Abstract
The filamentous fungus Neurospora crassa is a model organism for the genetic dissection of blue light photoreception and circadian rhythms. WHITE COLLAR-1 (WC-1) and WC-2 are considered necessary for all light responses, while FREQUENCY (FRQ) is required for light-regulated asexual development (conidia formation); without any of the three, self-sustained (circadian) rhythmicity in constant conditions fails. Here we show that light-regulated and self-sustained development occur in the individual or mutant white collar strains. These strains resemble wild type in their organization of the daily bout of light-regulated conidiation. Molecular profiles of light- induced genes indicate that the individual white collar-1 and white collar-2 mutants utilize distinct pathways, despite their similar appearance in all aspects. Titration of fluence rate also demonstrates different light sensitivities between the two strains. The data require the existence of an as-yet-unidentified photoreceptor. Furthermore, the extant circadian clock machinery in these mutant strains supports the notion that the circadian system in Neurospora involves components outside the WC–FRQ loop.
Keywords: circadian/frequency/light/Neurospora/white collar
Introduction
The most dependable of environmental signals that organisms receive in daily life are light and darkness, which come and go with great precision. Light supplies energy for photosynthesis, generates warmth for growth and metabolism, and enables vision. It also fulfills the complex signaling function in communicating time of day to an endogenous molecular network called the circadian system, or clock. A circadian system is recognizable by its continued ∼24-h rhythm after release into constant conditions (free-running rhythmicity). In a number of experimental systems, it appears that many, if not most, molecules, enzymes and processes are part of this oscillating, circadian timeline, all ultimately regulated by light. The features of the light environment (e.g. intensity or spectral composition) that are involved in this task are not well understood, and neither are the light-processing components at the cellular level. An excellent model system for defining both the circadian system and light input pathways is that of the simple, filamentous fungus Neurospora crassa. Insights into the molecular mechanisms of light and circadian regulation are parallel to those of more complex organisms.
Qualitatively, light responses in Neurospora appear to be limited to the blue and UV parts of the spectrum (De Fabo et al., 1976; West, 1976; Dharmananda, 1980; Linden et al., 1999). Quantitatively, conidium (asexual spore) formation is regulated by light levels that approximate moonlight, whereas light induction of carotenogenesis needs approximately two log orders more. This indicates a functional bifurcation in the Neurospora light input pathway that reflects either distinct photoreceptors or signaling pathways (Merrow et al., 2001). Numerous mutant screens for defects in light reception have revealed only two candidates for photoreceptors; WHITE COLLAR-1 (WC-1) and WC-2. Both mutants are blind for numerous photoresponses, including mycelial carotenoid production (Harding and Turner, 1981), sexual structure formation and their phototropism (Degli-Innocenti and Russo, 1984; Harding and Melles, 1984), gene expression (Sommer et al., 1989; Arpaia et al., 1993; Li and Schmidhauser, 1995; Crosthwaite et al., 1997; Collett et al., 2002), as well as temporal regulation of conidium (asexual spore) formation, following release from light into constant darkness (Russo, 1988), and overall levels of conidia (Lauter et al., 1997).
The WC proteins transduce the light signal, in part, by binding to light-regulated promoters and allowing transcription (Ballario et al., 1996; Linden and Macino, 1997). WC-1 and WC-2 form complexes with each other and with a third protein called FREQUENCY (FRQ) (Talora et al., 1999; Denault et al., 2001; Merrow et al., 2001), a critical component of the Neurospora circadian system. Evidence for functional links between light and circadian pathways is provided by the loss of circadian qualities (such as the ability to free-run) without either WC-1 or WC-2, on one hand, and the absence of light-regulated conidial band formation without FRQ on the other (Chang and Nakashima, 1997; Merrow et al., 1999; Lakin-Thomas and Brody, 2000). Similar features have been described in other organisms. In plants, the career photoreceptors Phy and Cry physically interact and also impact free-running circadian period (Ahmad and Cashmore, 1998; Somers et al., 1998). In cyanobacteria, SasA also modulates free-running rhythms (Iwasaki et al., 2000). Finally, in animals, the Per proteins control light-induced resetting of the clock (Albrecht et al., 2001). In Neurospora, circadian and light input pathway components are inextricably joined by function, by formation of a protein complex, and by interdependent regulation of expression (Crosthwaite et al., 1997; Lee et al., 2000; Merrow et al., 2001; Cheng et al., 2002). The blindness in conidiation of the FRQ-less strains is selective because frq mutants retain the ability to produce light-induced carotenoids. Again, a bifurcated light input pathway is defined, this time on the genetic level, with carotenogenesis and conidiation as readouts (Merrow et al., 2001).
Conidiation is regulated by the circadian system, as well as by environmental factors, including light. In constant darkness, conidia accumulate in discrete bands about once every 22 h, i.e. it is an output of a free-running circadian rhythm (Pittendrigh et al., 1959). The timing of this band formation in darkness is changed with the administration of light (a phase shift). Both the amount of phase shift (Dharmananda, 1980) and the amount of light-induced frq RNA expression (Crosthwaite et al., 1995) correlate with the amount of light delivered, suggesting a relationship between the two. In constant light, the consolidation of conidiation in discrete bands is absent, with conidia produced approximately continuously (Pittendrigh et al., 1959). In 24-h cycles of alternating light and dark, the conidia are produced within a precise temporal window (Chang and Nakashima, 1997; Merrow et al., 1999; Lakin-Thomas and Brody, 2000) that is systematically related to the cycle, as a phase-specific synchronization.
It is this last protocol that we have employed in order to characterize further light input components with respect to regulation of conidiation: assays that utilize release to constant conditions rely on a self-sustained rhythm to subsequently show an effect of light, whereas in the 24-h cycles, light-induced effects do not depend on an ongoing oscillation. In these light–dark (LD) cycles, we find that the wc mutants, individually or combined in the same strain, regulate conidiation in response to light. Investi gation of the underlying molecular events reveals that the two mutant strains have distinct gene activation profiles, despite their phenotypic similarity on all levels of light responsiveness or non-responsiveness. Fluence titration experiments reveal differential sensitivity to light between the mutants. The data support the existence of a photoreceptor that has not yet been identified in Neurospora. The description of a robust, light-regulated output in the white collar mutants is a critical step towards understanding molecular and genetic aspects of light input in Neurospora, and how they interact with the circadian system. To this end, once race tube conditions were optimized for LD cycles they were used to assay the wc mutants in free-running conditions, demonstrating a self-sustained rhythmicity. We conclude that although the FRQ and WC proteins have a large impact on the circadian and light input systems, their absence entails selective, not global, blindness and arhythmicity.
