Abstract
Signal transducer and activator of transcription (STAT) proteins are cytoplasmic transcription factors that translocate to the nucleus and regulate gene expression upon activation of cytokine or growth factor receptors. While this translocation event is essential for gene regulation by STATs, their mechanism of transport through the cytoplasm to the nucleus has remained elusive. We now report that cytoplasmic transport of Stat3 is an active process that requires receptor-mediated endocytosis. Stat3 co-localizes with endocytic vesicles in transit from the cell membrane to the perinuclear region in response to growth factor stimulation. Consistent with a role for receptor endocytosis in growth factor signaling, disruption of endocytosis with specific inhibitors blocks Stat3 nuclear translocation and Stat3-dependent gene regulation. These results indicate that receptor-mediated endocytosis may be a general mechanism of transport through the cytoplasm for a subset of cytoplasmic signaling proteins destined for the nucleus.
Keywords: endocytosis/growth factor/nuclear translocation/receptor/Stat3
Introduction
STATs (signal transducers and activators of transcription) are transcription factors that regulate genetic programs controlling development, differentiation, proliferation and apoptosis (Schindler and Darnell, 1995; Darnell, 1997). Stat3 is a STAT family member that can be activated by diverse cytokines, growth factors or oncoproteins, and has a critical role in cell growth and survival (Bowman et al., 2000). Upon stimulation of cell surface receptors, Stat3 protein is recruited to activated receptors through an interaction between the Stat3 SH2 domain and phosphotyrosine docking sites on the receptors (Darnell, 1997). Subsequent tyrosine phosphorylation of Stat3 occurs directly via the receptor kinase itself or indirectly by activation of intermediary kinases, including members of the Janus kinase (JAK) family, thereby inducing Stat3 dimerization (Darnell, 1997; Bowman et al., 2000). Following dimerization, Stat3 translocates to the nucleus and binds to specific DNA sequences in the promoters of genes and induces their expression through interactions with other transcriptional regulatory components (Darnell, 1997). The cellular mechanism by which STAT family proteins translocate to the nucleus following their activation in the cytoplasm has remained unclear since their discovery.
Evidence for an active role of endocytosis in signal transduction pathways has been accumulating (Ceresa and Schmid, 2000; Leof, 2000; McPherson et al., 2001). For example, protein phosphorylation and signaling during growth factor receptor endocytosis occurs following association of epidermal growth factor (EGF) receptor, mSos, Ras and Raf-1 on endosomes (Di Guglielmo et al., 1994). Conversely, activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase (Erk) is attenuated when receptor endocytosis is inhibited (Vieira et al., 1996; Ceresa and Schmid, 2000). To determine the mechanism by which Stat3 translocates through the cytoplasm to the nucleus, we investigated the hypothesis that Stat3 is actively targeted to the nucleus by transport on vesicles derived from endocytosis.
Receptor-mediated endocytosis allows the specific removal of cell surface receptors and their associated proteins from the plasma membrane and accumulates them in endosomes, where they are sorted for downregulation or recycling (Robinson et al., 1996; Takei et al., 1999; Slepnev et al., 2000). This process is initiated upon ligand binding by recruitment of the receptor complex into a clathrin-coated pit at the plasma membrane, a structure formed by assembly of clathrin and clathrin adaptor protein 2 (AP-2) into a protein lattice on the membrane’s cytosolic face (Robinson et al., 1996). Clathrin and AP-2 bind to multiple components of the endocytic complex such as amphiphysin and epsin (Di Fiore and Gill, 1999), which in turn bind to additional proteins that modulate formation and function of clathrin-coated pits (Di Fiore and Gill, 1999). Expression of a fragment of amphiphysin 1 (Amph A1) leads to mislocalization of AP-2 and clathrin, with a resulting block in clathrin-mediated endocytosis (Slepnev et al., 2000). Overexpression of full-length Epsin 2a, another protein important for receptor-mediated endocytosis, mislocalizes endocytic complexes and also inhibits clathrin-mediated endocytosis (Chen et al., 1998).
Using various specific inhibitors of endocytosis, we examined the role of receptor-mediated endocytosis in Stat3 activation and function. We demonstrate that receptor endocytosis is essential for Stat3 nuclear translocation and Stat3-dependent gene regulation. Accord ingly, Stat3 co-localizes with receptor–ligand complexes in endocytic vesicles targeted to the perinuclear region from the cell membrane following growth factor stimulation. Collectively, our findings provide evidence that endocytosis is the transport machinery for Stat3 translocation through the cytoplasm to the nucleus.
Results
Stat3 translocation into the nucleus following growth factor stimulation requires functional endocytosis
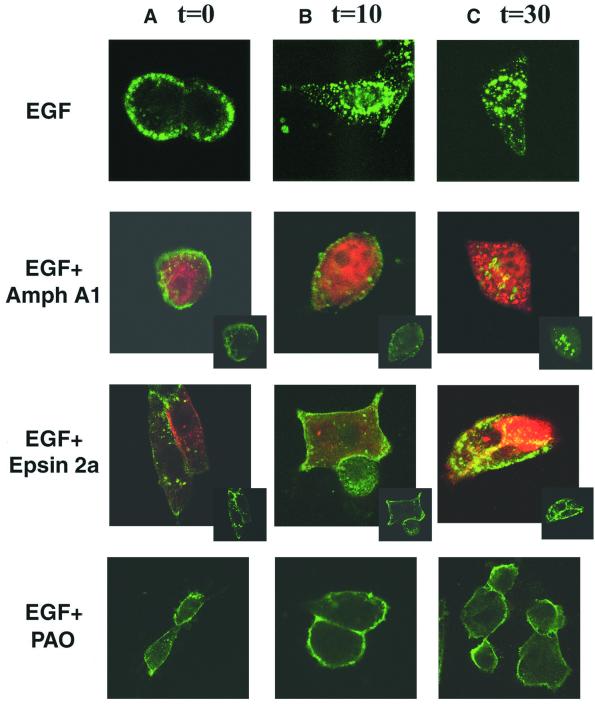
Previous studies showed that overexpression of either the Amph A1 fragment or Epsin 2a protein results in mis localization of the endocytic components and a subsequent block in clathrin-mediated endocytosis (Chen et al., 1998; Slepnev et al., 2000). Specifically, overexpression of the Amph A1 fragment disrupts the co-localization of AP-2 and clathrin from the typical punctate endosomal structures, causing AP-2 to form large aggregates and clathrin to redistribute into a diffuse pattern (Slepnev et al., 2000). Accordingly, overexpression of Epsin 2a causes a striking collapse of the clathrin coat, and a redistribution of AP-2 that results in a block in transferrin internalization (Chen et al., 1998). To further define the role of Amph A1 and Epsin 2a overexpression in blocking growth factor receptor-mediated endocytosis, NIH-3T3 cells overexpressing EGF receptor (NIH-3T3/EGFR cells) were analyzed for EGF receptor–ligand internalization in the presence of these inhibitory agents. Confocal microscopy experiments with a fluorescent EGF conjugate (Alexa Fluor EGF, Molecular Probes) were performed to detect the localization of EGF receptor–ligand complexes in NIH-3T3/EGFR cells, and overexpression of the endocytic inhibitors Amph A1 and Epsin 2a was detected using antibodies to a hemagglutinin (HA) tag (Figure 1).
