Abstract
Cytoplasmic polyadenylation stimulates the translation of several dormant mRNAs during oocyte maturation in Xenopus. Polyadenylation is regulated by the cytoplasmic polyadenylation element (CPE), a cis-acting element in the 3′-untranslated region of responding mRNAs, and its associated factor CPEB. CPEB also binds maskin, a protein that in turn interacts with eIF4E, the cap-binding factor. Here, we report that based on antibody and mRNA reporter injection assays, maskin prevents oocyte maturation and the translation of the CPE-containing cyclin B1 mRNA by blocking the association of eIF4G with eIF4E. Dissociation of the maskin–eIF4E complex is essential for cyclin B1 mRNA translational activation, and requires not only cytoplasmic polyadenylation, but also the poly(A)-binding protein. These results suggest a molecular mechanism by which CPE- containing mRNA is activated in early development.
Keywords: CPEB/maskin/oocyte maturation/polyadenylation/translation
Introduction
Early animal development is regulated in part by mRNAs that are synthesized and stored during oogenesis and inherited by the fertilized egg. These maternal mRNAs are not translated en masse at any one stage, but instead are activated at specific times in the developing organism. For example, some mRNAs are activated only when prophase I-arrested oocytes re-enter the meiotic divisions (oocyte maturation), while other mRNAs are translated at fertilization or during subsequent embryogenesis (reviewed by Richter 2000; Wickens et al., 2000; Mendez and Richter, 2001). In vertebrates such as frogs (Xenopus) and mice, mRNAs encoding the key proteins Mos, Cdk2 and cyclin B are translated during oocyte maturation and regulate such processes as release from meiotic arrest at the end of prophase I, meiotic arrest at metaphase II and mitosis in the embryo (Sheets et al., 1994, 1995; Stebbins-Boaz et al., 1996; de Moor and Richter, 1997, 1999; Groisman et al., 2000, 2002). Generally, these and other mRNAs have short poly(A) tails when they are dormant in oocytes and elongated poly(A) tails when they are activated as maturation and/or mitosis proceed (McGrew et al., 1989; Vassalli et al., 1989; Sheets et al., 1994).
In Xenopus oocytes, two cis elements in the 3′-untranslated region (UTR) of responding mRNAs direct cytoplasmic polyadenylation, the cytoplasmic polyadenylation element (CPE) and the polyadenylation hexanucleotide AAUAAA. The CPE is bound by CPEB, a zinc finger- and RRM-containing protein (Paris et al., 1991; Hake and Richter, 1994; Hake et al., 1998), while the hexanucleotide is bound by a cleavage and polyadenylation specificity factor (CPSF) (Bilger et al., 1994; Dickson et al., 1999). Polyadenylation is initiated when progesterone interacts with its receptor, which may be associated with the cell surface (Bayaa et al., 2000; Tian et al., 2000). This interaction activates aurora (also known as Eg2) (Andresson and Ruderman, 1998), a kinase that then phosphorylates CPEB on Ser174 (Mendez et al., 2000a). This event induces CPEB to bind and recruit CPSF, possibly stabilizing it on the AAUAAA sequence (Mendez et al., 2000b), which in turn attracts poly(A) polymerase (PAP) to the end of the mRNA.
CPEB interacts with an additional factor, maskin, simultaneously with the cap-binding factor eIF4E (Stebbins-Boaz et al., 1999). Because it contains a short amino acid stretch similar to those in eIF4G and other eIF4E-binding proteins (Gingras et al., 1999a), maskin would, in theory, preclude the binding of eIF4E to eIF4G. This hypothetical exclusion of eIF4G would prevent translation because it is this factor that is necessary to establish the 40S ribosomal subunit-containing initiation complex (Gingras et al., 1999a; Mendez and Richter, 2001). If this configuration of factors (i.e. CPEB–maskin–eIF4E), which is observed in meiotically arrested oocytes, inhibits translation, it stands to reason that the displacement of maskin from eIF4E would be a necessary prelude to the association of eIF4G with eIF4E and translational activation. In mature oocytes (eggs), when many CPE-containing mRNAs have undergone polyadenylation-induced translation, there is at least a partial dissociation of maskin from eIF4E (Stebbins-Boaz et al., 1999). While compelling, there is no extant evidence that maskin has any function in translation, or if it did have such a function, how its dissociation from eIF4E would be controlled.
In this study, we have investigated the involvement of maskin in CPE-mediated translation in Xenopus oocytes. We find that as maturation proceeds, maskin is displaced from eIF4E and replaced by eIF4G. The injection of affinity-purified maskin antibody into oocytes induces the synthesis of cyclin B1, Wee1 and Mos, all of which are encoded by CPE-containing mRNAs. The induction of translation by maskin antibody appears to be due to the displacement of maskin from eIF4E. Following progesterone stimulation of oocytes, the inhibition of cytoplasmic polyadenylation by 3′-deoxyadenosine (cordycepin) prevents the dissociation of maskin from eIF4E and also prevents oocyte maturation. However, while injected cyclin B protein rescues maturation even in the presence of cordycepin, it does not induce polyadenylation or the dissociation of maskin from eIF4E. These data suggest that cytoplasmic polyadenylation is responsible for the dissociation of maskin from eIF4E. Moreover, the effect of the poly(A) tail in controlling the switch from the maskin–eIF4E complex to the eIF4E–eIF4G complex is mediated by the poly(A)-binding protein (PABP).
Results
Maskin is a negative regulator of translation
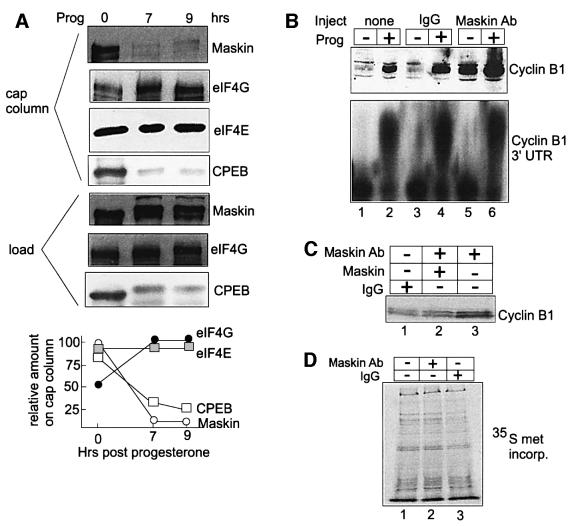
Stebbins-Boaz et al. (1999) demonstrated an interaction between CPEB, maskin and eIF4E by co-immunoprecipitation, protein ‘pull-down’ assays and yeast two-hybrid analysis. In addition, because eIF4E simultaneously binds the cap structure and maskin, chromatography on 7m-GTP–Sepharose (i.e. a cap column) was found to be an effective method for observing the association between these two factors. The interaction between eIF4E and maskin is direct; it requires the maskin eIF4E-binding motif, and is disrupted by a peptide that encompasses this motif (Stebbins-Boaz et al., 1999). An example of cap column chromatography using extracts prepared from non-stimulated oocytes (supplemented with GTP to reduce non-specific binding) is depicted in Figure 1. A western blot analysis shows that maskin, eIF4E and CPEB, as well as eIF4G, were all detected in the material retained on the column. When extracts prepared from progesterone-stimulated oocytes that had matured as far as metaphase I were applied to the column, the same amount of eIF4E was retained, but the amount of CPEB and maskin decreased by ∼70–90%. While CPEB is partially destroyed late during maturation (Mendez et al., 2002), much of that which remained stable (∼70%) was not retained on the column. The decrease in maskin on the column was not due to degradation since the same amount was detected in the load fraction at time 0 (i.e. at prophase I) and 7 h (i.e. at metaphase I) post-progesterone. Commensurate with the decrease in maskin binding at 7 h post-progesterone was an increase in the retention of eIF4G. These same profiles were also evident when the oocytes were exposed to progesterone for 9 h (i.e. at metaphase II). These data suggest that the maskin associated with eIF4E was replaced by eIF4G during oocyte maturation.