Results
The wc mutants synchronize conidiation in response to light
The wc mutants fail a panel of assays for light-induced physiological responses. However, on transfer from either constant light, or from brief light exposures, to constant darkness, it is possible to see subtle changes associated with the time of transfer, although no sustained effect of the transition is apparent (Ninneman, 1991; Crosthwaite et al., 1997). This result could either reflect blindness of wc mutant strains or it could derive from their failure to support an ongoing, self-sustained rhythm.
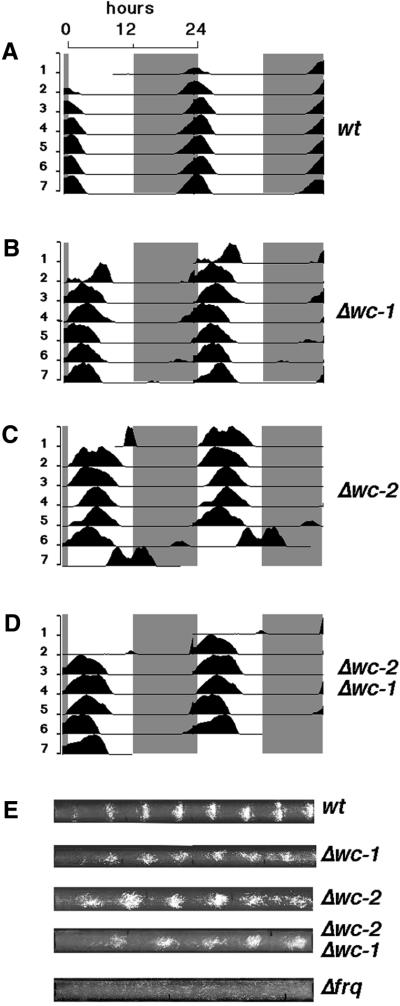
In the experiments described here, these possibilities were initially distinguished by submitting the wc mutants to 24-h LD cycles. In these protocols, a robust, light-regulated physiology is seen for both wc mutants, with conidia production consolidated to a discrete phase of the LD cycle, similar to the wild-type strain (Figure 1A–C and E); the frq mutant, as previously reported, shows no response to the same light cycles (Chang and Nakashima, 1997; Merrow et al., 1999; Lakin-Thomas and Brody, 2000). The phase of conidiation in the mutant strains is, however, delayed relative to wild type (by 2.8 h in Δwc-1 and by 3 h in Δwc-2).
Fig. 1. Conidiation in Δwc-1 and Δwc-2 is synchronized in LD cycles. Data from 1 week of 24-h LD cycles are double plotted (2 days per line, with the day on the right side of the graph repeated on the left side of the next line down). The dark period is indicated by gray shading. The peaks on the graphs represent consolidation of conidiation, and the corresponding Neurospora race tubes are shown in (E). (A) Wild type (wt); (B) Δwc-1; (C) Δwc-2; (D) Δwc-2 Δwc-1; and (E) race tubes for the above graphs and also for Δfrq (only a race tube is shown for this strain, as it fails to synchronize conidiation to light) (Chang and Nakashima, 1997; Lakin-Thomas and Brody, 2000; Merrow et al., 2001). Fluence rates for (A–C) and (D) and (E) are 4 and 35 µE/m2/s, respectively. The race tube shown for wild type runs for longer than the 7 days shown on the graph; the loss of synchrony seen in the last day of graph (C) reflects early removal of the race tubes from experimental conditions.
Nutrition has been shown to play a role in light reception (Nakashima and Fujimura, 1982; Sokolovsky et al., 1992) and clock mutants do not have the nutritional compensation that wild-type strains have (Loros and Feldman, 1986). Therefore, several media formulations were compared for appearance of conidial bands in the mutant strains. Media containing either glucose, quinic acid (a carbon source encountered in nature by Neurospora) or no carbon source were tested. Although responses to light are clearly seen in cycles run under all three conditions (data not shown), the bands were most discrete on media with quinic acid or with no carbon source. Titration experiments demonstrate that lower glucose levels decrease background conidiation and, thus, enhance conidial band appearance without necessarily increasing light sensitivity. The bands produced by the mutant strains increase in density over the course of the week-long race tube experiment, similar to what is seen for the free-running rhythm in FRQ-less strains (Loros et al., 1986). Thus, long race tubes were used to improve data acquisition possibilities.
The double mutant Δwc-2 Δwc-1 is synchronized by light
Δwc-1 is blind for most of the same outputs as Δwc-2. Several scenarios accommodate these observations, including the possibility that one or both protein products act as photoreceptors. Given the observation that either of the single mutants can synchronize conidiation to light, a double mutant, Δwc-2 Δwc-1, was generated and submitted to 24-h LD cycles. Like the single mutants, the double mutant strain consolidates conidia production in the light phase (Figure 1D and E), with a phase similar to the single mutants (delayed by 2.8 h relative to wild type).
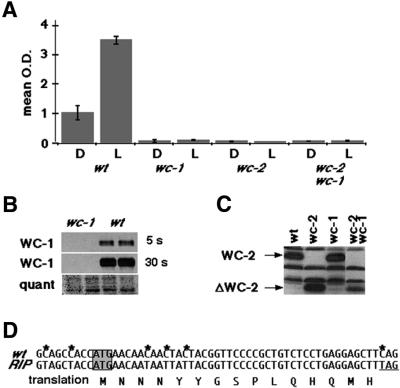
Given the robustness of the unpredicted light-regulated conidiation in the Δwc-1 and Δwc-2 mutants, verification of strain identity was essential. Light-induced mycelial carotenogenesis was used as a functional assay for the WC proteins. As described previously (Harding and Turner, 1981), wc mutants fail to generate carotenoids in mycelial tissue both in constant darkness and in response to light (Figure 2A), whereas the wild-type strain responds strongly to light, increasing basal mycelial carotenoids by >3-fold.
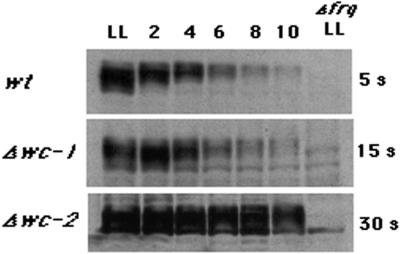
Fig. 2. Verification of the wc mutant strains. (A) Light-induced mycelial carotenogenesis fails in the single and double mutant strains. The optical density (OD) values were corrected for dilution in the case of the wild-type strain. (B) Western blot for WC-1 protein shows no detectable protein in the Δwc-1 RIP mutant (two left lanes) compared with wild type (two right lanes, wt). The top panel is a 5 s exposure and the middle panel is overexposed (30 s). The blot was stained with Ponceau S for quantitation purposes (bottom panel). (C) Western blot for WC-2 protein, with arrows on the left showing the full-length protein in comparison with the truncated version (ΔWC-2) in this mutant strain (Linden and Macino, 1997). The background bands indicate even loading of the wells. (D) The area surrounding the putative translation start site (gray box) for wc-1 was cloned and sequenced from the Δwc-1 strain. The sequence in the top line corresponds to the wild-type wc-1 DNA sequence, and the mutant sequence is immediately below it. Asterisks indicate the location of point mutations, and the last row of sequence corresponds to the putative translation of the sequence to protein, which reflects silent mutations until the stop codon.