Fig. 1. Disruption of the endocytic machinery blocks EGFR internalization. Immunofluorescence analysis of NIH-3T3/EGFR cells was performed after transfection of expression vectors encoding HA-tagged Amph A1 or Epsin 2a, or treatment with 5 µM PAO. Antibodies to the HA tag of Amph A1 and Epsin 2a were used to detect localization of these proteins (red). Control (EGF alone) or treated cells (transfected or PAO treated) were incubated with 2 µg/ml Alexa Fluor EGF (green) for 45 min at 4°C to recruit ligand-bound receptor at the cell surface (A, time 0), and then warmed to 37°C for 10 min (B) and 30 min (C) to enable endocytosis. Localization of the EGF receptor–ligand complexes was analyzed using the LSM 510 program on a Zeiss confocal microscope. Insets show green fluorescence alone.
NIH-3T3/EGFR cells were treated with EGF for 45 min at 4°C, which allows accumulation of EGF-bound re ceptors at the plasma membrane, but is a non-permissive temperature for endocytosis. Cells were then shifted to 37°C for the times indicated, to enable endocytosis. EGF receptor–ligand complexes localize at the plasma membrane following incubation at 4°C with EGF (Figure 1A, EGF alone), consistent with targeting of the receptor tyrosine kinase to forming clathrin-coated vesicles upon growth factor binding to its receptor. After a 10 min treatment with growth factor at 37°C, EGF receptor– ligand complexes localize with endocytic vesicles in the cytosol of control cells (Figure 1B, EGF alone). Subsequently, EGFR localized primarily to endocytic vesicles in the perinuclear region after a 30 min treatment with EGF at 37°C (Figure 1C, EGF alone). In contrast, cells overexpressing Amph A1 at all time points have a more diffuse staining overall of EGF receptor–ligand complexes (Figure 1A–C, Amph A1). After a 10 min treatment with EGF in cells overexpressing Amph A1, the receptor–ligand complex localizes predominately at the cell membrane, with some aggregates forming in the cytosol (Figure 1B, Amph A1). Following a 30 min EGF treatment, the majority of EGF receptor–ligand complexes localize to aggregates in the cytosol, similarly to the AP-2 aggregates which were seen to form upon Amph A1 overexpression (Figure 1C, Amph A1) (Slepnev et al., 2000). Further, overexpression of Epsin 2a inhibits internalization of the EGF receptor–ligand complex, causing retention of EGF receptor–ligand complexes at the plasma membrane 10 and 30 min following EGF treatment (Figure 1A–C, Epsin 2a). Treatment of cells with a pharmacological inhibitor of endocytosis, phenylarsine oxide (PAO) (Hertel et al., 1985; Tong et al., 2000), results in complete inhibition of EGF receptor–ligand internalization from the cell membrane following EGF treatment (Figure 1A–C, PAO). Collectively, these experiments show that the Amph A1, Epsin 2a and PAO endocytic inhibitors extensively disrupt and block functional growth factor receptor endocytosis.
To examine the role of receptor-mediated endocytosis in nuclear translocation of Stat3, we specifically blocked endocytosis of growth factor receptors by ectopic expression of Amph A1 or Epsin 2a, or by treatment with PAO. NIH-3T3/EGFR cells were transfected with expression vectors encoding either of these endocytic inhibitor proteins or control empty vector and then stimulated with EGF following serum starvation for 12 h. In some experiments, 5 µM PAO was added for the last one-half hour of the serum-free treatment and included in the media during the time of growth factor addition. Transfection efficiencies of 50–70% were confirmed by quantification of either Epsin 2a–GFP or control red fluorescent protein (RFP), prior to carrying out electrophoretic mobility shift assay (EMSA) or western blotting analysis (data not shown).
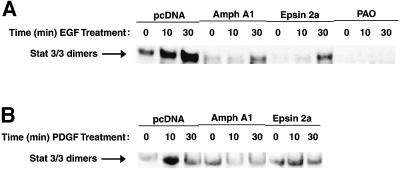
Nuclear extracts were prepared and incubated with a 32P-labeled high-affinity sis-inducible element (hSIE) oligonucleotide probe (Yu et al., 1995) and the resultant DNA–protein complexes were analyzed by EMSA for activated Stat3 dimers. Nuclear DNA-binding activity of endogenous Stat3 dimers in response to EGF treatment was greatly inhibited by overexpression of Amph A1 or Epsin 2a (Figure 2A). Further, blocking endocytosis of EGFR with PAO resulted in a complete loss of nuclear Stat3 DNA-binding activity in response to EGF. Analysis of cytoplasmic Stat3 in response to EGF shows a block in dimer formation and cytoplasmic DNA-binding activity following inhibition of endocytosis (data not shown). Functional endocytosis was also required for nuclear Stat3 DNA-binding activity following platelet-derived growth factor (PDGF) treatment of Balb/c-3T3 cells expressing endogenous levels of PDGFR (Figure 2B). The purity of the EMSA nuclear fractions was confirmed using the nuclear transcription factor, Sp1, and the cytosolic signaling protein, Raf-1, as markers (data not shown). These results demonstrate that translocation of active Stat3 to the nucleus in response to growth factor stimulation is dependent on the endocytic pathway.