Fig. 1. Translational control by maskin. (A) Extracts prepared from oocytes exposed to progesterone for 0–9 h were supplemented with GTP, chromatographed on 7mGTP–Sepharose (cap column), eluted with SDS sample buffer and analyzed by western blotting for maskin, eIF4E, CPEB and eIF4G. The maskin, CPEB and eIF4G in the starting material (load) were also examined. The oocytes exposed to progesterone for 7 h reached metaphase I, while those exposed to progesterone for 9 h reached metaphase II; the oocytes not exposed to progesterone remained at prophase I. The lower part of the figure shows a quantification of the western blot results; relative amount refers to the percentage of maximum of each particular protein retained on the column. (B) Affinity-purified maskin antibody or IgG was injected into oocytes, some of which were also injected with 32P-labeled cyclin B1 3′-UTR. One-half of each group of oocytes was then stimulated with progesterone. The oocytes were analyzed for cyclin B1 accumulation by western blotting and for polyadenylation by urea–acrylamide gel electrophoresis and phosphoimaging. (C) Affinity-purified maskin antibody was injected into oocytes as above, or mixed with E.coli-expressed maskin prior to injection. Other oocytes were injected with IgG. An extract was then prepared and analyzed for cyclin B1 protein accumulation by a western blot. (D) Oocytes injected with IgG or maskin antibody were incubated with [35S]methionine and the resulting radioactive proteins were displayed on an SDS gel.
To determine whether maskin is a negative regulator of translation, cyclin B1 protein levels were examined following the injection of affinity-purified maskin antibody. Cyclin B1 mRNA contains CPEs within its 3′-UTR, and its translation during maturation is controlled by CPEB-dependent cytoplasmic polyadenylation (Stebbins-Boaz et al., 1996). Figure 1B demonstrates that cyclin B1 levels were very low in non-stimulated oocytes irrespective of whether IgG was injected (lanes 1 and 3). As expected, progesterone stimulation induced a substantial increase in the accumulation of this protein in both groups of oocytes (lanes 2 and 4). Maskin antibody injection, on the other hand, induced cyclin B1 accumulation even when the oocytes were not exposed to progesterone (lane 5). When these antibody-injected oocytes were exposed to progesterone, there was a further increase in cyclin B1 levels (lane 6). In all cases, progesterone stimulation was required to induce cyclin B1 RNA polyadenylation (lanes 2, 4 and 6). These results indicate that injected maskin antibody can neutralize the activity of this repressor protein and overcome the normal requirement for polyadenylation for cyclin B1 mRNA to be activated. To rule out non-specific activity of the maskin antibody, it was mixed with Escherichia coli-expressed maskin prior to injection; there was no induction of cyclin B1 synthesis compared with when the antibody alone was injected (Figure 1C, lanes 2 and 3). Finally, Figure 1D demonstrates that neither maskin antibody nor IgG had any significant effect on the amount or general types of prevalent proteins that were synthesized in oocytes. These data are consistent with the notion that maskin is a negative regulator of CPE-containing mRNA translation.
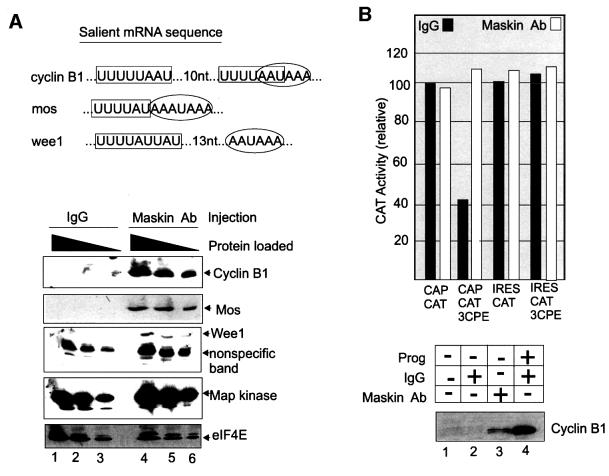
In addition to cyclin B1, the mRNAs encoding Mos and Wee1 are dormant in oocytes, are activated upon progesterone stimulation and contain CPEs that regulate their translation (Figure 2A) (Sheets et al., 1994; de Moor and Richter, 1997; Nakajo et al., 2000). To determine whether maskin influences the translation of these mRNAs, affinity-purified maskin antibody, or IgG, was injected into oocytes. Only maskin antibody induced the accumulation of all three of these proteins, but had no significant effect on the levels of mitogen-activated protein (MAP) kinase, eIF4E or an unidentified protein (Figure 2A). These results indicate that maskin regulates the translation of Mos and Wee1 mRNAs as well as cyclin B1 mRNA.
Fig. 2. Maskin antibody activates the translation of CPE-containing mRNAs. (A) The salient 3′-UTR sequences of cyclin B1, Mos and Wee1 mRNAs are depicted (top); the CPEs (boxed) and polyadenylation hexanucleotides (ovals) are designated. Maskin antibody or IgG was injected into oocytes, which were then cultured overnight (no progesterone). Extracts were then prepared and three concentrations of protein were analyzed by western blots probed for cyclin B1, Mos, Wee1, MAP kinase and eIF4E. (B) In vitro synthesized CAT reporter RNAs that contained a 5′ cap (CAP CAT) or the EMCV IRES (IRES CAT) were appended with 3′-UTRs composed of either a polylinker sequence or a polylinker sequence harboring three repeated CPE sequences (3CPE) (de Moor and Richter, 1999). The RNAs were injected into oocytes followed by a second injection of IgG or maskin antibody; extracts were then prepared and CAT activity was assessed. Each experiment was performed five times. The bottom portion of the figure shows that injected maskin antibody, but not injected IgG, induced endogenous cyclin B1 synthesis in the same oocytes that were injected with the reporter RNAs.
The observation that the CPE inhibited the translation of a reporter mRNA when it contained a 5′ cap but not when it contained an internal ribosome entry site (IRES) (de Moor and Richter, 1999) indicated the existence of a molecule (maskin) that abrogated the activity of eIF4E. This phenomenon is shown in Figure 2B (top), where three CPEs appended to the 3′-UTR of CAT RNA (which also contains a 5′ cap) inhibited translation in oocytes. Injected maskin antibody, but not IgG, activated the translation of this reporter as well as endogenous cyclin B1 RNA (top and bottom). When the reporter was placed under the control of the IRES from encephalomyocarditis virus (EMCV), the CPEs had no deleterious effect on translation, as shown previously (de Moor and Richter, 1999). Because IRES-directed translation does not utilize eIF4E, maskin would not be expected to have any effect on mRNA expression, and indeed injected maskin antibody did not significantly affect resulting CAT activity (Figure 2B). Taken together, these data suggest that maskin mediates CPE-dependent and cap-dependent translation.