The mutant strains were evaluated for the presence of either WC-1 or WC-2 protein. The Δwc-1 strain is a ‘RIP’ mutant (Talora et al., 1999), generated by methylation and subsequent mutation of a gene when two copies are present (Selker and Garrett, 1988). In this case, the promoter region is also RIPed, resulting in no detectable RNA (L.Franchi, personal communication; data not shown). No WC-1 protein was found in the mutant strain (Figure 2B). As a further molecular verification of strain identity, the 5′ end of the wc-1 gene from the Δwc-1 strain was cloned and sequenced (Figure 2D). The results show that in the event that any RNA is produced from the RIPed promoter, translation would stop after 15 amino acids. Numerous additional stop codons follow (data not shown). The Δwc-2 mutant produces a truncated WC-2 protein, lacking the zinc finger (Linden and Macino, 1997). The results shown in Figure 2C confirm the absence of a full-length WC-2 protein in the Δwc-2 mutant, while the truncated, smaller form is detectable. Given the presence of a truncated WC-2 protein, and despite the lack of light-induced carotenoids, the light response in the Δwc-2 mutant could be due to residual activity deriving from the N-terminus of the protein. To confirm that the light-induced behavior of the Δwc-2 mutant does not derive from this protein fragment, a complete wc-2 knockout from an independent source (Collett et al., 2002) was tested in the same experiments and found to regulate conidiation according to the LD cycle (data not shown).
We verified the double mutant strain in several ways: namely, failure to produce light-induced carotenoids (Figure 2A), lack of WC-1 protein (data not shown) and production of truncated WC-2 protein (Figure 2C). Finally, we tested siblings from the same cross in light cycles. Of six double mutants, all isolates synchronized. Taken together, these observations verify the identity of the mutant strains and confirm that robust, light-regulated conidial band formation occurs in strains that completely fail to produce carotenoids in response to light. Neither WC-1 nor WC-2 is essential for regulation of conidiation by light.
Light-induced RNA and protein in Δwc-1, but not in Δwc-2
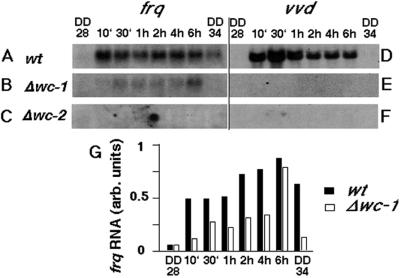
Several molecular events have been described with respect to the regulation of conidiation by light (Corrochano et al., 1995; Crosthwaite et al., 1995; Li and Schmidhauser, 1995; Heintzen et al., 2001). These include rapid induction of frq and vvd RNA and protein (see Figure 3A and D, respectively). To elucidate the molecular pathways involved in light-induced conidial band formation, these outputs were investigated in the mutant strains. In the Δwc-1 mutant, frq RNA is induced within 10 min of light exposure and remains elevated for the entire 6 h of light incubation (Figure 3B and G). The kinetics of induction are, in general, similar to those seen for wild type (Figure 3A and G). The low basal levels of frq RNA in the Δwc-1 strain are especially apparent at the DD34 time point, a time at which the circadian system in the wild-type strain is promoting increased frq RNA levels. This is consistent with previous reports and reflects the co-dependent regulation of these molecules by each other (Crosthwaite et al., 1997; Lee et al., 2000; Merrow et al., 2001). By the end of the 6 h light incubation, the amount of frq RNA in the Δwc-1 and wild-type strains is approximately equal, indicating that over the course of an extended incubation in light, frq RNA levels in Δwc-1 can approach those in the wild-type strain.
Fig. 3. Light-induced RNAs determined by northern blot analysis. (A–C) frq RNA levels; (D–F) vvd RNA levels. (A) and (D) are samples from wild-type cultures; (B) and (E) are from Δwc-1; and (C) and (F) are from Δwc-2, where the panels are overexposed. Loading of RNA was controlled by probing for rRNA (not shown), and these values were used for quantification. (G) Quantification of frq RNA induction as shown in (A) and (B). The harvest times are indicated, up to 6 h after lights-on, with DD28 (DD indicates constant darkness, e.g. 28 h) and DD34 representing the dark controls harvested at the beginning and the end of the light exposure.
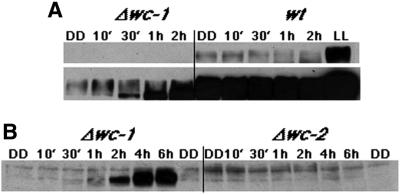
The kinetics of light-induced FRQ protein production in Δwc-1 (Figure 4A) again parallels the wild-type strain, first appearing within 30 min, with substantial amounts accumulating within 2 h (Figure 4). The qualitative aspects of FRQ protein induction are also similar when the two strains are compared: the high molecular weight form that is prevalent at DD28 is replaced after 2 h of light with newly made, low molecular weight (poorly phosphorylated) protein.
Fig. 4. Light-induced FRQ protein. (A) FRQ in Δwc-1 and wild-type protein extracts in short (top panel, 1 s) and long (bottom panel, 30 s) exposures of western blots to autoradiography film. Control samples at the left of both the wild-type (wt) and Δwc-1 series were harvested at DD28; constant light (LL) is from wild-type mycelia that were grown in constant light. Harvest times are indicated at the top, starting from lights-on at DD28. (B) FRQ protein is induced following light exposure in the Δwc-1 but not the Δwc-2 strain, as shown here in an extended film exposure (30 s). Control samples at the left and right of both Δwc-1 and Δwc-2 series were harvested at DD28 and DD34, the beginning and the end of each light incubation. Harvest times are indicated at the top, starting from lights-on at DD28.
In contrast to the results with the Δwc-1 strain, neither frq RNA (Figure 3C) nor FRQ protein (Figure 4B) is induced by light in the Δwc-2 strain (Collett et al., 2002). Neither of the wc mutant strains yielded light-induced vvd RNA (Figure 3C and F), consistent with previous reports of the failure of light-induced gene expression in the wc mutants (Sommer et al., 1989; Arpaia et al., 1993; Li and Schmidhauser, 1995). Thus, frq induction in Δwc-1 defines distinct signal transduction pathways.
