorescence-activated cell sorting (FACS; Figure 1A) showed that S-phase occurred with similar kinetics in the presence or absence of Cdc13, confirming that Cdc13 has only a minor role in the timing of S-phase (Fisher and Nurse, 1996). Cdc2-Tyr15 phosphorylation was clearly detectable during S-phase when cdc13+ was expressed, but was not detectable when Cdc13 was absent (Figure 1B and C). This result establishes that Cdc2 becomes Tyr15 phosphorylated during S-phase and that this appears to be mainly due to the presence of Cdc13/Cdc2 complexes.
Fig. 1. Cdc13-independent Cdc2 Tyr15 phosphorylation in S-phase. A cdc25-22 cdc13Δ pREP85 nmt::cdc13+ integrated (Z636) strain was synchronized in late G2 by a 4 h incubation at 36°C. Thiamine was added (cdc13 OFF) or not (cdc13 ON) during the last hour at 36°C. Cells were then released at 25°C with (cdc13 OFF + HU) or without the addition of HU. (A) DNA content analysis by FACS. Entry into S-phase was visualized by the appearance of a 4C peak. (B–D) Western blots of nmt::cdc13 ON (B), nmt::cdc13 OFF + HU (D) and nmt::cdc13 OFF (C) on boiled crude extracts [Cig2 profile is the same in (B) and (C); not shown]. AS, asynchronous.
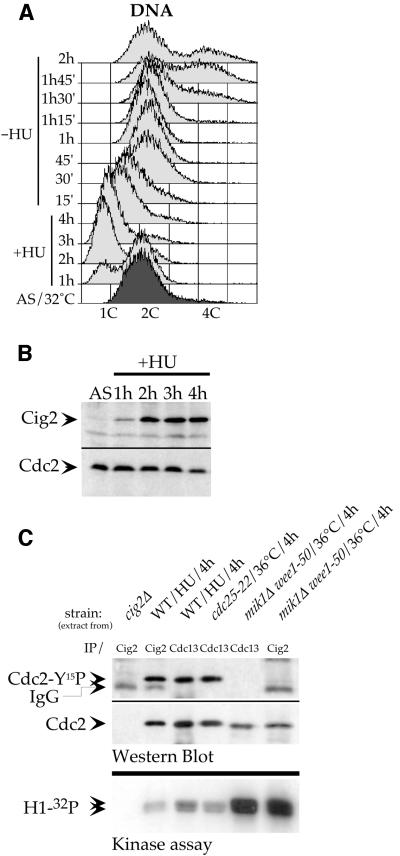
To investigate whether some Cdc2-Tyr15 phosphorylation can occur on Cig2/Cdc2 complexes in other situations known to invoke this regulation, we tested the effect of blocked DNA replication. HU treatment causes wild-type cells to stop in the first steps of DNA replication with an apparent DNA content of 1C as observed by FACS analysis (Figure 2A). In this situation we observed that Cig2 protein accumulates to a high level as cells become blocked in S-phase (Figure 2B). We confirmed that this accumulation occurred in the absence of Cdc13 cyclin when we added HU after release from the cdc25-22 block (Figure 1D) in cells lacking Cdc13 (promoter OFF). In this last situation, Cdc2-Tyr15 phosphorylation could easily be detected in these HU-blocked cells despite the absence of Cdc13, showing that Cdc2/cyclin complex(es) other than Cdc2/Cdc13 are subjected to Tyr15 phosphorylation. These results suggest that Cig2/Cdc2 complexes can become Tyr15 phosphorylated (Figure 1D). To confirm this possibility we carried out immunoprecipitations from wild-type cell extracts prepared from S-phase-blocked (HU-treated) cells. Figure 2C shows that the Cdc2 protein co-immunoprecipitated with Cig2 became Tyr15 phosphorylated during the S-phase block. We conclude that Cdc2 becomes phosphorylated in Cig2/Cdc2 complexes in cells blocked by HU in S-phase. Previous work had shown that in this situation, where the DNA replication checkpoint pathway is activated, the complete pool of Cdc13/Cdc2 complexes is inhibited by Tyr15 