Maskin–eIF4E dissociation correlates with translational activation
To determine the mechanism by which maskin antibody induces mRNA translation, extracts prepared from oocytes injected with either this antibody or IgG were supplemented with GTP to reduce non-specific adsorption, and in some cases free cap (7mGTP). The extracts were then analyzed on a cap column, and the bound material was detected by western blotting. Both maskin and eIF4E from IgG-injected oocytes were retained on the column in the presence of GTP, but not when free cap was also present (Figure 3, lanes 4–6). The eIF4E from maskin antibody-injected oocytes exhibited the same chromatographic profile. However, maskin was not retained when either GTP or free cap was added to the extract (lanes 1–3). These data suggest that maskin antibody induced translation by causing the dissociation of maskin from eIF4E. Complementary studies show that both maskin and eIF4E were eluted from the cap column with free cap (Figure 3B) and that maskin antibody co-immunoprecipitates both eIF4E and CPEB (Figure 3C).
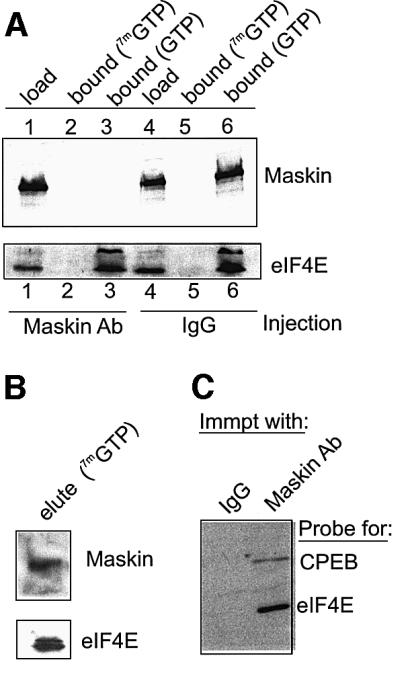
Fig. 3. Maskin antibody induces the dissociation of maskin from eIF4E. (A) Extracts from oocytes injected with maskin antibody or IgG were supplemented with GTP only, or GTP plus 7mGTP, and applied to a cap column. Following extensive washing, the bound material was eluted with SDS and, together with the load material, was analyzed for maskin and eIF4E by western blotting. (B) In a separate experiment, oocyte material bound to a cap column was eluted with free cap and then probed for maskin and eIF4E. (C) Oocyte extract was subjected to immunoprecipitation with either IgG or maskin antibody; the bound material was then probed for CPEB and eIF4E.
Polyadenylation is required for maskin–eIF4E dissociation
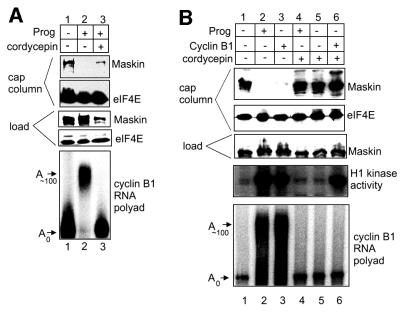
During oocyte maturation, polyadenylation of CPE- containing mRNAs occurs concomitantly with translational activation and maskin–eIF4E dissociation. One appealing hypothesis is that polyadenylation induces maskin–eIF4E dissociation. To address this possibility, we employed cordycepin (3′-deoxyadenosine), an adenosine analog that effectively terminates poly(A) elongation. Cordycepin has no detectable toxic effects in oocytes and does not obviously inhibit other ATP-requiring reactions (Kuge et al., 1995). Extracts prepared from oocytes incubated in the absence or presence of both cordypecin and progesterone were applied to a cap column; eIF4E was retained irrespective of the inhibition of polyadenylation by cordycepin (Figure 4A, lanes 1–3). Maskin, on the other hand, was retained when polyadenylation was inhibited by either the omission of progesterone or the addition of corcydepin (lanes 1–3). As expected, maskin was not retained on the column when progesterone alone was added and polyadenylation was stimulated (lane 2). Inspection of lanes 1 and 3 (Figure 4A) shows that the same relative amount of maskin was retained on the column when the amount of maskin in the initial load fraction is considered.
Fig. 4. Maskin–eIF4E dissociation requires cytoplasmic polyadenylation. (A) Oocytes were incubated in the absence or presence of progesterone or, in some cases, with progesterone and cordycepin. When the oocytes exposed to progesterone only had matured, extracts were prepared from all the oocytes and were applied to a cap column. The relative amounts of maskin and eIF4E on the column and in the initial load solution were analyzed by a western blot. Some oocytes injected with 32P-labeled cyclin B1 3′-UTR and treated with progesterone and/or cordycepin were used to examine cytoplasmic polyadenylation. (B) Oocytes were incubated with progesterone and/or cordycepin, some of which were injected with E.coli-expressed cyclin B1 protein. Extracts were then prepared and applied to a cap column; maskin and eIF4E in the bound fractions, as well as in the load fraction, were analyzed by western blotting. The extracts were also assessed for MPF activity as determined by the phosphorylation of histone H1. Finally, some oocytes treated with the agents noted above were also injected with labeled cyclin B1 3′-UTR and examined for cytoplasmic polyadenylation.
Because cordycepin blocks the progesterone-stimulated poly(A) addition of all CPE-containing mRNAs, including Mos, downstream events such as MAP kinase and M-phase-promoting factor (MPF) activation would not take place. Consequently, while the signaling events between progesterone interaction with its receptor and aurora-catalyzed CPEB phosphorylation would take place, entry into metaphase I would not occur (Kuge and Richter, 1995). Therefore, while cordycepin prevents maturation by blocking polyadenylation, the failure of maskin to dissociate from eIF4E could be due to the inhibition of maturation and not the inhibition of polyadenylation per se. To investigate the necessity for polyadenylation for maskin–eIF4E dissociation, E.coli-expressed cyclin B1 protein variant Δ90, a non-destructable form of the protein (Luca et al., 1991), was injected into oocytes incubated with cordycepin. Cyclin B1 not only acts downstream of Mos to stimulate MPF activation, but, through feedback loops, also induces upstream events including aurora activity (see below). These relationships are illustrated in Figure 4B. The first three lanes show that both progesterone and injected cyclin B1 protein induced the dissociation of maskin from eIF4E (cap column), activated MPF (i.e. phosphorylation of histone H1) and stimulated the polyadenylation of cyclin B1 RNA. It is important to note that injected cyclin B1 protein stimulated polyadenylation because this process requires the early event of aurora-catalyzed CPEB Ser174 phosphorylation (Mendez et al., 2000a), confirming that cyclin B exerts effects both downstream and upstream of Mos mRNA translation through feedback loops (Howard et al., 1999). The same parameters were examined in oocytes incubated in the presence of cordycepin (lanes 4–6). Neither progesterone nor injected cyclin B1 protein induced the dissociation of maskin from eIF4E (cap column). However, injected cyclin B1 did induce the downstream event of MPF activation (H1 kinase activity), even in the absence of cyclin mRNA polyadenylation. Because MPF activation requires that Cdc2 be modified by both phosphorylation and dephosphorylation events (Nebreda and Ferby, 2000), these results also indicate that cordycepin had no detectable deleterious effect on protein phosphorylation. These data indicate that polyadenylation, but not maturation per se, is necessary to induce the dissociation of maskin from eIF4E.