We have previously demonstrated that merely the presence of FRQ, not its acute induction, is required for regulation of conidiation by light (Merrow et al., 2001). In most wc-2 mutants, low levels of frq and FRQ are observed (Crosthwaite et al., 1997; Merrow et al., 2001; Collett et al., 2002), a pre-condition that apparently allows the light-regulated conidiation shown here (Figure 1C and D). But there might be quantitative or qualitative changes in FRQ that occur over an extended light incubation, e.g. in the 12 h of light used in these cycles that facilitate band formation versus the 6 h of light used in the light induction experiments of Figures 3 and 4. To investigate this possibility, FRQ protein was evaluated in mycelia from cultures that were transferred to darkness after an extended light incubation (∼44 h; Figure 5). In both Δwc-1 and wild type, FRQ accumulates to relatively high levels in constant light and degrades away in darkness until either an endogenous or an exogenous signal re-induces its production. In the Δwc-2 mutant, no quantitative or qualitative change in FRQ levels is seen. Thus, the regulation of conidiation by light, while dependent on FRQ, does not require its apparent modification.
Fig. 5. FRQ protein levels following light-to-dark transfer. Mycelia were grown in LL and then harvested for up to 10 h (indicated at top) following release into DD; proteins were extracted for western blotting. The wild-type (wt), Δwc-1 and Δwc-2 strains are compared for FRQ protein. Exposure times are shown on the right. Control samples were Δfrq grown in LL and are in the right lanes of all three panels. The difference in appearance of background bands derives from alternative development protocols, which were used on the bottom panel to decrease background levels for long exposures.
Fluence titration of light-induced conidiation
The molecular findings (Figures 34–5) provide evidence for distinct light transduction pathways in the wc-1 and wc-2 mutants, although the phase of conidiation in LD cycles is similar (Figure 1B and C). Significantly, the differences in the two strains is FRQ expression: there are no apparent changes in FRQ in the wc-2 mutant strain, despite a robust, light-regulated conidiation. This contrasts with previous observations that seem to directly link frq induction to phase shifts (Crosthwaite et al., 1997), and the frq allele to the timing of conidial band formation (Chang and Nakashima, 1997; Merrow et al., 1999). Thus, with different degrees of light-induced FRQ expression one might expect the phase of conidiation to be different in wc-1 and wc-2 strains; however, this prediction fails.
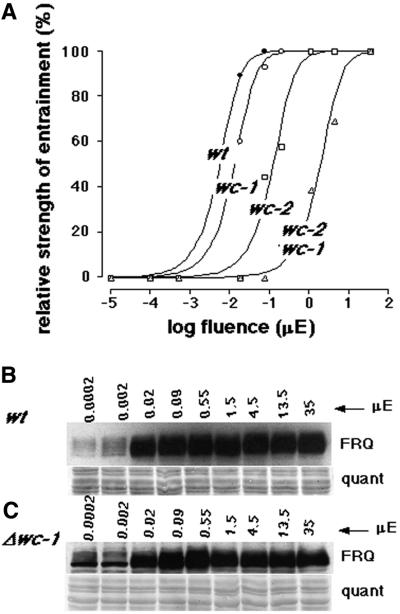
We used titration of fluence rate to determine whether the mutants are using the same pathway to synchronize conidiation (i.e. independent of FRQ in both cases). The fluence rate of light in the 24-h LD cycles was titrated in an attempt to functionally dissect the pathways. The fluence threshold for 50% synchronization of the wild-type strain (Figure 6A) is in good agreement with previously published data (Merrow et al., 1999). The Δwc-1 mutant is also efficiently synchronized, although it requires about twice as much light as wild type. Synchronization of the Δwc-2 mutant required 20-fold more light than wild type, while the double mutant, Δwc-2 Δwc-1, is ∼200 times less light sensitive than wild type. The large discrepancy in light sensitivity for synchronization of conidiation in the two single mutants indicates that the strains use distinct molecular mechanisms, to some extent.
Fig. 6. Fluence titration of conidial band synchronization by light. (A) Race tubes were held in 24-h LD cycles with graded fluence rates. The relative strength of synchronization (see Materials and methods) for different fluence rates is plotted for each strain. (B and C) FRQ induction was determined following a 4-h light incubation, beginning at DD28. The fluence rate was titrated, and wild-type (B, wt) and Δwc-1 (C) strains are compared.
The fluence titration for light induction of molecular components was also determined, using FRQ protein as a readout in the wild-type and Δwc-1 mutant strains (Figure 6B and C). FRQ induction is seen in wild type, with as little as 0.002 µE of light/m2/s, and in both strains almost full induction occurs with 0.02 µE/m2/s. Sensitivity of light induction of FRQ in both wild type and Δwc-1 correlates well with synchronization of conidiation by light, e.g. the sensitivity is similar, with a minor deficit in the mutant strain.
A self-sustained circadian rhythm in the wc mutants
Optimization of the race tube assay for light-regulated conidiation in the wc strains enhanced band appearance remarkably. In experiments using 24-h LD cycles, both circadian timing as well as direct light responses can contribute to the daily conidiation events. Although the wc mutants were repeatedly shown to be non-rhythmic (Russo, 1988; Ninneman, 1991; Crosthwaite et al., 1997), we investigated whether the combination of media and long race tubes can reveal self-sustained rhythmicity in these clock mutants. The null frq mutants are rhythmic, but only on long race tubes (Loros et al., 1986; Aronson et al., 1994a). When the wc mutants were grown in constant darkness without glucose and on long race tubes, they developed a self-sustained rhythm in conidia formation (Figure 7). Under these conditions, the period is longer than in wild type. Specifically, the tubes from a single experiment yielded periods of 21.5, 22.2, 23.3 and 29.3 h for Δwc-1, with standard deviations (SDs) of 1.6, 2.8, 2.6 and 2.4 h, respectively. In comparison, two wild-type control race tubes had periods of 22.9 ± 1.2 and 22.8 ± 1.7 h. The race tubes for Δwc-2 had periods of 24.8, 30.5, 33.8 and 34.7 h with SDs of 1.3, 5.7, 0.5 and 1.2 h, respectively. In general, the mutant race tubes had three to four conidial bands used for these calculations, while the wild type had seven to eight. These data reveal only a partial loss of circadian function in the wc mutants; while self-sustainment is still intact, precision appears to be lost.
Fig. 7. A self-sustained, ∼24-h rhythm of conidiation in the wc mutants. Race tubes incubated in constant darkness also consolidate conidia to a discrete time of day, indicating a remaining clock machinery.