Fig. 2. Nuclear Stat3 DNA-binding activity is inhibited by blocking endocytosis. (A and B) DNA-binding activity of Stat3 complexes in either EGF-treated NIH-3T3/EGFR (A) or PDGF-BB-treated Balb/c-3T3 (B). Cells were transfected with empty vector control (pcDNA) or vectors encoding the endocytosis inhibitors Amph A1 or Epsin 2a, or treated with 5 µM PAO. Nuclear extracts were prepared following treatment with growth factors for the indicated times and were then subjected to EMSA analysis to detect activated Stat3 dimers bound to a 32P-labeled hSIE probe.
Stat3 tyrosine phosphorylation is independent of nuclear translocation and receptor endocytosis
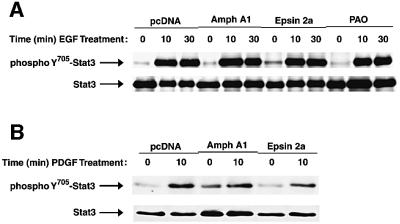
Following growth factor stimulation, EGFR autophosphorylation, Ras and Raf-1 activation and phosphorylation of Shc are not inhibited when endocytosis is blocked, suggesting that events which precede receptor endocytosis remain unaffected (Ceresa and Schmid, 2000; Tong et al., 2000; McPherson et al., 2001). Similarly, ligand-induced Stat3 phosphorylation, which could occur prior to receptor-mediated translocation, may be unaffected by inhibition of endocytosis. To further define the mechanism by which blocking endocytosis inhibits Stat3 DNA-binding activity, we examined tyrosine phosphorylation of Stat3 following EGF stimulation. NIH-3T3/EGFR cells expressing control empty vector, Amph A1, or Epsin 2a, or treated with PAO (as described above) were lysed following EGF stimulation, and Stat3 was immunoprecipitated from lysates. Western blotting was performed using an anti-phosphoY705-Stat3 antibody, which is specific for activated Stat3. Tyrosine phosphorylation of Stat3 in response to EGF was not affected by inhibition of endocytosis (Figure 3A). Additionally, inhibition of endocytosis does not block Stat3 tyrosine phosphorylation following PDGF stimulation of Balb/c-3T3 cells (Figure 3B). Together with results shown in Figure 2A and B, these findings demonstrate that growth factor receptor-mediated Stat3 tyrosine phosphorylation occurs even when receptor-mediated endocytosis is blocked. These results indicate that growth factors bind to and activate their receptors in the presence of endocytic inhibitors, thereby leading to Stat3 phosphorylation. Thus, Amph A1, Epsin 2a and PAO inhibit events subsequent to Stat3 phosphorylation, suggesting a requirement for receptor endocytosis in Stat3 nuclear translocation, and also that Stat3 tyrosine phosphorylation alone is not sufficient for nuclear translocation.
Fig. 3. Stat3 tyrosine phosphorylation is independent of nuclear translocation and receptor endocytosis. Western blot analyses were performed using antibodies to phosphoY705-Stat3 or total Stat3 protein to probe immunoprecipitates prepared with antibodies to total Stat3 protein from lysates of either EGF-treated NIH-3T3/EGFR (A) or PDGF-BB-treated Balb/c-3T3 (B) cells. Cells were treated with the endocytosis inhibitors followed by growth factor stimulation for the indicated times.
Stat3 transcriptional activity requires receptor-mediated endocytosis
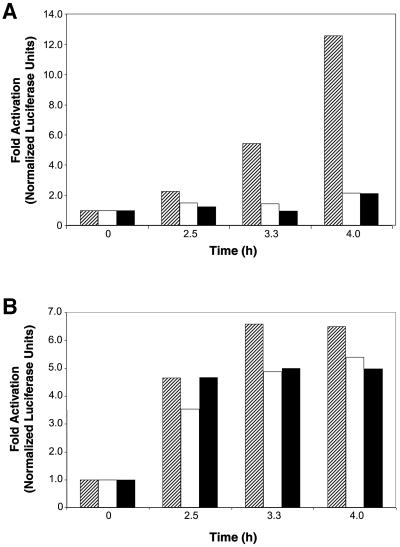
Because nuclear Stat3 DNA-binding activity was inhibited following a block in endocytosis, we investigated the role of endocytosis in Stat3-mediated gene regulation induced by growth factor stimulation. Balb/c-3T3 cells were transfected with empty vector or vectors encoding endocytic inhibitors and the Stat3-responsive reporter plasmid, pLucTKS3, which contains multiple copies of a Stat3-specific DNA-binding site driving luciferase expression (Turkson et al., 1998). As shown in Figure 4A, Stat3-mediated transcriptional activity in response to PDGF was abrogated by expression of the endocytic inhibitors, demonstrating that blocking endocytosis prevents nuclear translocation of functional Stat3 dimers. For a negative control, Balb/c-3T3 fibroblasts were transfected with vectors encoding endocytic inhibitors and a Stat3-independent luciferase reporter plasmid, pLucSRE, which is dependent on the activation of the c-fos serum response element (Cahill et al., 1996; Turkson et al., 1998; Gineitis and Treisman, 2001). Stat3-independent transcriptional activity in response to PDGF is not greatly affected by expression of endocytic inhibitors, ruling out the possibility that these endocytic inhibitors have non-specific or generalized effects on signaling pathways and gene regulation (Figure 4B). Collectively, the results shown in Figures 2 and 4 indicate a requirement of receptor-mediated endocytosis for induction of Stat3 DNA-binding and transcriptional activities in the nucleus.