Poly(A)-binding protein mediates maskin–eIF4E dissociation
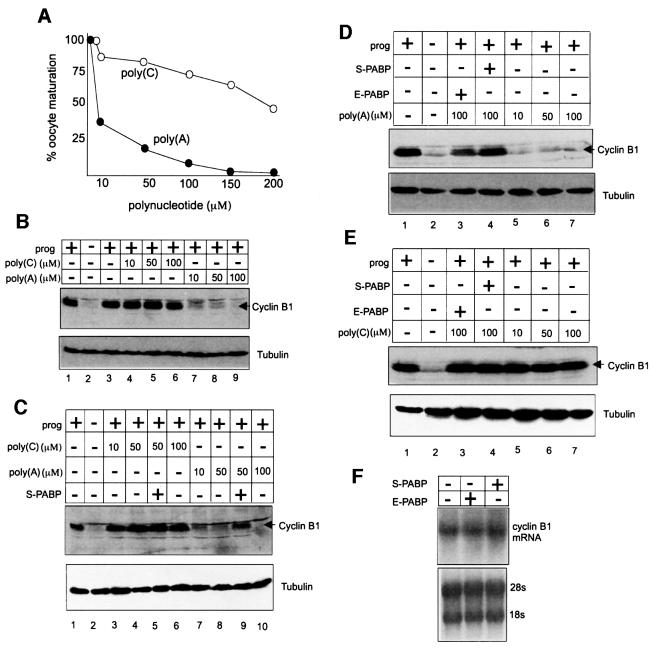
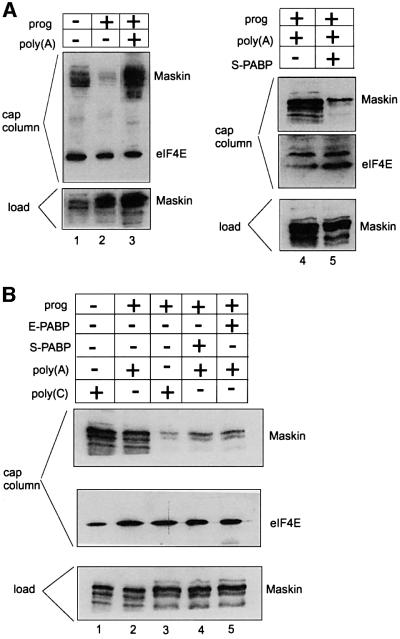
Data from a number of systems suggest that the poly(A) tail potentiates translation through an interaction with the poly(A)-binding protein (PABP) (Tarun and Sachs, 1996; Le et al., 1997; Wells et al., 1998; von der Haar et al., 2000; Wakiyama et al., 2000). PABP may help eIF4G bind eIF4E; eIF4G in turn is necessary for the positioning of the 40S ribosomal subunit on the mRNA. To determine whether the polyadenylation-mediated dissociation of maskin from eIF4E in mature oocytes involves PABP, we have performed two sets of experiments. The first set was to examine whether a poly(A)-binding titratable factor was involved in maskin–eIF4E dissociation and, if so, whether such a factor was PABP. Poly(A) and, as a control, poly(C) were injected into oocytes that were subsequently incubated with progesterone. When scored for germinal vesicle breakdown (GVBD), poly(A) inhibited maturation in a dose-dependent manner; injected poly(C) had only a modest effect (Figure 5A). Poly(A) also inhibited the progesterone-induced accumulation of cyclin B1, while poly(C) had no effect (Figure 5B, compare lanes 4–6 with lanes 7–9). These results indicate that injected poly(A) titrates a factor that is necessary for cyclin B1 mRNA translation and progesterone-induced maturation.
Fig. 5. PABP regulates the dissociation of maskin from eIF4E. (A) Several concentrations of poly(A) or poly(C) were injected into oocytes that were then stimulated with progesterone. Maturation was scored by the appearance of a white spot at the animal pole. (B) Oocytes were injected with poly(A) or poly(C) and some were then incubated with progesterone. Extracts were then analyzed for cyclin B1 accumulation by western blotting. In this and subsequent panels, the blots were also probed for tubulin, which served as a loading control. (C) Oocytes were injected with poly(A) and, in some cases, with E.coli-expressed S-PABP. Some of the oocytes were then incubated with progesterone and scored for the accumulation of cyclin B1 by western blotting. (D) Oocytes were injected with poly(A) and, in some cases, with E.coli-expressed S-PABP or E-PABP and scored for the accumulation of cyclin B1 by western blotting. (E) Oocytes were injected with poly(C) and, in some cases, with E.coli-expressed S-PABP or E-PABP and scored for the accumulation of cyclin B1 by western blotting. (F) RNA was extracted from S-PABP- and E-PABP-injected oocytes and used for a northern blot probed for cyclin B1. The rRNA subunits served as loading controls.
To assess whether this factor might be PABP, this protein, which is referred to as the ‘somatic’ type, was expressed in E.coli, purified and injected into oocytes together with poly(A). This S-PABP not only rescued the poly(A)-mediated inhibition of maturation, but also rescued the poly(A)-mediated inhibition of cyclin B1 protein accumulation (Figure 5C, lanes 8 and 9). Recently, a second type of PABP has been identified that is abundant in Xenopus oocytes and embryos (Voeltz et al., 2001), which we examined in a manner similar to S-PABP. This ‘embryonic’ type PABP (E-PABP) also overcame the poly(A)-induced inhibition of cyclin B synthesis (Figure 5D). As expected, neither S-PABP nor E-PABP had any effect on cyclin synthesis in oocytes injected with poly(C) (Figure 5E). Finally, Figure 5F shows that neither S-PABP nor E-PABP had any effect on cyclin B1 mRNA stability. These results indicate a conserved function between S-PABP and E-PABP in regulated cyclin mRNA translation.
We employed cap columns to investigate the influence of poly(A) as well as the two PABPs on the maskin–eIF4E interaction. While injected poly(A) prevented the progesterone-induced dissociation of maskin from eIF4E (Figure 6A, lanes 2 and 3), S-PABP overcame this block and restored the ability of progesterone to stimulate the dissociation of maskin from eIF4E (Figure 6A, lanes 4 and 5; Figure 6B, lanes 2 and 4). Injected E-PABP also restored the ability of progesterone to induce maskin– eIF4E dissociation (Figure 6A, lanes 2 and 5). Poly(C), which had no influence on progesterone-induced cyclin B1 synthesis (Figure 5B, compare lane 1 with lanes 4–6), had no effect on progesterone-induced maskin–eIF4E dissociation (Figure 6B, lanes 1 and 3). These results indicate that both S-PABP and E-PABP can mediate cyclin B1 mRNA translation by regulating the dissociation of maskin from eIF4E.
Fig. 6. Poly(A), PABP and the maskin–eIF4E interaction. (A) Oocytes injected with poly(A) and/or S-PABP were incubated in the absence or presence of progesterone. Extracts were then prepared from these oocytes and applied to a cap column as described in Figure 3. Maskin and eIF4E were analyzed by western blotting. (B) Oocytes were injected with combinations of poly(A), poly(C), S-PABP and E-PABP and incubated in the absence or presence of progesterone, followed by extract preparation, cap column chromatography and western blotting for maskin and eIF4E.