phosphorylation in order to prevent entry into mitosis (Rhind and Russell, 1998). Furthermore, these Cdc13/Cdc2 complexes are also entirely Tyr15 phosphorylated in cells arrested in late G2 phase when the Cdc25 phosphatase is inactivated (Hayles and Nurse, 1995). We therefore took advantage of these two results to estimate the proportion of the Tyr15 phosphorylated form in the total pool of Cig2/Cdc2 complexes in HU-blocked cells. Immunoprecipitations of Cdc13 and Cig2 were performed from wild-type cells blocked in S-phase by a 4 h HU treatment. Cdc13 was also immunoprecipitated from cdc25-22 mutant cells blocked in late G2 by a 4 h incubation at 36°C. Western blot analysis (Figure 2C) shows that for a similar amount of Cdc2 protein co-immunoprecipitated with each cyclin, the corresponding level of Tyr15 phosphorylation is also very similar for each cyclin/Cdc2 complex. This result establishes that in HU-arrested cells, both Cig2/Cdc2 and Cdc13/Cdc2 complexes are Tyr15 phosphorylated to the same extent. We conclude that most, if not all, of the Cig2/Cdc2 complexes are Tyr15 phosphorylated in HU-arrested cells.
Fig. 2. Cig2/Cdc2 complexes are Tyr15 phosphorylated and inhibited in HU-arrested cells. Wild-type (972) cells were synchronized by 4 h of 12 mM HU treatment and released. (A) DNA content analysis by FACS. (B) Cig2 accumulation in HU block (western blots on boiled crude extracts). AS, asynchronous. (C) Cig2/Cdc2 and Cdc13/Cdc2 complexes were immunoprecipitated from the strains shown and treated as indicated. Upper panel: western blot analysis of immunoprecipitated complexes (the same membrane was first probed with anti-Cdc2-Tyr15P polyclonal antibodies and reprobed with monoclonal anti-Cdc2 antibodies). Lower panel: histone H1 kinase assays (autoradiography) with the same immunoprecipitated complexes. cig2+-deleted strain (cig2Δ) = Z180, cdc25-22ts strain = Z335, mik1Δwee1-50 strain = Z484.
We next asked whether this Tyr15 phosphorylation on Cig2/Cdc2 complexes had the same inhibitory effect on kinase activity as it had on Cdc13/Cdc2 complexes. Figure 2C shows histone H1 kinase assays performed with either Cig2/Cdc2 or Cdc13/Cdc2 Tyr15-phosphorylated immunoprecipitated complexes isolated as described above, or with the same Tyr15-dephosphorylated complexes isolated from cells in which all Cdc2-Tyr15 kinases (Mik1 and Wee1) had been inactivated. These results show that the same level of inhibition of the H1 kinase activity is observed in both complexes (Cig2/Cdc2 and Cdc13/Cdc2) when Cdc2 is Tyr15 phosphorylated. We conclude that when DNA replication is inhibited, Cig2/Cdc2 complexes accumulate to a high level in a Tyr15-phosphorylated form, which inhibits their corresponding kinase activity.