Fig. 4. Disruption of endocytosis with specific inhibitors blocks Stat3-dependent gene regulation. Balb/c-3T3 cells were transfected with control empty vector DNA (hatched) or expression vectors encoding the endocytosis inhibitors Amph A1 (open) or Epsin 2a (solid), the β-galactosidase vector as an internal control for transfection efficiency, and either the Stat3-specific luciferase reporter construct pLucTKS3 (A) or the Stat3-independent reporter construct pLucSRE (B). The transfected cells were harvested 48 h post-transfection following serum starvation overnight and treatment with 50 ng/ml PDGF for the times indicated. Results are shown as fold luciferase activities normalized to the β-gal internal control and are representative of three independent experiments.
Stat3 translocates from the cell membrane to the perinuclear region in endosomes following growth factor treatment
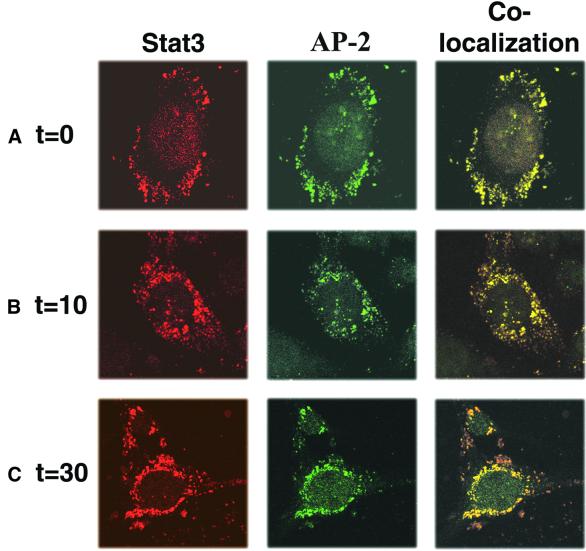
To further illustrate the contribution of growth factor receptor endocytosis in Stat3 signaling, confocal microscopy immunofluorescence studies with antibodies to either Stat3 or AP-2, a marker for endocytic vesicles, were used to examine co-localization of endogenous Stat3 with endosomes following PDGF stimulation (Figure 5A–C). NIH-3T3 cells were treated with PDGF for 45 min at 4°C, to allow accumulation of PDGF-bound receptors at the plasma membrane, and were then shifted to 37°C for the times indicated to enable endocytosis. Endogenous Stat3 localized with AP-2 at the plasma membrane following incubation at 4°C with PDGF (Figure 5A), consistent with targeting of Stat3 to forming clathrin-coated vesicles upon growth factor binding to its receptor. After a 10 min treatment with growth factor at 37°C, Stat3 co-localized with AP-2 in the cytosol, indicating that endogenous Stat3 is internalized with endocytic vesicles (Figure 5B). Subsequently, endogenous Stat3 localized to endocytic vesicles in the perinuclear region after a 30 min treatment with PDGF at 37°C (Figure 5C). Stat3 similarly co-localized with AP-2 following EGF treatment of NIH-3T3 cells (data not shown). To substantiate these findings, we used an independent method of Stat3 detection by expression of a recombinant Stat3–RFP chimera in NIH-3T3 cells. Following treatment of these cells with EGF, Stat3–RFP co-localized with AP-2 in endocytic vesicles in transit from the plasma membrane to the nucleus (data not shown). Together, these findings demonstrate that Stat3 localizes to endocytic vesicles that move sequentially in a time-dependent manner from the plasma membrane, through the cytosol and to the perinuclear region, following growth factor stimulation.
Fig. 5. Stat3 localizes sequentially to endocytic vesicles at the cell membrane (A), in the cytosol (B) or at the perinuclear region (C) following PDGF treatment. (A–C) NIH-3T3 cells were treated with 50 ng/ml PDGF for 45 min at 4°C to recruit ligand-bound receptors at the cell surface, and then warmed to 37°C for the times indicated, to enable endocytosis. Staining with rabbit anti-Stat3 (red) or mouse anti-AP-2 (green) antibodies shows Stat3 in endosomes following growth factor stimulation. Images were collected using the LSM 510 program on a Zeiss confocal microscope.
Growth factor receptor–ligand complexes facilitate transport of Stat3 from the plasma membrane to the perinuclear region
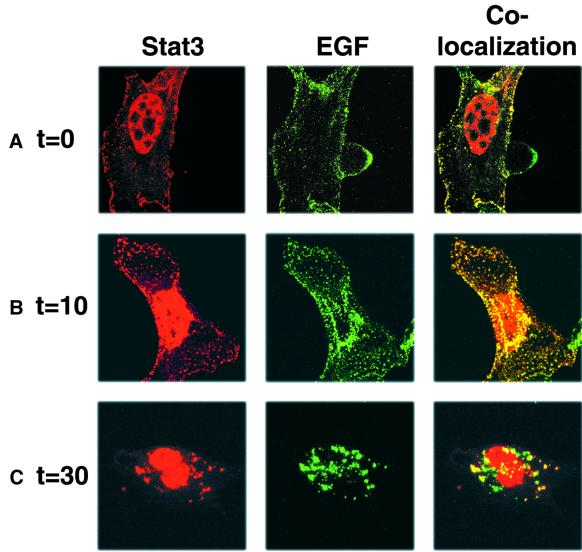
Previous studies showed direct interaction of Stat3 with EGF and PDGF receptors (Fu and Zhang, 1993; Wang et al., 2000), consistent with the possibility that growth factor receptors might mediate endocytotic transport of Stat3 through the cytoplasm. To examine the role of receptors in Stat3 translocation, confocal microscopy experiments with a fluorescent EGF conjugate (Alexa Fluor EGF) were performed to detect the localization of EGF receptor–ligand complexes in NIH-3T3/EGFR or Balb/c-3T3 cells. For these confocal microscopy experiments, Stat3 was overexpressed in order to achieve levels of brightness equivalent to the fluorescent EGF for co-localization studies. Following treatment of transfected cells with Alexa Fluor EGF, immunofluorescence studies with antibodies to Stat3 were performed to determine co-localization with EGF receptor–ligand complexes. As shown in Figure 6, Stat3 co-localizes with the EGF receptor–ligand complexes following treatment with Alexa Fluor EGF in endosomal compartments sequentially at the cell membrane at time 0 (A), in the cytoplasm at 10 min (B) and the perinuclear region at 30 min (C). Elevated Stat3 nuclear levels, which are detectable in the absence of EGF stimulation due to Stat3 overexpression, increase significantly during the 30 min time course following EGF stimulation. Similar results were obtained following co-transfection of expression vectors encoding recombinant EGFR fused to GFP and Stat3-RFP (data not shown). These results suggest that growth factor receptors serve as a scaffold for Stat3 trafficking. Altogether, our findings demonstrate that Stat3 comigrates with growth factor receptors from the plasma membrane to the perinuclear region during endocytosis.