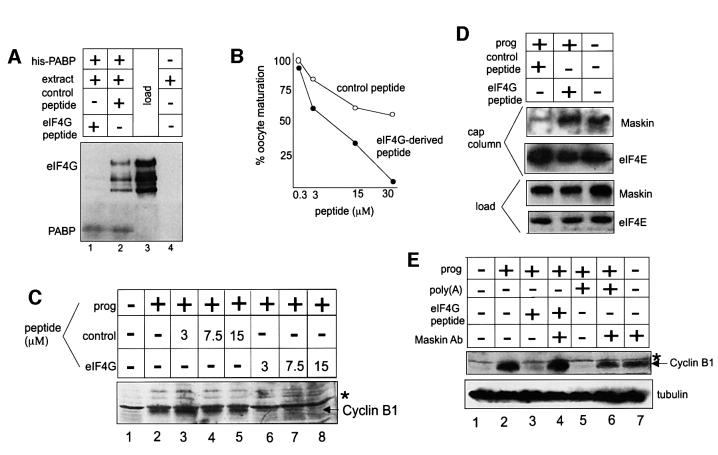
In other experiments, we focused on residues 132–156 of Xenopus eIF4G, which are necessary for the association of this protein with PABP (Wakiyama et al., 2000). A peptide consisting of these residues as well as a control peptide of irrelevant sequence were mixed with egg extracts and then applied to columns containing His-tagged E.coli-expressed PABP linked to Ni2+–agarose. Following washing, the bound material was eluted and probed on a western blot for eIF4G and PABP. Figure 7A shows that while eIF4G was not retained on a column lacking PABP (lane 4), it was retained on a PABP-containing column even when the extract was mixed with the control peptide (lane 2). However, eIF4G was not retained on the PABP column when the extract was mixed with the eIF4G-derived peptide prior to chromatography (lane 1). Thus, the peptide whose sequence is derived from the portion of eIF4G that interacts with PABP is able to disrupt the interaction between eIF4G and PABP.
Fig. 7. An eIF4G–PABP complex displaces maskin from eIF4E. (A) A peptide derived from eIF4G that is necessary for the binding of this protein to PABP was added to an egg extract, as was a control peptide of irrelevant sequence. The extract was then applied to an affinity column containing (lanes 1 and 2) or lacking (lane 4) recombinant His-tagged PABP. Following extensive washing, the columns were eluted with SDS and probed on a western blot for eIF4G and PABP. Lane 3 shows the profile of eIF4G in the extract prior to chromatography. (B) Various concentrations of the eIF4G-derived peptide and the control peptide were injected into oocytes that were subsequently incubated with progesterone. The incidence of oocyte maturation was then scored by the appearance of a white spot at the animal pole. (C) Oocytes injected with various concentrations of the control peptide or the eIF4G-derived peptide were incubated with progesterone, scored for the appearance of the white spot and then analyzed for cyclin B1 accumulation by western blotting. The control peptide-injected oocytes had matured whereas the eIF4G-derived peptide-injected oocytes had not. The asterisk denotes a non-specific band that serves as a loading control. (D) Oocytes injected with the control and eIF4G-derived peptides were incubated with progesterone, homogenized, mixed with free GTP and applied to a cap column. Following extensive washing, the column was eluted with SDS and probed on western blots for maskin and eIF4E. Maskin in the load fraction was also analyzed on a western blot. The control peptide-injected oocytes had matured whereas the eIF4G-derived peptide-injected oocytes had not. (E) Oocytes injected with poly(A) or the eIF4G peptide were also injected with maskin antibody and subsequently used for a cyclin B1 western blot. The asterisk denotes a non-specific band.
The two peptides were injected next into oocytes that were subsequently incubated with progesterone. This eIF4G-derived peptide inhibited oocyte maturation up to 10-fold more efficiently than a control peptide when a 30 µM concentration was injected (Figure 7B). The eIF4G-derived peptide also inhibited the accumulation of cyclin B1 protein in progesterone-stimulated oocytes compared with the control peptide (Figure 7C, compare lanes 3–5 with lanes 6–8). When extracts prepared from the progesterone-stimulated and peptide-injected oocytes were applied to cap columns, maskin retention was sub stantially reduced when the control peptide was injected, but was fully retained when the eIF4G-derived peptide was injected (Figure 7D). A western blot for eIF4E demonstrates that this protein was retained on the cap column under all conditions. Because the eIF4G-derived peptide induces the dissociation of PABP from eIF4G, represses the progesterone-induced translation of cyclin B1 mRNA and inhibits the dissociation of maskin from eIF4E, we infer that eIF4G, once bound to PABP, displaces maskin from eIF4E and initiates the translation of CPE-containing mRNA. Finally, Figure 7E shows that injected maskin antibody can overcome both the poly(A) and eIF4G peptide-induced inhibition of cyclin B1 synthesis, again indicating the importance of the maskin–eIF4E dissociation for translation.
Discussion
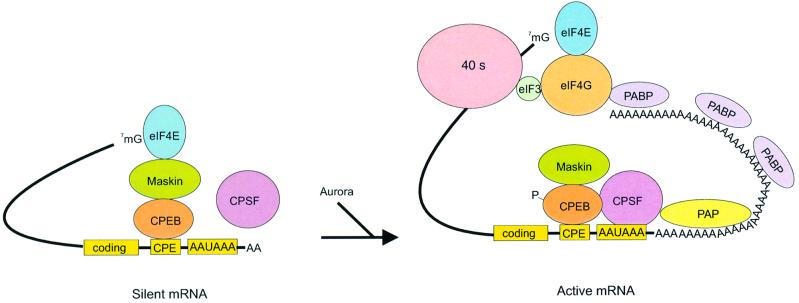
Taking into account the results reported in this study, a model for polyadenylation-induced translation in maturing oocytes is proposed (Figure 8). In immature oocytes, the CPEs of dormant mRNAs are bound by CPEB (Hake and Richter, 1994; Stebbins-Boaz et al., 1996; de Moor and Richter, 1999). The translational inactivity of these mRNAs is due to maskin, which, through an interaction with both CPEB and eIF4E, inhibits the assembly of the eIF4G-mediated 43S initiation complex on CPE-containing mRNAs. When oocytes are stimulated by progesterone, aurora (Eg2) phosphorylates CPEB on Ser174 (Mendez et al., 2000a), an event that may help CPEB stabilize CPSF on the AAUAAAA (Mendez et al., 2000b; see also Bilger et al., 1994; Dickson et al., 1999). By analogy with nuclear pre-mRNA polyadenylation, CPSF engages PAP at the end of the mRNA to catalyze poly(A) addition. The elongated poly(A) tail is then bound by PABP, which in turn associates with eIF4G (Tarun and Sachs, 1996; Gray et al., 2000; Wakiyama et al., 2000). The PABP–eIF4G complex displaces maskin from eIF4E, which allows for the association of eIF4G with eIF4E and the initiation of translation.
Fig. 8. Model of polyadenylation-induced translation. Dormant CPE-containing mRNAs (e.g. cyclin B1) in immature oocytes are bound by CPEB, which in turn is bound to maskin, which in turn is bound to eIF4E, the cap-binding factor. The binding of maskin to eIF4E precludes the binding of eIF4G to eIF4E, thus inhibiting the formation of the initiation complex. The cleavage and polyadenylation specificity factor (CPSF) may or may not be loosely associated with the hexanucleotide AAUAAA at this time. Following progesterone stimulation, the kinase aurora is activated and phosphorylates CPEB Ser174, an event that causes CPEB to bind and recruit CPSF into an active cytoplasmic polyadenylation complex, presumably helping it to associate with the AAUAAA. CPSF recruits poly(A) polymerase (PAP) to the end of the mRNA, where it catalyzes poly(A) addition. The newly elongated poly(A) tail is then bound by poly(A)-binding protein (PABP), which in turn associates with eIF4G. eIF4G, when associated with PABP, then displaces maskin from, and binds to, eIF4E, thereby initiating translation. eIF4G, through eIF3, interacts with the 40S ribosomal subunit.