Significance of Cig2/Cdc2-Tyr15 phosphorylation in S-phase-arrested cells
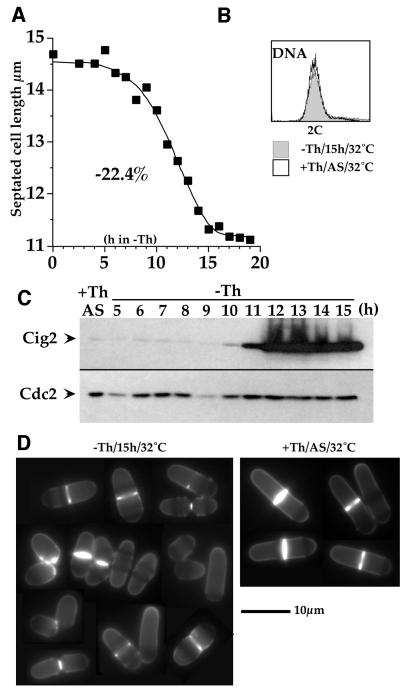
We have shown that Cig2/Cdc2 accumulates to a high level and becomes inhibited by Tyr15 phosphorylation when cells are arrested in S-phase. What is the significance of this phosphorylation during the cell cycle arrest? The fact that phosphorylation is total during S-phase arrest suggests that checkpoint activation may be involved. We therefore tested the possibility that the accumulation of phosphorylated Cdc2-Tyr15 in Cig2 complexes during S-phase arrest could have some inhibitory effect upon mitosis (as shown for Cdc13/Cdc2 complexes). In exponentially growing cells, Cig2 is normally present only during late G1 and S-phases, quickly disappearing in early G2 phase (Yamano et al., 2000). Constitutive cig2+ expression (even to a very high level) does not suppress the thermosensitivity of a cdc13ts mutant (data not shown), showing that Cig2 cannot substitute for Cdc13 to induce mitosis. To look for a possible indirect promoting effect of Cig2 on mitosis, we first analysed the consequences of producing Cig2 in G2 in exponentially growing wild-type cells by placing cig2+ under the control of the nmt1 promoter (Z588). We looked for a possible promoting effect by measuring the size of cells undergoing division (septated cells), given that advanced mitosis results in short septated cells in fission yeast. Figure 3 shows that when Cig2 accumulated, septated cell length dropped by nearly one-quarter (22.4%) (Figure 3A) and no G1 peak was observed in the DNA content analysis of these cells (FACS profile; Figure 3B), showing that there was no S-phase delay in these short cells. At later time points, cell viability dropped to 60%, and some cells began to swell while others became elongated. These experiments demonstrate that ectopic Cig2 accumulation during G2 advances mitotic onset, and thus strengthens the idea that the Cdc2/Cig2 protein kinase activity must be kept at a low level during checkpoint arrest to prevent mitotic onset.
Fig. 3. Cig2 overproduction accelerates entry into mitosis. A wild-type strain containing an integrated Rep5 nmt1::cig2+ plasmid (Z588) was used. Thiamine was removed from the medium at t0. Cells were followed for 15 h at 32°C. (A) Length of septated cells during Cig2 overproduction. (B) DNA content analysis (FACS) of cells overproducing Cig2 (-Th/15h/32°C) compared with same strain with the nmt1 promoter switched OFF (+Th/AS/32°C). (C) Western blot of Cig2 accumulation when nmt1::cig2+ is switched ON. (D) Calcofluor staining of cells overproducing Cig2 (-Th/15h/32°C) compared with the same strain in +thiamine medium (nmt1 promoter OFF) (+Th/AS/32°C).
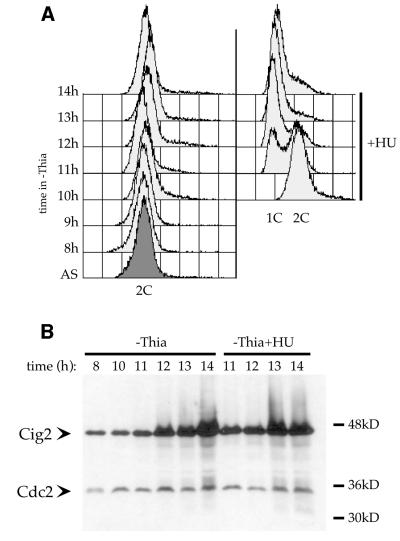
Given that Cig2 can stimulate mitotic onset in cycling cells, we asked what would be the effect of Cig2 accumulation in HU-arrested cells (where Cig2/Cdc2 complexes are entirely Tyr15 phosphorylated): would this accumulation be sufficient to drive the cells into mitosis and therefore overcome the checkpoint arrest or would the checkpoint arrest resist the mitotic promoting effect of Cig2 accumulation? To test this, we used the strain described above (Z588), where cig2+ expression is controlled by the nmt1 promoter. Ten hours after thiamine removal, the culture was split and HU was added to one half of it. Figure 4B shows that Cig2 starts to accumulate 11 h after thiamine depletion in both cultures and reaches maximal level by 14 h. DNA content analysis (FACS; Figure 4A) shows that despite the very high level of Cig2 protein, cells were normally blocked in early S-phase upon HU treatment. Microscopic analysis (DAPI and calcofluor staining) confirmed that no sign of mitotic induction was detectable in these cells. These results establish that despite its mitotic promoting effect when overexpressed in cycling cells, in a situation where Cdc2/Cig2 activity is inhibited by complete Tyr15 phosphorylation, even high levels of Cig2 protein are not able to overcome the HU-induced checkpoint arrest.