Fig. 6. Stat3 co-localizes with Alexa Fluor EGF sequentially at the cell membrane (A), in the cytoplasm (B) or the perinuclear region (C) following growth factor stimulation. (A–C) NIH-3T3/EGFR cells overexpressing Stat3 were treated with 2 µg/ml Alexa Fluor EGF for 45 min at 4°C to recruit ligand-bound receptor at the cell surface, and then warmed to 37°C for the times indicated, to enable endocytosis. Immunofluorescent staining with rabbit anti-Stat3 antibodies was performed as in Figure 5. Images were collected using the LSM 510 program on a Zeiss confocal microscope.
Inhibition of functional receptor endocytosis blocks Stat3 trafficking from the cell membrane to the perinuclear region
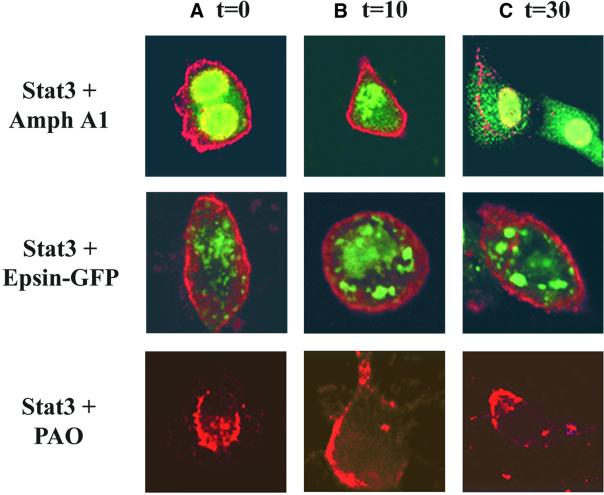
Our results above show that Stat3 localizes with endocytic vesicles and EGF receptor–ligand complexes following growth factor stimulation. To further characterize the requirement of endocytosis for Stat3 translocation, cells overexpressing Stat3 and either Amph A1 or Epsin 2a were treated with EGF and confocal microscopy immunofluorescence studies were used to determine the localization of Stat3. As shown in Figure 7, Stat3 transport through the cytoplasm following EGF treatment was blocked by overexpression of either Amph A1 or Epsin 2a or treatment with PAO at times 0 (A), 10 min (B) and 30 min (C). In the presence of Amph A1 (at 10 min) or Epsin 2a (at 10 and 30 min), Stat3 remains localized to the plasma membrane in a less punctate pattern as compared to cells with functional endocytosis (Figures 5 and 6). These results correspond to the pattern we see with EGF receptor–ligand complexes in the presence of the endocytic inhibitor proteins (Figure 1). Consistent with Figure 1, Stat3 forms aggregate structures in the cytosol 30 min following EGF treatment in Amph A1 overexpressing cells. The observed differences in the distribution of Stat3 following treatment with the various endocytic inhibitors most likely reflects differences in the mechanisms by which these inhibitors disrupt endocytosis. Importantly, however, no significant accumulation of Stat3 in the nucleus was detected during EGF treatment. Therefore, Stat3 translocation to the perinuclear region in response to EGF stimulation requires functional endocytosis.
Fig. 7. Inhibition of endocytosis blocks Stat3 translocation to the perinuclear region. Immunofluorescence analysis of NIH-3T3/EGFR cells was carried out after transfection of expression vectors encoding Stat3, Amph A1 or Epsin 2a–GFP. Antibodies to Stat3 or the HA tag of Amph A1 were used to detect localization of these proteins. Transfected cells were treated with 1 µg/ml EGF for 0 min (A), 10 min (B) and 30 min (C). Localization of Stat3 (red) and Amph A1 (green) or Epsin 2a–GFP (green) was analyzed using a Zeiss confocal microscope.
Discussion
How it is that polypeptide ligands bound to receptors at the cell surface ultimately lead to regulation of gene expression in the nucleus is a central question in signal transduction. In the specific case of STAT proteins, their mechanism of translocation through the cytoplasm to the nucleus has been unclear since their discovery. Studies presented here support a model in which Stat3 is transported through the cytoplasm by receptor-mediated endocytosis. We show that Stat3 co-localizes with growth factor receptors in vesicles derived from endosomes at three locations in a time-dependent manner while in transit: the cell membrane, the cytosol and the perinuclear region. We further show that inhibition of receptor-mediated endocytosis by Amph A1, Epsin 2a or PAO has the following effects: abrogation of Stat3 transport to the perinuclear region, exclusion of functional Stat3 DNA-binding activity from the nucleus and suppression of Stat3-mediated transcriptional events. These results together demonstrate the important role of growth factor receptor-mediated endocytosis in Stat3 signaling. In addition, the accumulation of tyrosine phosphorylated Stat3, combined with the greatly reduced Stat3 DNA-binding activity when receptor endocytosis is blocked, strongly suggests that tyrosine phosphorylated Stat3 does not exist as randomly-diffusing active dimers in the cytoplasm but rather as monomers that remain bound to receptors following inhibition of endocytosis.