Discussion
Neurospora is photoreceptive without both WC proteins
The wc mutants were identified in screens for defects in light reception (Harding and Turner, 1981; Degli-Innocenti and Russo, 1984). They have repeatedly been found to be non-responsive to light, leading to the conclusion that they are blind (Sommer et al., 1989; Ballario et al., 1996; Linden and Macino, 1997; Cheng et al., 2001b; Schwerdtfeger and Linden, 2001). Here, we report that the wc mutants, individually or when combined as a double mutant, readily synchronize conidial band formation at light levels that are far below those encountered in natural daytime conditions (Figures 1 and 6). The key to this observation was the use of entrainment protocols (24-h LD cycles). Previous experiments examining the effects of light on conidiation in the wc mutants (e.g. Lauter et al., 1997) used only constant light or constant darkness in combination with media containing high levels of carbon source. In other experiments, a light pulse delivered in otherwise constant darkness revealed a response to light that could not be evaluated because conditions did not favor self-sustained rhythms (Ninneman, 1991; Crosthwaite et al., 1997). The entrainment protocols used here clearly demonstrate a novel light input pathway in Neurospora.
A second deficiency reported for the wc strains is their lack of circadian rhythmicity (Crosthwaite et al., 1997). However, given that other clock mutants, at the frq locus, are rhythmic under restricted conditions (Loros et al., 1986; Aronson et al., 1994a), we tested the wc mutants for self-sustained rhythmicity with the media and race tubes used in the cycle protocols. In constant darkness, both wc-1 and wc-2 mutants exhibit a self-sustained circadian rhythm. As reported for the frq mutants, the rhythmicity lacks circadian qualities, in this case precision, and the period tends to be longer than wild type. This observation is helpful in modeling the circadian system; there is a circadian machinery beyond the frq/wc transcription– translation feedback loop that oscillates with a circadian period. The frq/wc feedback loop appends certain qualities (Roenneberg and Merrow, 1998).
What are the photoreceptors in Neurospora?
Current candidates for the blue-light photoreceptor in Neurospora are few, namely WC-1 and WC-2. They are both transcription factors with PAS and/or LOV (light, oxygen or voltage) domains (Ballario et al., 1996; Linden and Macino, 1997), features which are sometimes involved in heterodimerization, but have also been implicated in binding a flavin as a blue-light chromophore (Christie et al., 1999). The LOV domain in WC-1 is similar to that of NPH1 (a plant photoreceptor), and mutations therein result in blindness for light-induced mycelial carotenogenesis (Ballario et al., 1998). Our results do not eliminate either WC protein as a photoreceptor candidate, but rather introduce the possibility of a third one. Based on functional comparisons with the WC proteins, FRQ might be proposed for this role. This is unlikely, however, given that FRQ has no signature sequences that indicate photoreceptor function (McClung et al., 1989). Its biochemical characteristics include phosphorylation by a CAM kinase or casein kinase I ε species (Goerl et al., 2001; Yang et al., 2001), the timed appearance and degradation in the context of circadian rhythms (Garceau et al., 1997), and the negative feedback effect on self-transcription (Aronson et al., 1994b) via an unknown mechanism. The FRQ sequence features a coiled-coil domain, which mediates binding to self and to the WC proteins (Cheng et al., 2001a), thereby forming a protein complex (Denault et al., 2001; Merrow et al., 2001) which is central to transcriptional responses following photoreception (Linden et al., 1999). Given this information, we favor a model that calls for an as-yet-unidentified photoreceptor. Multiple photoreceptors in Neurospora have been suggested (Dharmananda, 1980) based on multiple peaks and discrepancies of action spectra when using different read-outs, namely phase shifting versus light-induced carotenogenesis (De Fabo et al., 1976).
Molecular complexity of light signal transduction
Regardless of whether either of the WC proteins is a photoreceptor or not, they are necessary for a subset of light signal transduction (e.g. carotenogenesis), and FRQ is necessary for another (e.g. conidiation). These two outputs have vastly different light sensitivities, supporting the existence of a branched light input pathway (Merrow et al., 2001). Other evidence for multiple light input pathways is seen in the complete absence of light-induced carotenogenesis in wc-2 mutant strains that are still capable of light-induced gene expression (Degli-Innocenti and Russo, 1984; Collett et al., 2002) or phosphorylation of WC-1 (Talora et al., 1999). The Δwc-2 strain used in these studies is not light induced for gene expression or for carotenogenesis.
Here, we continue to define genetically the branched light input pathways by demonstrating that light-regulated conidiation persists in the otherwise blind wc mutant strains. We also find that within the conidiation branch there are differences between the Δwc-1 and Δwc-2 strains in light-induced gene expression. In contrast to previous work (Crosthwaite et al., 1997), we find that frq RNA and protein are induced in the Δwc-1 mutant (Figures 3 and 4). However, the protein is not induced, nor is it modified, in Δwc-2 (Collett et al., 2002). So, while FRQ protein is essential for light-regulated conidiation (Chang and Nakashima, 1997; Merrow et al., 1999; Lakin-Thomas and Brody, 2000), its acute induction (Crosthwaite et al., 1995; Collett et al., 2002), its cycling (as for its role in the circadian clock) and its phosphorylation (Garceau et al., 1997) are not.
Unlike light induction of frq, vvd requires WC-1 (Figure 3)—a twig in the branched system that suggests VVD is part of the carotenogenesis branch, which it presumably shares with the panel of other light-induced genes whose expression fails in wc-1 mutants (Sommer et al., 1989; Arpaia et al., 1993; Li and Schmidhauser, 1995). The most conspicuous feature of a vvd mutation is the abnormally high carotenoid accumulation, but it also modulates the effects on light input to the circadian system. These effects are probably mediated by disrupting adaptation of the response under prolonged light exposure (Heintzen et al., 2001; Schwerdtfeger and Linden, 2001; Shrode et al., 2001). Functionally, WC-2 appears to act before the vvd/frq bifurcation because it is required for light-induced expression of both genes (see Figure 8).
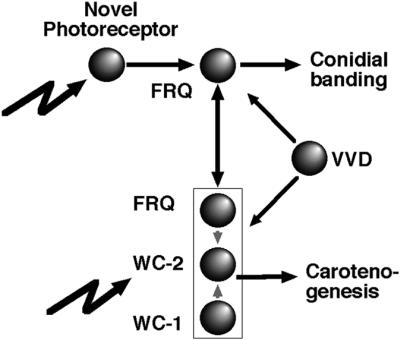
Fig. 8. Genetic evidence for a novel photoreceptor in Neurospora. Carotenogenesis and conidiation represent two light- and circadian-regulated outputs. Carotenogenesis requires both WC proteins, while regulation of conidiation by light fails without FRQ. All three proteins are part of a complex that modulates their gene expression (not indicated here for reasons of clarity) (Crosthwaite et al., 1997; Lee et al., 2000; Goerl et al., 2001; Merrow et al., 2001; Cheng et al., 2002). The diagram is not intended to summarize the known inter-regulatory effects, but rather the defined light signaling pathways. Symbols: filled circles, protein products; lightning, exogenous light; rectangle, protein complex formation.