Fig. 4. Cig2 overproduction in HU-arrested cells. A wild-type strain containing an integrated Rep5 nmt1::cig2+ plasmid (Z588) was used. Thiamine was removed from the medium at t0, and HU (12 mM) was added at t = 10 h to one-half of the cells. (A) S-phase arrest was followed by DNA content analysis (FACS). (B) Western blot analysis showing Cig2 accumulation in both blocked and cycling cells. Cdc2 was used as loading control.
These experiments indicate that inhibition of Cig2/Cdc2 complexes must be maintained during the HU-induced checkpoint arrest in order to restrain their mitotic promoting ability.
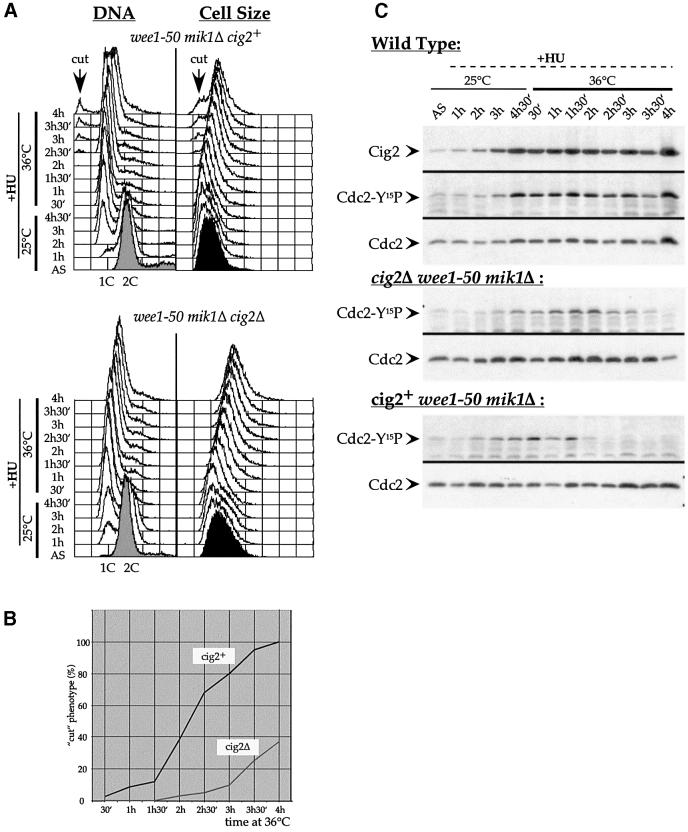
To test this model and to determine the effect of Tyr15 phosphorylation of this complex on the checkpoint control, we constructed a strain that lacked Mik1 and was conditional for Wee1, the protein kinases responsible for Tyr15 phosphorylation-dependent Cdc2 inhibition during HU treatment (Rhind and Russell, 1998). At 36°C, a mik1Δ wee1-50 (thermosensitive for wee1) double mutant blocked in S-phase with HU entered mitosis to generate a lethal ‘cut phenotype’ because the inhibitory protein kinases were lacking (Rhind and Russell, 1998; Figure 5A and B). This experiment was repeated using a wee1-50 mik1Δ cig2Δ triple mutant blocked in S-phase by HU treatment at 25°C (Figure 5A) and shifted to 36°C. Figure 5B shows that the cut phenotype appeared 2 h earlier in the cig2+ strain compared with the cig2Δ strain. Thus, in an S-phase block, the presence of Cig2 advances cells into mitosis if Cdc2 cannot be phosphorylated on Tyr15. Figure 5C shows that Cdc2-Tyr15 phosphorylation disappears between 2 and 2.5 h at 36°C in the cig2+ strain, whereas it persists for at least 1 h longer in the cig2Δ strain. This result shows that the presence of Cdc2/Cig2 complexes has a strong effect on the kinetics of Cdc2-Tyr15 global dephosphorylation. We conclude that Tyr15 phosphorylation on Cig2/Cdc2 complexes plays a role in delaying mitotic onset in S-phase-blocked cells, and that this is achieved at least in part through the maintenance of Tyr15 phosphorylation on Cdc13/Cdc2 complexes.