eIF4E-binding proteins
Several related eIF4E-binding proteins (eIF4EBPs) that mediate translation by reversibly binding to eIF4E have been identified (Lin et al., 1994; Pause et al., 1994; Fadden et al., 1997; reviewed in Gingras et al., 1999a; Raught et al., 2000). These eIF4EBPs all contain a peptide motif (consensus YDRKFLL) that is similar to one in eIF4G that binds to eIF4E. This peptide sequence in the eIF4EBPs has spawned the idea that ‘molecular mimicry’ controls cap-dependent translation by establishing a competition between eIF4G and the eIF4EBPs for binding to eIF4E (Haghighat et al., 1995; Marcotrigiano et al., 1999). The data presented here and in Stebbins-Boaz et al. (1999) demonstrate that maskin is another, albeit unconventional, eIF4EBP. While the critical functions of the eIF4EBPs and maskin are similar, the mechanisms by which they accomplish this task are different, as are the mRNA substrates upon which they act. The eIF4EBPs bind eIF4E when they are hypophosphorylated, but dissociate from eIF4E when they are hyperphosphorylated by at least two different kinases (Pause et al., 1994; Fadden et al., 1997). The first kinase, FRAP/mTOR, phosphorylates eIF4EBP1 on two critical residues, which, while having no direct effect on the ability of eIF4EBP1 to bind eIF4E, act as priming phosphorylations for subsequent modifications that directly mediate eIF4E–eIF4EBP1 interactions (Gingras et al., 1999b, 2001). A kinase that might be more directly responsible for controlling eIF4EBP1– eIF4E interactions is ataxia telangiectasia (ATM). ATM directly phosphorylates a key residue of eIF4EBP1 in vitro, which is also phosphorylated in vivo. In cells lacking ATM, this residue is not phosphorylated, which correlates with reduced dissociation of eIF4EBP1 from eIF4E (Yang and Kastan, 2000). Maskin, like the eIF4EBPs, is also phosphorylated (D.Barnard and J.D.Richter, unpublished data), although the extent to which these modifications affect its binding to eIF4E during oocyte maturation is unknown.
Because the eIF4EBPs lack any obvious RNA-binding motif and are not known to interact with any RNA-specific binding protein, they could in theory regulate the translation of most cap-containing mRNAs. However, mRNAs with long 5′-UTRs that contain stable secondary structures or upstream initiation codons might be particularly susceptible to eIF4EBP regulation because they would be more likely to require the RNA helicase activity associated with eIF4F, a complex of proteins that includes eIF4E and eIF4G (Pyronnet and Sonenberg, 2001). Such mRNAs often encode growth factors whose translational control could be important for cell cycle progression. In support of this possibility, eIF4EBP1 is hypophosphorylated during mitosis (Pyronnet and Sonenberg, 2001), which is when cap-dependent translation is substantially reduced but IRES-dependent translation is relatively robust (Cornelis et al., 2000; Pyronnet et al., 2001).
Because of its association with CPEB, maskin would be predicted to modulate the translation of only CPE-containing mRNAs, many of which control the meiotic and early mitotic cell divisions. (Maskin could control the translation of non-CPE-containing mRNAs if it also associates with other RNA-binding proteins.) For example, we show here that dormant cyclin B1, Mos and Wee1 mRNAs are all activated when oocytes are injected with maskin antibody. Similar phenomena also occur in the early Xenopus embryo, where maskin antibody injection leads to an arrest of cell division (Groisman et al., 2000). In cycling extracts derived from Xenopus eggs, maskin antibody blocks cell cycle progression at M phase by promoting maximal cyclin B1 mRNA translation. The robust translation of cyclin B1 mRNA that results presumably offsets the anaphase-promoting complex-stimulated destruction of cyclin B1 protein at this time; reduced cyclin B1 levels are, of course, necessary for the M-phase to interphase transition. The same block in M-phase and vigorous cyclin B1 mRNA translation also occur when maskin synthesis is blocked by an antisense oligonucleotide (Groisman et al., 2002). Therefore, these results are entirely consistent with those presented in this study showing that cyclin B1 mRNA translation is regulated by the reversible association of maskin with eIF4E.
Poly(A)-binding proteins in development
Wakiyama et al. (2000) have shown that Xenopus oocytes injected with an mRNA encoding an altered form of eIF4GI that is unable to interact with PABP reduces translation of poly(A)-containing mRNA and inhibits oocyte maturation. Those authors, as well as Gray et al. (2000) who performed complementary experiments, concluded that PABP plays as essential a role in translation in Xenopus oocytes, through its interaction with eIF4G, as it does in yeast (Tarun and Sachs, 1996; Tarun et al., 1997; Wells et al., 1998). Our data agree with those of Wakiyama et al. (2000) and Gray et al. (2000), and further suggest that at least one function of the PABP–eIF4G complex is to out-compete maskin for binding to eIF4E.
While late stage Xenopus embryos contain an amount of S-PABP that seems roughly equivalent to that present in most somatic cells, oocytes contain virtually undetectable amounts of this protein (Zelus et al., 1989; Voeltz et al., 2001). However, they do contain an embryonic-specific PABP, termed E-PABP (Voeltz et al., 2001). E-PABP, whose developmental expression profile is reciprocal to that of S-PABP, was identified initially as a factor that interacts with the ARE, an AU-rich element that mediates stability of a variety of mRNAs. E-PABP was also found to interact with the poly(A) tail, and Voeltz et al. (2001) have suggested that it could be a functional substitute for S-PABP in oocytes. Our data indicate that this is indeed the case, because E-PABP not only restores progesterone-induced cyclin B synthesis in the presence of excess poly(A), but it also leads to maskin–eIF4E dissociation under similar circumstances. Thus, it seems likely that E-PABP, as well as S-PABP, would interact with eIF4G.
Our data also suggest a mechanism whereby mRNA deadenylation, which occurs in early Xenopus development, particularly for cyclin B1 mRNA during the M to S phase transition (Groisman et al., 2002), mediates translational repression. When the poly(A) tail is lost, PABP may dissociate from eIF4G, which in turn could destabilize the interaction of eIF4G with eIF4E. Because maskin remains bound to CPEB even during periods of deadenylation (Stebbins-Boaz et al., 1999), it would be in sufficiently close proximity to out-compete the now PABP-less eIF4G and re-associate with eIF4E to inhibit translation. Such a model for the reversible inhibition of translation by deadenylation is presently under investigation.
Materials and methods
Oocyte extracts and cap column
Oocytes, some of which were induced to mature with progesterone (1 µg/ml), were homogenized in buffer (one oocyte per 2 µl of buffer) (0.1 M KCl, 1 mM MgCl2, 50 mM Tris–HCl pH 7.5, 80 mM β-glycerophosphate and 10 µg/µl each of pepstatin, chymostatin and leupeptin) and centrifuged briefly in a microfuge (10 000 r.p.m., 10 min) to remove insoluble material. The supernantant was supplemented with 0.1 mM GTP to reduce non-specific adsorption and applied in batch to an 7mGTP–Sepharose (cap) column that had been equilibrated with the homogenization buffer plus bovine serum albumin (BSA; 0.1 mg/ml) and RNase A (2 µg/ml). Following mixing for 1 h at 4°C, the slurry was packed into a mini-column, washed extensively (100 bed volumes) with 0.1 M KCl in 0.05 M Tris pH 7.5, and the bound material eluted with SDS gel sample buffer. In some cases, the maskin–eIF4E complexes were eluted with free cap (5 mM). Generally, ∼10% of total oocyte eIF4E was retained on the column; the amounts of maskin, eIF4G and CPEB retained were about one-tenth that of eIF4E.