Circadian modulation of light responses: response strength versus sensitivity
A circadian modulation of the light input to the circadian clock has been reported in several systems (Roenneberg and Merrow, 2000). In Neurospora, the time-of-day differences in response strength of light-induced gene expression and carotenogenesis (Heintzen et al., 2001; Merrow et al., 2001) could be attributed to the regulation of WC-1 levels by FRQ, presuming WC-1 is a photoreceptor. The response strength of light-induced carotenogenesis is greater with FRQ than without, while the fluence rate threshold is unchanged in either case, i.e. there is no sensitivity defect, consistent with quantitative rather than qualitative changes in the photoreceptor. Thus, FRQ functions as a response amplifier without changing sensitivity. In contrast, in the case of conidiation, fluence titrations reveal sensitivity defects in the WC mutants (Figure 6). Such an observation indicates that photoreception is modified uniquely in the two wc mutant strains. In the wild-type strain then, photoreception for conidiation is a composite of FRQ, WC-1, WC-2 and the function of a novel photoreceptor. That FRQ participates in this process is suggested here by the fact that the wc-1 strain approaches wild-type sensitivity, and it also can generate ∼50% of FRQ protein levels relative to the wild type in constant light (Figures 4 and 5). In contrast, the strains with the lowest sensitivities (Δwc-2 and Δwc-1 Δwc-2) have the least FRQ (∼5% of wild-type levels). Given that WC-1 and FRQ levels change over the course of the day, the circadian modulation in light input may impact both sensitivity (fluence thresholds) and response strength.
Double vision in Neurospora
The basic observation that at least one light-regulated phenotype is intact in the wc mutants re-forms our concepts of photoreception in Neurospora. We are left with many new questions and some new tools with which to attack them. First, what is the photoreceptor that regulates the conidiation pathway? To find its gene, the double mutant strain is an excellent candidate for mutagenesis and subsequent screening in LD cycles. Secondly, does circadian modulation of light input impact sensitivity of light reception, as proposed? To address this question, high density fluence titration curves have to be constructed throughout the circadian cycle. Thirdly, which aspects of asexual development are regulated by light via FRQ, WC-1 or WC-2? Profiling with microarrays should reveal unique sets of genes that are light induced in each of the mutant strains. This information will supplement the known events and gene expression profiles that have been described previously (Sachs and Yanofsky, 1991; Madi et al., 1994). It is also possible that the wc mutants regulate conidiation via metabolic (non-transcriptional) mechanisms. Finally, given that conidiation in the wc mutants exhibits self-sustained rhythmicity in constant darkness which can be synchronized by light, what additional molecular components are responsible for these residual circadian qualities? Mutagenesis of double mutants and screening in appropriate conditions will reveal additional circadian clock components.
Materials and methods
Strains
The strain referred to as wild type is the standard laboratory strain used for circadian experiments, and for much work on light reception in Neurospora. It harbors a single mutation called band (bd), which results in daily consolidation of conidium formation (Sargent et al., 1956). The Δwc-1 strain was obtained by crossing the RIP mutant (Talora et al., 1999) onto a bd background. The Δwc-2 strain used here (234w, FGSC 3187; Fungal Genetics Stock Center, Kansas City, KS) is a point mutant that results in production of a truncated protein (Linden and Macino, 1997), and was also crossed to obtain wc-2 234w;bd (called Δwc-2). All results for Δwc-2 were confirmed using a complete knockout strain (Collett et al., 2002). The double mutant wc-2 234w;bd;wc-1 RIP (called Δwc-1 Δwc-2 in this work) was obtained by crossing the bd;Δwc-1 RIP and Δwc-2;bd strains.
Race tube experiments and data analysis
‘Race tubes’ (40 cm long, 1.2 cm in diameter) permit visualization of linear growth for ∼1 week and are thus ideal for experiments aimed at describing timed developmental events. Race tube medium for these experiments is 1× Vogels salts (Vogel, 1964), 2% agar, 0.5% arginine and 10 ng/ml biotin (i.e. no glucose added, resulting in clearer band formation in the mutant strains). LD cycles were started with inoculation of race tubes, incubation for 1 day in constant light, and subsequent transfer to cycles of 12 h light and 12 h darkness (24-h LD cycles). Experiments ended when the growth reached the far end of the tube (>1 week). Light from cool white fluorescent bulbs was used (Osram, Germany); fluence rates are 4 µE/m2/s, except where indicated (Figures 1 and 6). All experiments were strictly controlled and permanently recorded for temperature maintenance at 25°C to exclude temperature effects. For experiments in constant conditions, tubes were germinated, as above, overnight in constant light and then transferred to constant darkness, with growth rate marked under dim red light. Race tubes were marked regularly to facilitate correlation of growth and conidial development with elapsed time, as determined with the CHRONO program (Roenneberg and Taylor, 2000).
Fluence titration experiments were analyzed by averaging the race tube data for each strain at each fluence rate (per fluence, two race tubes for the wild type, and four to six race tubes for each mutant; all race tubes were used, i.e. no selection was made). The degree of light-regulated synchronization of conidiation was assessed by periodogram analysis (Sokolove and Bushell, 1978) for each averaged time series using CHRONO (Roenneberg and Taylor, 2000). Results were expressed as percentage of the highest Qp value for a given strain. Fluence response curves were fitted as described in Merrow et al. (2001).
Molecular analyses
Liquid cultures were used for evaluation of light-induced molecular components. Mycelia were grown in liquid medium by inoculating conidia from 7–10 day cultures into the same medium described for race tubes, but with 2% glucose added and agar omitted. Petri dishes were inoculated with 105 conidia/ml, allowed to germinate in constant light for 16 h, then transferred to darkness at 25°C until a light incubation was initiated at DD28. The pads were harvested either at the indicated times or after 4 h in light (fluence titration).
RNA and protein were prepared and processed for northern and western analysis as described previously (Goerl et al., 2001; Merrow et al., 2001). SDS–PAGE gels of 5, 7.5 and 10% polyacrylamide were used for resolution of WC-1, FRQ and WC-2, respectively. For detection of WC-1 and FRQ, monoclonal antibodies were used, and for detection of WC-2, affinity purified polyclonal anti-WC-2 was used.
Light-induced mycelial carotenogenesis in disk cultures was measured as described previously (Merrow et al., 2001).