Fig. 5. Cig2 accelerates mitotic induction in HU-blocked cells. wee1-50 mik1Δ (Z484) and wee1-50 mik1Δ cig2Δ (Z463) strains were synchronized in S-phase by incubation in 12 mM HU for 4.5 h at 25°C. A second dose of HU (12 mM) was added while cultures were shifted to 36°C to inactivate Wee1-50. (A) FACS analysis (DNA, left panel) and forward scatter (Cell Size, right panel). The arrows indicate the appearance of a <1C DNA content peak and the appearance of short cells: two characteristics of a cut phenotype. (B) Quantification of the appearance of the cut phenotype in both strains. Cells were stained with calcofluor. The percentage of septated cells was determined every 30 min from the 36°C shift up. (C) Western blot on crude boiled extracts: Cdc2-Tyr15 phosphorylation was followed in wild-type (972), cig2+wee1-50 mik1Δ (Z484) and cig2Δ wee1-50 mik1Δ (Z463) strains during S-phase arrest and wee1-50 inactivation. Cdc2 was used as loading control.
Discussion
The aim of this work was to investigate the role of Cdc2-Tyr15 phosphorylation at the G1/S transition. We focused our study on the regulation of Cig2/Cdc2 complexes, which control the initiation of S-phase in fission yeast. Previously, it has been shown that when DNA replication is blocked by HU treatment, Cdc13/Cdc2 complexes are kept inactive by Cdc2-Tyr15 phosphorylation inhibiting entry into mitosis (Rhind and Russell, 1998). In HU-treated cells lacking Cdc13, Cdc2 still becomes Tyr15 phosphorylated (Figure 1A and D), showing that forms of Cyclin/Cdc2 complexes other than Cdc13/Cdc2 are subject to Tyr15 phosphorylation. In this situation, Cig2 accumulates to a high level (Figure 1D), and immunoprecipitation experiments show that Cig2/Cdc2 complexes become completely phosphorylated (Figure 2C). Kinase assays demonstrate that Tyr15 phosphorylation strongly inhibits the kinase activity of these complexes, establishing that in HU-blocked cells, both Cig2/Cdc2 and Cdc13/Cdc2 complexes become inhibited by Tyr15 phosphorylation to a similar extent (Figure 6).
Fig. 6. Cig2/Cdc2 complexes are inhibited in DNA-replication-arrested cells to restrain mitotic induction.
Our results have established that ectopic (G2) expression of the cig2+ gene can lead to advanced mitotic entry (Figure 3), and that cig2+ overexpression cannot overcome the HU-imposed checkpoint block over mitosis. Also, the prevention of Cdc2-Tyr15 inhibitory phosphorylation in HU-blocked cells led to a faster onset of mitosis in a cig2+ strain compared with an isogenic cig2 deleted strain. We conclude that the Cig2/Cdc2 complexes have an activating effect on mitosis, and inhibition of this CDK complex is required when replication is blocked to fully maintain the checkpoint block over mitosis (Figure 6).