Western blotting and polyadenylation
Western blotting was performed as described by de Moor and Richter (1997). The primary antibodies were directed against maskin (Stebbins-Boaz et al., 1999), cyclin B1 (a gift from J.Maller, University of Colorado Medical School), Xenopus eIF4E (a gift from S.Morley, University of Sussex), PABP (a gift from D.Schoenberg, Ohio State University), Xenopus eIF4G (a gift from B.Keiper and R.Rhoads, Louisiana State University Medical School), MAP kinase (Santa Cruz Biotechnology), Mos (Santa Cruz Biotechnology) or Wee1 (Zymed). The secondary antibodies were horseradish peroxidase-conjugated goat anti-rabbit IgGs. The signals were detected by enhanced chemiluminescence. For some experiments, maskin antibody was pre-mixed with purified His-tagged E.coli-expressed maskin prior to oocyte injection and subsequent cyclin B1 analysis by western blotting.
Cyclin B1 RNA (3′-UTR) was synthesized in vitro with T7 RNA polymerase in the presence of [32P]UTP as described previously (Stebbins-Boaz et al., 1996). Following oocyte injection of the radio labeled RNA, total RNA was extracted and analyzed by denaturing PAGE and autoradiography (Stebbins-Boaz et al., 1996).
H1 kinase assays, which detect MPF activity, have been described previously (de Moor and Richter, 1997).
CAT assays
CAT reporter RNAs containing or lacking the EMCV IRES and containing or lacking three CPES have been described previously (de Moor and Richter, 1999). RNA injection, CAT assays and quantification have been described previously (de Moor and Richter, 1999).
Antibody and cyclin B1 protein injection
Maskin antibody was affinity purified (Stebbins-Boaz et al., 1996) and brought to a concentration of 5 µg/µl. An 80 nl aliquot of this affinity-purified antibody or an identical concentration of protein A–Sepharose-purified rabbit IgG was injected into each oocyte. The injected oocytes were incubated with progesterone until the control oocytes exhibited a white spot at the animal pole, which indicates GVBD and oocyte maturation. The oocytes were then homogenized and processed for western blotting or cap column chromatography.
Cyclin B1 protein variant Δ90, a non-destructible form of the protein (a gift from J.Ruderman, Harvard Medical School), was synthesized in and purified from E.coli. It was brought to a concentration of ∼1 µg/µl and injected into oocytes, some of which had previously been incubated overnight with 10 mM cordycepin. The oocytes were then homogenized and processed for western blotting or cap column chromatography.
Polynucleotide and poly(A)-binding protein injection and chromatography
Poly(A) and poly(C) were dissolved in water at concentrations ranging from 10 to 100 µM and injected into oocytes (80 nl/oocyte). The oocytes were then incubated with progesterone and the incidence of oocyte maturation was scored by the appearance of a white spot at the animal pole. Some oocytes were then processed for western blotting, or injected with an 80 nl solution containing 2.5 µg/µl purified His-tagged E.coli-expressed human somatic S-PABP (a gift from N.Sonenberg, McGill University) or Xenopus embryonic E-PABP (a gift of J.Steitz, Yale University). The oocytes were then processed for western blotting or cap column chromatography.
Oocytes were homogenized in 150 mM NaCl, 25 mM Tris–HCl pH 7.5, 0.2% NP-40 and 10 mM imidazole (5 µl of buffer per oocyte) and briefly centrifuged to remove insoluble material. The homogenate from 30 oocytes was applied to an Ni2+ column containing or lacking purified His-tagged PABP that had previously been equilibrated in the homogenization buffer. After binding, the column was washed with 100 bed volumes of buffer containing 20 mM imidazole and the bound material then eluted in SDS sample buffer.
The extract from some oocytes was mixed with ∼30 µM eIF4G-derived peptide (Xenopus residue 132-APKRERKTIRIRDPNQGGKDITEEIC; the terminal C residue is not a part of the Xenopus sequence), or an irrelevant peptide (DHKLRAQIDAAAKTATTGVC) and then applied to the PABP column or western blotted.
Acknowledgments
Acknowledgements
We are grateful to B.Keiper, J.Maller, S.Morley, R.Rhoads, J.Ruderman, D.Schoenberg, J.Steitz and N.Sonenberg for gifts of reagents. We also thank members of the Richter laboratory for their valuable input into the project and for comments on the manuscript. This work was supported by grants from the NIH.
References
- Andresson T. and Ruderman,J.V. (1998) The kinase Eg2 is a component of the Xenopus oocyte progesterone-activated signaling pathway. EMBO J., 17, 5627–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayaa M., Booth,R.A., Sheng,Y. and Liu,X.J. (2000) The classical progesterone receptor mediates Xenopus oocyte maturation through a nongenomic mechanism. Proc. Natl Acad. Sci. USA, 97, 12607–12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger A., Fox,C.A., Wahle,E. and Wickens,M. (1994) Nuclear poly adenylation factors recognize cytoplasmic polyadenylation elements. Genes Dev., 8, 1106–1116. [DOI] [PubMed] [Google Scholar]
- Cornelis S., Bruynooghe,Y., Denecker,G., Van Huffel,S., Tinton,S. and Beyaert,R. (2000) Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Mol. Cell, 5, 597–605. [DOI] [PubMed] [Google Scholar]
- de Moor C.H. and Richter,J.D. (1997) The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol. Cell. Biol., 17, 6419–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor C.H. and Richter,J.D. (1999) Cytoplasmic polyadenylation elements mediate masking and unmasking of cyclin B1 mRNA. EMBO J., 18, 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson K.S., Bilger,A., Ballantyne,S. and Wickens,M.P. (1999) The cleavage and polyadenylation specificity factor in Xenopus laevis oocytes is a cytoplasmic factor involved in regulated polyadenylation. Mol. Cell. Biol., 19, 5707–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadden P., Haystead,T.A. and Lawrence,J.C.,Jr (1997) Identification of phosphorylation sites in the translational regulator, PHAS-I, that are controlled by insulin and rapamycin in rat adipocytes. J. Biol. Chem., 272, 10240–10247. [DOI] [PubMed] [Google Scholar]
- Gingras A.C., Raught,B. and Sonenberg,S. (1999a) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem., 68, 913–963. [DOI] [PubMed] [Google Scholar]
- Gingras A.C., Gygi,S.P., Raught,B., Polakiewicz,R.D., Abraham,R.T., Hoekstra,M.F., Aebersold,R. and Sonenberg,N. (1999b) Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev., 13, 1422–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A.C., Raught,B. and Sonenberg,N. (2001) Regulation of translation initiation by FRAP/mTOR. Genes Dev., 15, 807–826. [DOI] [PubMed] [Google Scholar]
- Gray N.K., Coller,J.M., Dickson,K.S. and Wickens,M. (2000) Multiple portions of poly(A)-binding protein stimulate translation in vivo. EMBO J., 19, 4723–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman I., Huang,Y.S., Mendez,R., Cao,Q., Theurkauf,W. and Richter,J.D. (2000) CPEB, maskin and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell, 103, 435–447. [DOI] [PubMed] [Google Scholar]
- Groisman I., Jung,M.-Y., Sarkissian,M. and Richter,J.D. (2002) Translational control of the embryonic cell cycle. Cell, 109, 473–483. [DOI] [PubMed] [Google Scholar]
- Haghighat A., Mader,S., Pause,A. and Sonenberg,N. (1995) Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J., 14, 5701–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake L.E. and Richter,J.D. (1994) CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell, 79, 617–627. [DOI] [PubMed] [Google Scholar]