The mechanism by which Stat3 translocates across the nuclear membrane remains to be determined, although there is evidence from other studies for several potential mechanisms. Recent studies suggest that EGF is released from its receptor in late endosomes before degradation, which may provide a mechanism by which Stat3 is released from the receptor–ligand complex and is subsequently imported into the nucleus (Burke et al., 2001). However, like other STAT family members Stat3 lacks a classical nuclear localization sequence. In the case of Stat1, nuclear import in response to IFNγ has been shown to be mediated by the importin/Ran transport system (Sekimoto et al., 1997). Furthermore, Stat1 was found to interact with the nuclear pore-targeting protein NPI-1, an importin-α-like molecule associated with nuclear pore complexes (Sekimoto et al., 1997). Other studies suggest that Stat1 contains a non-classical nuclear localization arginine/lysine-rich element required for nuclear import (Melen et al., 2001). Alternatively, it has been suggested that a polybasic nuclear localization sequence in IFNγ regulates Stat1 nuclear translocation (Subramaniam et al., 2000). In this model, ligand–receptor complexes assist in nuclear translocation of Stat1. There have been numerous other reports of nuclear translocation of transmembrane receptors, including EGFR and PDGFR (Jans and Hassan, 1998; Lin et al., 2001). Together with earlier studies showing that stabilized ligand–EGFR complexes on the cell surface are not sufficient to induce DNA synthesis (Wakshull and Wharton, 1985), these findings suggest that nuclear translocation of transmembrane receptors is of functional importance.
Intriguingly, a recent report confirms the presence of EGFR in the nucleus and suggests that nuclear EGFR regulates expression of genes, including cyclin D1 (Lin et al., 2001). One possibility is that EGFR directly shuttles Stat3 into the nucleus, where Stat3 (perhaps still in a complex with EGFR) regulates gene expression. Consistent with this possibility is the finding that Stat3 regulates cyclin D1 gene expression (Bromberg et al., 1999; Sinibaldi et al., 2000). If this model is correct, then the primary role of EGFR translocation to the nucleus would be to serve as a scaffold for transport of signaling proteins like Stat3 directly to their nuclear targets involved in mediating growth factor responses. This raises the question of how receptors like EGFR and their associated proteins in the endocytic vesicles might translocate across the nuclear membrane. Certain components of the endo cytic machinery, including Eps15, Eps15R and AP-180 possess FG repeats (Chen et al., 1998; Slepnev et al., 2000). FG-containing repeat regions have been shown to interact with several members of the importin family (Ohno et al., 1998). Furthermore, Epsin 1 undergoes nucleo-cytoplasmic shuttling and its ENTH domain is structurally similar to Armadillo and HEAT repeats of the nuclear shuttling proteins β-catenin and karyopherin-β, respectively (Hyman et al., 2000). Therefore, endocytic proteins may interact directly with the nuclear pore complex, thereby connecting the cell machineries that govern vesicle transport to those that mediate nucleo-cytoplasmic shuttling.
In summary, our results demonstrate that endocytosis is necessary for Stat3 translocation from the cytoplasm to the nucleus. Stat3 tyrosine phosphorylation is not sufficient for full activation of Stat3 function. Furthermore, endocytosis of growth factor receptors serves to transport Stat3 from the plasma membrane to the perinuclear region following ligand stimulation. Together, these results indicate for the first time a mechanism by which Stat3 is actively transported through the cytoplasm to the nucleus via endocytic vesicles. Receptor-mediated endocytosis may also be the mechanism by which unrelated signaling proteins such as Erk and Smad undergo nuclear translocation following cytoplasmic activation.
Materials and methods
Cells, plasmids and transfections
As specified, either NIH-3T3, Balb/c-3T3 or NIH-3T3/EGFR cells were used. NIH-3T3 cells were cultured in DMEM supplemented with l-glutamine, sodium pyruvate, HEPES buffer and 5% bovine calf serum (BCS). NIH-3T3/EGFR cells were cultured in the same media with 10% BCS. Balb/c-3T3 cells were cultured in DMEM supplemented with l-glutamine, essential amino acids and 10% BCS. In some experiments, 5 µM PAO, a pharmacologic inhibitor of endocytosis (Hertel et al., 1985; Tong et al., 2000), was added for the last one-half hour of the serum-free treatment and included in the media during the time of growth factor addition. Human EGF was obtained from Molecular Probes. Amph A1 and Epsin 2a expression vectors were generously provided by Dr P.De Camilli. The Stat3 reporter, pLucTKS3, driving expression of firefly luciferase has been described previously (Turkson et al., 1998). The pLucTKS3 plasmid harbors seven copies of a sequence corresponding to the Stat3-specific binding site in the promoter of the human C-reactive protein gene. Cells were transfected using Lipofectamine Plus (Gibco-BRL).
Nuclear extract preparation and gel shift assays
Nuclear extracts were prepared from NIH-3T3 or Balb/c-3T3 fibroblasts treated with or without growth factor (0.8 µg/ml EGF or 50 ng/ml PDGF-BB) for the times indicated, and EMSAs were carried out as previously described (Yu et al., 1995). Cells treated with growth factor were serum starved overnight in 0.1% media. The 32P-radiolabeled oligonucleotide probe is hSIE (high affinity sis-inducible element, m67 variant, 5′-AGCTTCATTTCCCGTAAATCCCTA) that binds Stat1 and Stat3 (Yu et al., 1995). Protein–DNA complexes were resolved by non-denaturing PAGE and detected by autoradiography.