It is not clear how the Cig2/Cdc2 complexes induce onset of mitosis. Even when overexpressed, Cig2 cannot bring about onset and completion of mitosis in the absence of Cdc13 (Obara-Ishihara and Okayama, 1994; data not shown). When Cig2 is absent, Cdc2-Tyr15 phosphorylation of Cdc13 complexes persists at a high level for a longer time (Figure 5C), suggesting that Cig2/Cdc2 mediates its effects, at least in part, through Cdc13/Cdc2 by regulating the kinetics of its Tyr15 dephosphorylation. Without Cig2, the dephosphorylation of Cdc13/Cdc2 complexes is delayed and there is also a delay of mitotic onset. Therefore Cig2/Cdc2 may act as a starter protein acting upstream of Cdc25 activation of Cdc13/Cdc2, analogous to the role proposed for the Cdk2 CDK in mammalian cells (Guadagno and Newport, 1996).
We conclude that Tyr15 phosphorylation of the G1/S CDK Cig2/Cdc2 plays a role in the S-phase checkpoint control, which restrains mitotic onset when DNA replication is incomplete. This was unexpected but is important because ectopic activation of Cig2/Cdc2 can bring about onset of mitosis, acting in part through the G2/M CDK Cdc13/Cdc2. Tyr15 phosphorylation of the Cdk2 has also been observed in mammalian cells after DNA damage (Bartek and Lukas, 2001), suggesting that phosphorylation of G1/S CDKs may be a more general feature of checkpoint controls in higher eukaryotes. However, the requirement of this phosphorylation to maintain a proper G2/M checkpoint arrest has not been reported. In the light of these results it will be interesting to investigate the role of G1/S-phase CDK inhibition by Tyr15 phosphorylation (mainly Cdk2) in metazoan eukaryotes and the implication of this inhibition in maintaining the mitotic checkpoint arrest.
Materials and methods
Yeast strains and media
Growth and genetic manipulation of S.pombe were performed using the standard methods described previously (http://www.bio.uva.nl/pombe/handbook). Cells were grown routinely at 25°C (permissive conditions for thermosensitive mutants) or 36°C (restrictive conditions) in minimal EMM2 medium with or without the addition of 5 µg/ml thiamine to repress/activate the nmt1 promoter. Strains used in this study were: 972, wild-type h–; Z636, cdc25-22 pREP85::cdc13+ (Sup3-5) int. cdc13::ura4+ ura4-D18 leu1-32 ade6-704; Z588, pRep5::cig2+ (Sup3-5) int ade6-704; Z484, wee1-50 mik1::LEU2 leu1– h–; Z463, wee1-50 mik1::LEU2 cig2::ura4+ ura4D-18 leu1-32; Z180, cig2+::ura4+ ura4-D18 h–; Z335, cdc25-22 h–. pREP85 contains the lowest expression version of the nmt1 promoter, pREP45 and pREP41 contain the medium expression version of the nmt1 promoter, and pREP5 contains the strongest (wild-type) version of the nmt1 promoter.
Western blotting and immunoprecipitation assays
The techniques used have been described previously (http://www.bio.uva.nl/pombe/handbook). For immunoprecipitation assays, 2 mg of cell extract proteins were incubated with anti-Cig2 polyclonal serum MOC8 (M.O’Connell and J.Correa-Bordes, unpublished data; Stern and Nurse, 1997) (15 µl/IP of affinity-purified antibody) precipitated with 10 µl of protein A–Sepharose or 0.5 mg of cell extract proteins were incubated with anti-Cdc13 monoclonal serum 6F11 (5 µl/IP) precipitated with 10 µl of protein G–Sepharose. Proteins were separated on a 10% SDS–polyacrylamide gel (Laemmli, 1970) and blotted onto Immobilon™-P membrane (Millipore). For western blot analysis, the antibodies (Abs) used were anti-Cdc13 monoclonal Ab 6F11 (1:500; J.Hayles and G.Steel, unpublished data), anti-Cig2 monoclonal Ab 5E3/1+3A11/7 (1:500+1:500; H.Yamano, unpublished data), anti-Cdc2 monoclonal Ab Y63 (1:1000; Yamano et al., 1996) and polyclonal anti-Cdc2 (Tyr15 phosphorylated, 1:1000; New England Biolabs; 9111L), which specifically detects Cdc2 and Cdk2 when catalytically inactivated by phosphorylation at Tyr15. Immunoreactive bands were detected using enhanced chemiluminescence (ECL; Amersham).