Acknowledgments
Acknowledgements
We thank Vera Schiewe and Astrid Bauer for their support, Moyra Mason for technical help, G.Macino, L.Franchi, H.Prokisch and W.Neupert for critical comments on the manuscript, and J.Loros and M.Collett for sending a wc-2 knockout strain prior to its description in a publication. We acknowledge the fine craftsmanship of Helmut Klaussner, on whom we depend for our demanding and complicated infrastructure. This work was supported by the Deutsche Forschungsgemeinchaft, the Friedrich-Bauer- and Meyer-Struckmann-Stiftungs, and the Eppendorf Company, Hamburg.
Note added in proof
Since acceptance of this manuscript, Professor Yi Liu has informed us that a wc-1 point mutant can reinitiate translation. We thank him for sending us a complete wc-1 RIP strain. With this strain we confirmed entrainment by light cycles in the absence of WC-1. Professor J.Loros has also suggested that the ‘Δ’ symbol should only be used for knockout alleles constructed by complete ORF replacement. We regret any confusion, and refer the reader to the Materials and methods and Results sections, as well as Figure 2, for a complete description of strains.
References
- Ahmad M. and Cashmore,A.R. (1998) The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell, 1, 939–948. [DOI] [PubMed] [Google Scholar]
- Albrecht U., Zheng,B., Larkin,D., Sun,Z.S. and Lee,C.C. (2001) mPer1 and mPer2 are essential for normal resetting of the circadian clock. J. Biol. Rhythms, 16, 100–104. [DOI] [PubMed] [Google Scholar]
- Aronson B.D., Johnson,K.A. and Dunlap,J.C. (1994a) The circadian clock locus frequency: a single ORF defines period length and temperature compensation. Proc. Natl Acad. Sci. USA, 91, 7683–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson B.D., Johnson,K.A., Loros,J.J. and Dunlap,J.C. (1994b) Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science, 263, 1578–1584. [DOI] [PubMed] [Google Scholar]
- Arpaia G., Loros,J.J., Dunlap,J.C., Morelli,G. and Macino,G. (1993) The interplay of light and the circadian clock. Plant Physiol., 102, 1299–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballario P., Vittorioso,P., Magrelli,A., Talora,C., Cabibbo,A. and Macino,G. (1996) White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J., 15, 1650–1657. [PMC free article] [PubMed] [Google Scholar]
- Ballario P., Talora,C., Galli,D., Linden,H. and Macino,G. (1998) Roles in dimerization and blue light photoresponse of the PAS and LOV domains of Neurospora crassa white collar proteins. Mol. Microbiol., 29, 719–729. [DOI] [PubMed] [Google Scholar]
- Chang B. and Nakashima,H. (1997) Effects of light dark cycles on the circadian conidiation rhythm in Neurospora crassa. J. Plant Res., 110, 449–453. [Google Scholar]
- Cheng P., Yang,Y., Heintzen,C. and Liu,Y. (2001a) Coiled-coil domain mediated FRQ–FRQ interaction is essential for its circadian clock function in Neurospora. EMBO J., 20, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P., Yang,Y. and Liu,Y. (2001b) Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc. Natl Acad. Sci. USA, 98, 7408–7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P., Yang,Y., Gardner,K.H. and Liu,Y. (2002) PAS domain-mediated WC-1/WC-2 interaction is essential for maintaining the steady-state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora. Mol. Cell. Biol., 22, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie J.M., Salomon,M., Nozue,K., Wada,M. and Briggs,W.R. (1999) LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc. Natl Acad. Sci. USA, 96, 8779–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M.A., Garceau,N., Dunlap,J.C. and Loros,J.J. (2002) Light and clock expression of the Neurospora clock gene frequency is differentially driven by but not dependent on WHITE COLLAR-2. Genetics, 160, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrochano L.M., Lauter,F.R., Ebbole,D.J. and Yanofsky,C. (1995) Light and developmental regulation of the gene con-10 of Neurospora crassa. Dev. Biol., 167, 190–200. [DOI] [PubMed] [Google Scholar]
- Crosthwaite S.K., Loros,J.J. and Dunlap,J.C. (1995) Light-induced resetting of a circadian clock is mediated by a rapid increase in frequency transcript. Cell, 81, 1003–1012. [DOI] [PubMed] [Google Scholar]
- Crosthwaite S.K., Dunlap,J.C. and Loros,J.J. (1997) Neurospora wc-1 and wc-2: transcription, photoresponses and the origin of circadian rhythmicity. Science, 276, 763–769. [DOI] [PubMed] [Google Scholar]
- De Fabo E.C., Harding,R.W. and Shropshire,W.,Jr (1976) Action spectrum between 260 and 800 nanometers for the photoinduction of carotenoid biosynthesis in Neurospora crassa. Plant Physiol., 57, 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli-Innocenti F. and Russo,V.E. (1984) Isolation of new white collar mutants of Neurospora crassa and studies on their behavior in the blue light-induced formation of protoperithecia. J. Bacteriol., 159, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denault D.L., Loros,J.J. and Dunlap,J.C. (2001) WC-2 mediated WC-1–FRQ interaction within the PAS protein-linked circadian feedback loop of Neurospora. EMBO J., 20, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmananda S. (1980) Studies of the circadian clock of Neurospora crassa: light-induced phase shifting. PhD thesis, University of California, Santa Cruz, CA.