Western blot analyses
Western blot analysis was performed using rabbit polyclonal antibodies to phosphoY705-Stat3 (Cell Signaling Technology, 9131) or mouse monoclonal antibodies to total Stat3 protein (BD Biosciences, 610190) to probe immunoprecipitates prepared with antibodies to total Stat3 protein (Santa Cruz Biotechnology, Stat3 C-20, sc-482) from lysates of either EGF-treated NIH-3T3/EGFR or PDGF-BB-treated Balb/c-3T3 cells. Immunoprecipitations and western blot analyses were performed as described previously (Turkson et al., 1998).
Stat3-dependent luciferase reporter gene expression
To measure PDGF-BB-induced, Stat3-mediated transcriptional activation with or without functional endocytosis, Balb/c-3T3 cells were transfected with control empty vector, or expression vectors encoding the endocytosis inhibitors Amph A1 or Epsin 2a, and the Stat3 luciferase reporter construct pLucTKS3, together with β-galactosidase vector as an internal control for transfection efficiency. The transfected cells were harvested 48 h post-transfection following serum starvation overnight and treatment with 50 ng/ml PDGF for the times indicated. Cytosolic extract preparation from fibroblasts and luciferase assays were performed as described previously (Turkson et al., 1998). Briefly, after two washes with PBS and equilibration for 5 min with 0.5 ml of PBS–0.5 mM EDTA, cells were scraped off the dishes and the cell pellet was obtained by centrifugation (4500 g, 2 min, 4°C). Cells were resuspended in 0.4 ml of low-salt HEPES buffer (10 mM HEPES pH 7.8, 10 mM KCl, 0.1 mM EGTA, 0.1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride and 1 mM dithiothreitol) for 15 min, lysed by the addition of 20 µl of 10% NP-40 and centrifuged (10 000 g, 30 s, 4°C) to obtain the cytosolic supernatant, which was used for luciferase assays (Promega) measured with a luminometer. Results are shown as fold luciferase activities normalized to the β-gal internal control.
Confocal microscopy
To examine the ability of the endocytic inhibitors to block growth factor-mediated endocytosis, NIH-3T3/EGFR cells were transiently transfected with either HA-tagged Amph A1 or HA-tagged Epsin 2a expression vectors at least 36 h prior to EGF stimulation. Following 0.1% serum starvation for 12 h, cells were treated with 2 µg/ml Alexa Fluor EGF (Molecular Probes). Cells treated with PAO were pretreated 30 min prior to and during growth factor addition. For visualization of Stat3 localization to endosomes, NIH-3T3 cells expressing endogenous levels of PDGFR were grown on glass coverslips. After 0.1% serum starvation for 12 h, cells were treated with 50 ng/ml PDGF. To determine co-localization of Stat3 with receptor–ligand complexes, NIH-3T3/EGFR cells were grown on glass coverslips after transfection with Stat3 expression vector. Following 0.1% serum starvation for 12 h, cells were treated with 2 µg/ml Alexa Fluor EGF. To visualize Stat3 localization following inhibition of endocytosis, NIH-3T3/EGFR cells were transfected with expression vectors encoding Stat3, Amph A1 or Epsin 2a–GFP. After 0.1% serum starvation for 12 h, cells were treated with 1 µg/ml EGF (Molecular Probes). All cells were treated with growth factor for 45 min at 4°C to recruit ligand-bound receptor at the cell surface, and then warmed to 37°C for the times indicated, to enable endocytosis. Cells were fixed in 4% paraformaldehyde for 10 min and permeabilized with 0.2% Triton X-100. After washing in PBS, cells were blocked with filtered culture medium and incubated with rabbit polyclonal anti-Stat3 (Santa Cruz Biotechnology, Stat3 C-20, sc-482), and either mouse monoclonal anti-AP-2 (Affinity BioReagents, MA3-061) or anti-HA monoclonal antibodies to the HA tag of either Amph A1 or Epsin 2a (BabCo, HA.11, MMS-101P) to detect localization of these proteins. The coverslips were then extensively washed and incubated with secondary antibodies (Sigma, TRITC-conjugated anti-rabbit or FITC-conjugated anti-mouse, T-5268 or F-9006, respectively). Images were collected using the LSM 510 program on a Zeiss confocal microscope.
Acknowledgments
Acknowledgements
We thank P.De Camilli for Amph A1 and Epsin 2a expression vectors, W.J.Pledger for Balb/c-3T3 cells and PDGF-BB, P.McPherson, W.Wharton and T.Guadagno for insightful comments on the manuscript, E.Sejo and N.Valkov of the Moffitt Analytic Microscopy core facility for confocal microscopy assistance, L.Kuba of the Molecular Imaging core facility for assistance with figures, M.Morris for computer technical support, and members of the laboratory for stimulating discussion. A.H.B. was supported by DOD predoctoral fellowship DAMD17-98-1-8297. This work was supported in part by NCI grant CA55652 (to R.J.).