Kinase assays
H1 kinase assays were performed with immunoprecipitated Cdc2/cyclin (Cig2 or Cdc13) complexes (as described above using conditions where the same amount of Cdc2 protein was co-immunoprecipitated with each cyclin). Immunoprecipitations were divided in two; one half was used for western blot analysis (Cdc2 quantitation) and the second half was used for H1 kinase assays in the following conditions: 20 µl of KIN buffer [1 mg/ml H1 histone (Boehringer), 200 µM [γ-32P]ATP (100 c.p.m./pmol = 40 µCi/ml), 1 mM ATP in HB buffer] were added to the beads and incubated for 20 min at 30°C.
FACS analysis
DNA content was analysed as described previously (http://www. bio.uva.nl/pombe/handbook/section3/section3-4.html) using a Becton Dickinson FACScan.
Cell length measurement
The length of septated cells was determined as described previously (http://www.bio.uva.nl/pombe/handbook/section3/section3-1.html). Two hundred cells were measured for each point.
Acknowledgments
Acknowledgements
We would like to thank all members of the Cancer Research UK London Cell Cycle Laboratory and members of the Curie Institute ‘Génotoxicité et Cycle Cellulaire’ Laboratory for their help and support. P.Z. was supported by Cancer Research UK and the Association pour la Recherche contre le Cancer fellowships, and A.D. was supported by an EC Biotechnology Training Grant.
References
- Bartek J. and Lukas,J. (2001) Pathways governing G1/S transition and their response to DNA damage. FEBS Lett., 490, 117–122. [DOI] [PubMed] [Google Scholar]
- Blomberg I. and Hoffmann,I. (1999) Ectopic expression of Cdc25A accelerates the G1/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol. Cell. Biol., 19, 6183–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R.N., Alfa,C.E., Hyams,J.S. and Beach,D.H. (1989) The fission yeast cdc2/cdc13/suc1 protein kinase: regulation of catalytic activity and nuclear localization. Cell, 58, 485–497. [DOI] [PubMed] [Google Scholar]
- Correa-Bordes J., Gulli,M.P. and Nurse,P. (1995) p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell, 83, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Correa-Bordes J., Gulli,M.P. and Nurse,P. (1997) p25rum1 promotes proteolysis of the mitotic B-cyclin p56cdc13 during G1 of the fission yeast cell cycle. EMBO J., 16, 4657–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creanor J. and Mitchison,J.M. (1996) The kinetics of the B cyclin p56cdc13 and the phosphatase p80cdc25 during the cell cycle of the fission yeast Schizosaccharomyces pombe. J. Cell Sci., 109, 1647–1653. [DOI] [PubMed] [Google Scholar]
- Falck J., Mailand,N., Syljuasen,R.G., Bartek,J. and Lukas,J. (2001) The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature, 410, 842–847. [DOI] [PubMed] [Google Scholar]
- Fisher D.L. and Nurse,P. (1996) A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J., 15, 850–860. [PMC free article] [PubMed] [Google Scholar]
- Francesconi S., Grenon,M., Bouvier,D. and Baldacci,G. (1997) p56(chk1) protein kinase is required for the DNA replication checkpoint at 37°C in fission yeast. EMBO J., 16, 1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaktionov K., Lee,A.K., Eckstein,J., Draetta,G., Meckler,J., Loda,M. and Beach,D. (1995) CDC25 phosphatases as potential human oncogenes. Science, 269, 1575–1577. [DOI] [PubMed] [Google Scholar]
- Gould K.L. and Nurse,P. (1989) Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis [see comments]. Nature, 342, 39–45. [DOI] [PubMed] [Google Scholar]
- Gould K.L., Moreno,S., Owen,D.J., Sazer,S. and Nurse,P. (1991) Phos phorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J., 10, 3297–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno T.M. and Newport,J.W. (1996) Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2-cyclin B kinase activity. Cell, 84, 73–82. [DOI] [PubMed] [Google Scholar]