- Garceau N.Y., Liu,Y., Loros,J.J. and Dunlap,J. (1997) Alternative initiation of translation and time specific phosphorylation yield multiple forms of essential clock protein FREQUENCY. Cell, 89, 469–476. [DOI] [PubMed] [Google Scholar]
- Goerl M., Merrow,M., Huttner,B., Johnson,J., Roenneberg,T. and Brunner,M. (2001) A PEST-like element in FREQUENCY determines the length of the circadian period in Neurospora crassa. EMBO J., 20, 7074–7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R.W. and Melles,S. (1984) Genetic analysis of the phototrophism of Neurospora crassa parethecial beaks using white collar and albino mutants. Plant Physiol., 72, 996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R.W. and Turner,R.V. (1981) Photoregulation of the carotenoid biosynthetic pathway in albino and white collar mutants of Neurospora crassa. Plant Physiol., 68, 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzen C., Loros,J.J. and Dunlap,J.C. (2001) The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating and regulates clock resetting. Cell, 104, 453–464. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Williams,S.B., Kitayama,Y., Ishiura,M., Golden,S.S. and Kondo,T. (2000) A KaiC-interacting sensory hisidine kinase, SasA, necessary to sustain robust circadian oscillation in cyanobacteria. Cell, 101, 223–233. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas P.L. and Brody,S. (2000) Circadian rhythms in Neurospora crassa: lipid deficiencies restore robust rhythmicity to null frequency and white-collar mutants. Proc. Natl Acad. Sci. USA, 97, 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter F.-R., Yamashiro,C.T. and Yanofsky,C. (1997) Light stimulation of conidiation in Neurospora crassa: studies with wild-type strain and mutants wc-1, wc-2 and acon-2. J. Photochem. Photobiol. B Biol., 37, 203–211. [Google Scholar]
- Lee K., Loros,J.J. and Dunlap,J.C. (2000) Interconnected feedback loops in the Neurospora circadian system. Science, 289, 107–110. [DOI] [PubMed] [Google Scholar]
- Li C. and Schmidhauser,T.J. (1995) Developmental and photoregulation of al-1 and al-2, structural genes for two enzymes essential for carotenoid biosynthesis in Neurospora. Dev. Biol., 169, 90–95. [DOI] [PubMed] [Google Scholar]
- Linden H. and Macino,G. (1997) White collar 2, a partner in blue-light signal transduction controlling expression of light-regulated genes in Neurospora crassa. EMBO J., 16, 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden H., Ballario,P., Arpaia,G. and Macino,G. (1999) Seeing the light: news in Neurospora blue light signal transduction. Adv. Genet., 41, 35–54. [DOI] [PubMed] [Google Scholar]
- Loros J.J. and Feldman,J.F. (1986) Loss of temperature compensation of circadian period length in the frq-9 mutant of Neurospora crassa. J. Biol. Rhythms, 1, 187–198. [DOI] [PubMed] [Google Scholar]
- Loros J.J., Richman,A. and Feldman,J.F. (1986) A recessive circadian clock mutation at the frq locus of Neurospora crassa. Genetics, 114, 1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madi L., Ebbole,D.J., White,B.T. and Yanofsky,C. (1994) Mutants of Neurospora crassa that alter gene expression and conidia development. Proc. Natl Acad. Sci. USA, 91, 6226–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C.R., Fox,B.A. and Dunlap,J.C. (1989) The Neurospora clock gene frequency shares a sequence element with the Drosophila clock gene period. Nature, 339, 558–562. [DOI] [PubMed] [Google Scholar]
- Merrow M., Brunner,M. and Roenneberg,T. (1999) Assignment of circadian function for the Neurospora clock gene frequency. Nature, 399, 584–586. [DOI] [PubMed] [Google Scholar]
- Merrow M., Franchi,L., Dragovic,Z., Görl,M., Johnson,J., Brunner,M., Macino,G. and Roenneberg,T. (2001) Circadian regulation of the light input pathway in Neurospora crassa. EMBO J., 20, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H. and Fujimura,Y. (1982) Light-induced phase shifting of the circadian clock in Neurospora crassa ammonium salts in high pH. Planta, 155, 431–436. [DOI] [PubMed] [Google Scholar]
- Ninneman H. (1991) Photostimulation of conidiation in mutants of Neurospora crassa. J. Photochem. Photobiol. B Biol., 9, 189–199. [DOI] [PubMed] [Google Scholar]
- Pittendrigh C.S., Bruce,V.G., Rosensweig,N.S. and Rubin,M.L. (1959) Growth patterns in Neurospora crassa. Nature, 184, 169–170. [Google Scholar]
- Roenneberg T. and Merrow,M. (1998) Molecular circadian oscillators—an alternative hypothesis. J. Biol. Rhythms, 13, 167–179. [DOI] [PubMed] [Google Scholar]
- Roenneberg T. and Merrow,M. (2000) Circadian light input: omnes viae Romam ducunt. Curr. Biol., 10, R742–R745. [DOI] [PubMed] [Google Scholar]
- Roenneberg T. and Taylor,W. (2000) Automated recordings of bioluminescence with special reference to the analysis of circadian rhythms. Methods Enzymol., 305, 104–119. [DOI] [PubMed] [Google Scholar]
- Russo V.E. (1988) Blue light induces circadian rhythms in the bd mutant of Neurospora: double mutants bd,wc-1 and bd,wc-2 are blind. Photochem. Photobiol., 2, 59–65. [DOI] [PubMed] [Google Scholar]
- Sachs M.S. and Yanofsky,C. (1991) Developmental expression of genes involved in conidiation and amino acid biosynthesis in Neurospora crassa. Dev. Biol., 148, 117–128. [DOI] [PubMed] [Google Scholar]
- Sargent M.L., Briggs,W.R. and Woodward,D.O. (1956) Circadian nature of a rhythm expressed by an invertaseless strain of Neurospora crassa. Plant Physiol., 41, 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdtfeger C. and Linden,H. (2001) Blue light adaptation and desensitization of light signal transduction in Neurospora crassa. Mol. Microbiol., 39, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Selker E.U. and Garrett,P.W. (1988) DNA sequence duplications trigger gene inactivation in Neurospora crassa. Proc. Natl Acad. Sci. USA, 85, 6870–6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrode L.B., Lewis,Z.A., White,L.D., Bell-Pedersen,D. and Ebbole,D.J. (2001) vvd is required for light adaptation of conidiation-specific genes of Neurospora crassa, but not circadian conidiation. Fungal Genet. Biol., 32, 169–181. [DOI] [PubMed] [Google Scholar]
- Sokolove P.G. and Bushell,W.N. (1978) The chi square periodogram: its utility for analysis of circadian rhythms. J. Theor. Biol., 72, 131–160. [DOI] [PubMed] [Google Scholar]
- Sokolovsky V.Y., Lauter,F.-R., Müller-Röber,B., Ricci,M., Schmidhauser,T.J. and Russo,V.E.A. (1992) Nitrogen regulation of blue-light-inducible genes in Neurospora crassa. J. Gen. Microbiol., 138, 2045–2049. [Google Scholar]
- Somers D.E., Devlin,P.F. and Kay,S.A. (1998) Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science, 282, 1488–1490. [DOI] [PubMed] [Google Scholar]
- Sommer T., Chambers,J.A., Eberle,J., Lauter,F.R. and Russo,V.E. (1989) Fast light-regulated genes of Neurospora crassa. Nucleic Acids Res., 17, 5713–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talora C., Franchi,L., Linden,H., Ballario,P. and Macino,G. (1999) Role of a white collar-1–white collar-2 complex in blue-light signal transduction. EMBO J., 18, 4961–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H.J. (1964) Distribution of lysine pathways among fungi: evolutionary implications. Am. Nat., 98, 435–446. [Google Scholar]
- West D.J. (1976) Phase shifting of circadian rhythm of conidiation in response to ultraviolet light. Neurospora Newsl., 23, 17–18. [Google Scholar]
- Yang Y., Cheng,P., Zhi,G. and Liu,Y. (2001) Identification of a calcium/calmodulin-dependent protein kinase that phosphorylates the Neurospora circadian clock protein FREQUENCY. J. Biol. Chem., 276, 41064–41072. [DOI] [PubMed] [Google Scholar]