- Hake L.E., Mendez,R. and Richter,J.D. (1998) Specificity of RNA binding by CPEB: requirement for RNA recognition motifs and a novel zinc finger. Mol. Cell. Biol., 18, 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard E.L., Charlesworth,A., Welk,J. and MacNicol,A.M. (1999) The mitogen-activated protein kinase signaling pathway stimulates mos mRNA cytoplasmic polyadenylation during Xenopus oocyte maturation. Mol. Cell. Biol., 19, 1990–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge H. and Richter,J.D. (1995) Cytoplasmic 3′ poly(A) addition induces 5′ cap ribose methylation: implications for translational control of maternal mRNA. EMBO J., 14, 6301–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H., Tanguay,R.L., Balasta,M.L., Wei,C.C., Browning,K.S., Metz,A.M., Goss,D.J. and Gallie,D.R. (1997) Translation initiation factors eIF-iso4G and eIF-4B interact with the poly(A)-binding protein and increase its RNA binding activity. J. Biol. Chem., 272, 16247–16255. [DOI] [PubMed] [Google Scholar]
- Lin T.A., Kong,X., Haystead,T.A., Pause,A., Belsham,G., Sonenberg,N. and Lawrence,J.C.,Jr (1994) PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science, 266, 653–656. [DOI] [PubMed] [Google Scholar]
- Luca F.C., Shibuya,E.K., Dohrmann,C.E. and Ruderman,J.V. (1991) Both cyclin A Δ60 and B Δ97 are stable and arrest cells in M-phase, but only cyclin B Δ97 turns on cyclin destruction. EMBO J., 10, 4311–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotrigiano J., Gingras,A.C., Sonenberg,N. and Burley,S.K. (1999) Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol. Cell, 3, 707–716. [DOI] [PubMed] [Google Scholar]
- McGrew L.L., Dworkin-Rastl,E., Dworkin,M.B. and Richter,J.D. (1989) Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev., 3, 803–815. [DOI] [PubMed] [Google Scholar]
- Mendez R. and Richter,J.D. (2001) Translational control by CPEB: a means to an end. Nat. Rev. Mol. Cell. Biol., 2, 521–529. [DOI] [PubMed] [Google Scholar]
- Mendez R., Hake,L.E., Andresson,T., Littlepage,L.E., Ruderman,J.V. and Richter,J.D. (2000a) Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature, 404, 302–307. [DOI] [PubMed] [Google Scholar]
- Mendez R., Murthy,K.G., Ryan,K., Manley,J.L. and Richter,J.D. (2000b) Phosphorylation of CPEB by Eg2 mediates the recruitment of CPSF into an active cytoplasmic polyadenylation complex. Mol. Cell, 6, 1253–1259. [DOI] [PubMed] [Google Scholar]
- Mendez R., Barnard,D. and Richter,J.D. (2002) Differential mRNA translation and meiotic progression require cdc2-mediated CPEB destruction. EMBO J., 21, 1833–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo N., Yoshitome,S., Iwashita,J., Iida,M., Uto,K., Ueno,S., Okamoto,K. and Sagata,N. (2000) Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev., 14, 328–338. [PMC free article] [PubMed] [Google Scholar]
- Nebreda A.R. and Ferby,I. (2000) Regulation of the meiotic cell cycle in oocytes. Curr. Opin. Cell Biol., 12, 666–675. [DOI] [PubMed] [Google Scholar]
- Paris J., Swenson,K., Piwnica-Worms,H. and Richter,J.D. (1991) Maturation-specific polyadenylation: in vitro activation by p34cdc2 and phosphorylation of a 58-kD CPE-binding protein. Genes Dev., 5, 1697–1708. [DOI] [PubMed] [Google Scholar]
- Pause A., Belsham,G.J., Gingras,A.C., Donze,O., Lin,T.A., Lawrence,J.C.,Jr and Sonenberg,N. (1994) Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature, 371, 762–767. [DOI] [PubMed] [Google Scholar]
- Pyronnet S. and Sonenberg,N. (2001) Cell-cycle-dependent translational control. Curr. Opin. Genet. Dev., 11, 13–18. [DOI] [PubMed] [Google Scholar]
- Pyronnet S., Dostie,J. and Sonenberg,N. (2001) Suppression of cap-dependent translation in mitosis. Genes Dev., 15, 2083–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B., Gingras,A.C. and Sonenberg,N. (2000) Regulation of ribosomal recruitment in eukaryotes. In Mathews,M.B., Hershey,J. and Sonenberg,N. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 245–293.
- Richter J.D. (2000) The influence of polyadenylation-induced translation on metazoan development and neuronal synaptic function. In Mathews,M.B., Hershey,J. and Sonenberg,N. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 785–805.
- Sheets M.D., Fox,C.A., Hunt,T., Vande Woude,G. and Wickens,M. (1994) The 3′-untranslated regions of c-mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. Genes Dev., 8, 926–938. [DOI] [PubMed] [Google Scholar]
- Sheets M.D., Wu,M. and Wickens,M. (1995) Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature, 374, 511–516. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Cao,Q., de Moor,C.H., Mendez,R. and Richter,J.D. (1999) Maskin is a CPEB-associated factor that transiently interacts with elF4E. Mol. Cell, 4, 1017–1027. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Hake,L.E. and Richter,J.D. (1996) CPEB controls the cytoplasmic polyadenylation of cyclin, cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J., 15, 2582–2592. [PMC free article] [PubMed] [Google Scholar]
- Tarun S.Z. Jr and Sachs,A.B. (1996) Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J., 15, 7168–7177. [PMC free article] [PubMed] [Google Scholar]
- Tarun S.Z. Jr, Wells,S.E., Deardorff,J.A. and Sachs,A.B. (1997) Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc. Natl Acad. Sci. USA, 94, 9046–9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Kim,S., Heilig,E. and Ruderman,J.V. (2000) Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation. Proc. Natl Acad. Sci. USA, 97, 14358–14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J.D., Huarte,J., Belin,D., Gubler,P., Vassalli,A., O’Connell,M.L., Parton,L.A., Rickles,R.J. and Strickland,S. (1989) Regulated polyadenylation controls mRNA translation during meiotic maturation of mouse oocytes. Genes Dev., 3, 2163–2171. [DOI] [PubMed] [Google Scholar]
- Voeltz G.K., Ongkasuwan,J., Standart,N. and Steitz,J.A. (2001) A novel embryonic poly(A) binding protein, ePAB, regulates mRNA deadenylation in Xenopus egg extracts. Genes Dev., 15, 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Der Haar T., Ball,P.D. and McCarthy,J.E. (2000) Stabilization of eukaryotic initiation factor 4E binding to the mRNA 5′-cap by domains of eIF4G. J. Biol. Chem., 275, 30551–30555. [DOI] [PubMed] [Google Scholar]
- Wakiyama M., Imataka,H. and Sonenberg,N. (2000) Interaction of eIF4G with poly(A)-binding protein stimulates translation and is critical for Xenopus oocyte maturation. Curr. Biol., 10, 1147–1150. [DOI] [PubMed] [Google Scholar]
- Wells S.E., Hillner,P.E., Vale,R.D. and Sachs,A.B. (1998) Circular ization of mRNA by eukaryotic translation initiation factors. Mol. Cell, 2, 135–140. [DOI] [PubMed] [Google Scholar]
- Wickens M., Goodwin,E., Kimble,J., Strickland,S. and Hentze,M. (2000) Translational control of developmental decisions. In Mathews,M.B., Hershey,J. and Sonenberg,N. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 295–370.
- Yang D.Q. and Kastan,M.B. (2000) Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat. Cell Biol., 2, 893–898. [DOI] [PubMed] [Google Scholar]
- Zelus B.D., Giebelhaus,D.H., Eib,D.W., Kenner,K.A. and Moon,R.T. (1989) Expression of the poly(A)-binding protein during development of Xenopus laevis. Mol. Cell. Biol., 9, 2756–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]