- Hayles J. and Nurse,P. (1995) A pre-start checkpoint preventing mitosis in fission yeast acts independently of p34cdc2 tyrosine phosphorylation. EMBO J., 14, 2760–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavarone A. and Massagué,J. (1997) Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-β in cells lacking the CDK inhibitor p15. Nature, 387, 417–422. [DOI] [PubMed] [Google Scholar]
- Jinno S., Hung,S.C. and Okayama,H. (1999) Cell cycle start from quiescence controlled by tyrosine phosphorylation of Cdk4. Oncogene, 18, 565–571. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Mailand N., Falck,J., Lukas,C., Syljuåsen,R.G., Welcker,M., Bartek,J. and Lukas,J. (2000) Rapid destruction of human Cdc25A in response to DNA damage. Science, 288, 1425–1429. [DOI] [PubMed] [Google Scholar]
- Martin-Castellanos C., Labib,K. and Moreno,S. (1996) B-type cyclins regulate G1 progression in fission yeast in opposition to the p25rum1 cdk inhibitor. EMBO J., 15, 839–849. [PMC free article] [PubMed] [Google Scholar]
- Mondesert O., McGowan,C.H. and Russell,P. (1996) Cig2, a B-type cyclin, promotes the onset of S in Schizosaccharomyces pombe. Mol. Cell. Biol., 16, 1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S. and Nurse,P. (1994) Regulation of progression through the G1 phase of the cell cycle by the rum1+ gene [see comments]. Nature, 367, 236–242. [DOI] [PubMed] [Google Scholar]
- Moser B.A. and Russell,P. (2000) Cell cycle regulation in Schizosaccharomyces pombe. Curr. Opin. Microbiol., 3, 631–636. [DOI] [PubMed] [Google Scholar]
- Norbury C. and Nurse,P. (1992) Animal cell cycles and their control. Annu. Rev. Biochem., 61, 441–470. [DOI] [PubMed] [Google Scholar]
- Nurse P. (1990) Universal control mechanism regulating onset of M-phase. Nature, 344, 503–508. [DOI] [PubMed] [Google Scholar]
- Obara-Ishihara T. and Okayama,H. (1994) A B-type cyclin negatively regulates conjugation via interacting with cell cycle ‘start’ genes in fission yeast. EMBO J., 13, 1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N. and Russell,P. (1998) Tyrosine phosphorylation of cdc2 is required for the replication checkpoint in Schizosaccharomyces pombe. Mol. Cell. Biol., 18, 3782–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N., Furnari,B. and Russell,P. (1997) Cdc2 tyrosine phosphoryla tion is required for the DNA damage checkpoint in fission yeast. Genes Dev., 11, 504–511. [DOI] [PubMed] [Google Scholar]
- Stern B. and Nurse,P. (1996) A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet., 12, 345–350. [PubMed] [Google Scholar]
- Stern B. and Nurse,P. (1997) Fission yeast pheromone blocks S-phase by inhibiting the G1 cyclin B-p34cdc2 kinase. EMBO J., 16, 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y., Tatsuka,M., Jinno,S. and Okayama,H. (1995) Requirement for tyrosine phosphorylation of Cdk4 in G1 arrest induced by ultraviolet irradiation. Nature, 376, 358–362. [DOI] [PubMed] [Google Scholar]
- Yamano H., Gannon,J. and Hunt,T. (1996) The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J., 15, 5268–5279. [PMC free article] [PubMed] [Google Scholar]
- Yamano H., Tsurumi,C., Gannon,J. and Hunt,T. (1998) The role of the destruction box and its neighbouring lysine residues in cyclin B for anaphase ubiquitin-dependent proteolysis in fission yeast: defining the D-box receptor. EMBO J., 17, 5670–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H., Kitamura,K., Kominami,K., Lehmann,A., Katayama,S., Hunt,T. and Toda,T. (2000) The spike of S phase cyclin Cig2 expression at the G1–S border in fission yeast requires both APC and SCF ubiquitin ligases. Mol. Cell, 6, 1377–1387. [DOI] [PubMed] [Google Scholar]