References
- Bowman T., Garcia,R., Turkson,J. and Jove,R. (2000) STATs in oncogenesis. Oncogene, 19, 2474–2488. [DOI] [PubMed] [Google Scholar]
- Bromberg J.F., Wrzeszczynska,M.H., Devgan,G., Zhao,Y., Pestell,R.G., Albanese,C. and Darnell,J.E.,Jr (1999) Stat3 as an oncogene. Cell, 98, 295–303. [DOI] [PubMed] [Google Scholar]
- Burke P., Schooler,K. and Wiley,H.S. (2001) Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol. Biol. Cell, 12, 1897–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill M.A., Janknecht,R. and Nordheim,A. (1996) Signalling pathways: Jack of all cascades. Curr. Biol., 6, 16–19. [DOI] [PubMed] [Google Scholar]
- Ceresa B.P. and Schmid,S.L. (2000) Regulation of signal transduction by endocytosis. Curr. Opin. Cell Biol., 12, 204–210. [DOI] [PubMed] [Google Scholar]
- Chen H., Fre,S., Slepnev,V.I., Capua,M.R., Takei,K., Butler,M.H., Di Fiore,P.P. and De Camilli,P. (1998) Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature, 394, 793–797. [DOI] [PubMed] [Google Scholar]
- Darnell J.E. Jr (1997) STATs and gene regulation. Science, 277, 1630–1635. [DOI] [PubMed] [Google Scholar]
- Di Fiore P.P. and Gill,G.N. (1999) Endocytosis and mitogenic signaling. Curr. Opin. Cell Biol., 11, 483–488. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo G.M., Baass,P.C., Ou,W.J., Posner,B.I. and Bergeron,J.J. (1994) Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J., 13, 4269–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X.Y. and Zhang,J.J. (1993) Transcription factor p91 interacts with the epidermal growth factor receptor and mediates activation of the c-fos gene promoter. Cell, 74, 1135–1145. [DOI] [PubMed] [Google Scholar]
- Gineitis D. and Treisman,R. (2001) Differential usage of signal transduction pathways defines two types of serum response factor target gene. J. Biol. Chem., 276, 24531–24539. [DOI] [PubMed] [Google Scholar]
- Hertel C., Coulter,S.J. and Perkins,J.P. (1985) A comparison of catecholamine-induced internalization of β-adrenergic receptors and receptor-mediated endocytosis of epidermal growth factor in human astrocytoma cells. Inhibition by phenylarsine oxide. J. Biol. Chem., 260, 12547–12553. [PubMed] [Google Scholar]
- Hyman J., Chen,H., Di Fiore,P.P., De Camilli,P. and Brunger,A.T. (2000) Epsin 1 undergoes nucleocytosolic shuttling and its eps15 interactor NH2-terminal homology (ENTH) domain, structurally similar to Armadillo and HEAT repeats, interacts with the transcription factor promyelocytic leukemia Zn2+ finger protein (PLZF). J. Cell Biol., 149, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans D.A. and Hassan,G. (1998) Nuclear targeting by growth factors, cytokines, and their receptors: a role in signaling? BioEssays, 20, 400–411. [DOI] [PubMed] [Google Scholar]
- Leof E.B. (2000) Growth factor receptor signalling: location, location, location. Trends Cell Biol., 10, 343–348. [DOI] [PubMed] [Google Scholar]
- Lin S.Y., Makino,K., Xia,W., Matin,A., Wen,Y., Kwong,K.Y., Bourguignon,L. and Hung,M.C. (2001) Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol., 3, 802–808. [DOI] [PubMed] [Google Scholar]
- McPherson P.S., Kay,B.K. and Hussain,N.K. (2001) Signaling on the endocytic pathway. Traffic, 2, 375–384. [DOI] [PubMed] [Google Scholar]
- Melen K., Kinnunen,L. and Julkunen,I. (2001) Arginine/lysine-rich structural element is involved in interferon-induced nuclear import of STATs. J. Biol. Chem., 276, 16447–16455. [DOI] [PubMed] [Google Scholar]
- Ohno M., Fornerod,M. and Mattaj,I.W. (1998) Nucleocytoplasmic transport: the last 200 nanometers. Cell, 92, 327–336. [DOI] [PubMed] [Google Scholar]
- Robinson M.S., Watts,C. and Zerial,M. (1996) Membrane dynamics in endocytosis. Cell, 84, 13–21. [DOI] [PubMed] [Google Scholar]
- Schindler C. and Darnell,J.E.,Jr (1995) Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem., 64, 621–651. [DOI] [PubMed] [Google Scholar]
- Sekimoto T., Imamoto,N., Nakajima,K., Hirano,T. and Yoneda,Y. (1997) Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J., 16, 7067–7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinibaldi D., Wharton,W., Turkson,J., Bowman,T., Pledger,W.J. and Jove,R. (2000) Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene, 19, 5419–5427. [DOI] [PubMed] [Google Scholar]
- Slepnev V.I., Ochoa,G.C., Butler,M.H. and De Camilli,P. (2000) Tandem arrangement of the clathrin and AP-2 binding domains in amphiphysin 1 and disruption of clathrin coat function by amphiphysin fragments comprising these sites. J. Biol. Chem., 275, 17583–17589. [DOI] [PubMed] [Google Scholar]
- Subramaniam P.S., Larkin,J., Mujtaba,M.G., Walter,M.R. and Johnson,H.M. (2000) The COOH-terminal nuclear localization sequence of interferon γ regulates STAT1 α nuclear translocation at an intracellular site. J. Cell Sci., 113, 2771–2781. [DOI] [PubMed] [Google Scholar]
- Takei K., Slepnev,V.I., Haucke,V. and De Camilli,P. (1999) Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol., 1, 33–39. [DOI] [PubMed] [Google Scholar]
- Tong X.K., Hussain,N.K., Adams,A.G., O’Bryan,J.P. and McPherson, P.S. (2000) Intersectin can regulate the Ras/MAP kinase pathway independent of its role in endocytosis. J. Biol. Chem., 275, 29894–29899. [DOI] [PubMed] [Google Scholar]
- Turkson J., Bowman,T., Garcia,R., Caldenhoven,E., De Groot,R.P. and Jove,R. (1998) Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell Biol., 18, 2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira A.V., Lamaze,C. and Schmid,S.L. (1996) Control of EGF receptor signaling by clathrin-mediated endocytosis. Science, 274, 2086–2089. [DOI] [PubMed] [Google Scholar]
- Wakshull E.M. and Wharton,W. (1985) Stabilized complexes of epidermal growth factor and its receptor on the cell surface stimulate RNA synthesis but not mitogenesis. Proc. Natl Acad. Sci. USA, 82, 8513–8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.Z., Wharton,W., Garcia,R., Kraker,A., Jove,R. and Pledger,W.J. (2000) Activation of Stat3 preassembled with platelet-derived growth factor β receptors requires Src kinase activity. Oncogene, 19, 2075–2085. [DOI] [PubMed] [Google Scholar]
- Yu C.L., Meyer,D.J., Campbell,G.S., Larner,A.C., Carter-Su,C., Schwartz,J. and Jove,R. (1995) Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science, 269, 81–83. [DOI] [PubMed] [Google Scholar]