Abstract
Soluble N-ethylmaleimide sensitive factor-attachment protein receptors (SNAREs) and Sec1p/Munc18-homologs (SM proteins) play key roles in intracellular membrane fusion. The SNAREs form tight four-helix bundles (core complexes) that bring the membranes together, but it is unclear how this activity is coupled to SM protein function. Studies of the yeast trans-Golgi network (TGN)/endosomal SNARE complex, which includes the syntaxin-like SNARE Tlg2p, have suggested that its assembly requires activation by binding of the SM protein Vps45p to the cytoplasmic region of Tlg2p folded into a closed conformation. Nuclear magnetic resonance and biochemical experiments now show that Tlg2p and Pep12p, a late- endosomal syntaxin that interacts functionally but not directly with Vps45p, have a domain structure characteristic of syntaxins but do not adopt a closed conformation. Tlg2p binds tightly to Vps45p via a short N-terminal peptide motif that is absent in Pep12p. The Tlg2p/Vps45p binding mode is shared by the mammalian syntaxin 16, confirming that it is a Tlg2p homolog, and resembles the mode of interaction between the SM protein Sly1p and the syntaxins Ufe1p and Sed5p. Thus, this mechanism represents the most widespread mode of coupling between syntaxins and SM proteins.
Keywords: SM proteins/SNAREs/syntaxins/Tlg2p/Vps45p
Introduction
All types of intracellular membrane fusion are believed to share a common protein machinery (Bennett and Scheller, 1993; Ferro-Novick and Jahn, 1994). Particularly important to execute membrane fusion appear to be the soluble NSF-attachment protein receptors (SNAREs) and the Sec1/munc18 homologs (SM proteins) (Jahn and Südhof, 1999; Lin and Scheller, 2000). The SNAREs are characterized by sequences called SNARE motifs that generally precede C-terminal transmembrane regions. One SNARE motif from one membrane (v-SNARE) and three from the apposed membrane (t-SNAREs) assemble into tight four-helix bundles known as core complexes (Poirier et al., 1998; Sutton et al., 1998). Because of the parallel arrangement of the helices, core complex formation should bring the opposing membranes together and may catalyze membrane fusion (Hanson et al., 1997). Reconstitution experiments have suggested that SNAREs alone can indeed cause bilayer merger but at a very slow rate (Weber et al., 1998), suggesting that additional factors are required for physiological membrane fusion.
The function of SM proteins is less well understood but their importance is emphasized by the complete blocks in neurotransmitter release and/or general secretion caused by mutations in these proteins (Ossig et al., 1991; Hosono et al., 1992; Schekman, 1992; Harrison et al., 1994; Verhage et al., 2000). The SM protein involved in neuronal exocytosis, munc18-1, binds tightly to the SNARE syntaxin 1 (Hata et al., 1993). Syntaxin 1 contains an N-terminal region that precedes the SNARE motif and includes an autonomously folded three-helical domain termed the Habc domain (Fernandez et al., 1998). The Habc domain folds back onto the SNARE motif, forming a ‘closed conformation’ that is incompatible with the core complex but is essential for munc18-1 binding (Dulubova et al., 1999; Misura et al., 2000). Since munc18-1 is essential for exocytosis (Verhage et al., 2000), it was suggested that munc18-1 provides a platform that somehow assists in core complex assembly (Misura et al., 2000; Munson et al., 2000). These results led to the general belief that all SM proteins function by binding to the closed conformation of the corresponding syntaxin. Indeed, genetic and/or direct interactions between several SM proteins and syntaxins have been observed in yeast (see Table I). However, while the yeast plasma membrane syntaxin Sso1p adopts a closed conformation (Munson et al., 2000), the corresponding SM protein Sec1p binds to core complexes containing Sso1p rather than to isolated Sso1p (Carr et al., 1999). In addition, the yeast vacuolar syntaxin Vam3p does not adopt a closed conformation and its SNARE motif is sufficient to capture the SM protein Vps33p from yeast extracts (Dulubova et al., 2001). More recently, we showed that the yeast ER and Golgi syntaxins, Ufe1p and Sed5p, bind to the SM protein Sly1p through a novel, evolutionarily conserved mode that involves a short peptide motif at the very N-terminus of Ufe1p and Sed5p (Yamaguchi et al., 2002).
Table I. Interactions between syntaxins and SM proteins in yeasta.
| Syntaxin | SM protein | Cellular compartment | Comments |
|---|---|---|---|
| Sso1p, Sso2p | Sec1p | Plasma membrane | Sec1p binds to core complexes rather than to isolated Sso1pb |
| Ufe1p | Sly1p | ER | Ufe1p binds directly to Sly1p via an N-terminal peptide motifc |
| Sed5p | Sly1p | Golgi | Sed5p binds directly to Sly1p via an N-terminal peptide motifc,d |
| Tlg2p | Vps45p | TGN/early endosome | Tlg2p binds directly to Vps45pe, this study |
| Pep12p | Vps45p | Late endosome | Pep12p interacts functionally but not directly with Vps45pe,f, this study |
| Vam3p | Vps33p | Vacuole | Vps33p probably binds indirectly to Vam3p while incorporated into a large protein complexg |
aFor most syntaxins and SM proteins, the cellular compartments where they function have been identified, as indicated, but in some cases they are still uncertain (reviewed in Pelham, 1999). bCarr et al. (1999); cYamaguchi et al. (2002); dGrabowski and Gallwitz (1997) and Kosodo et al. (1998); eNichols et al. (1998) and Bryant and James (2001); fBurd et al. (1997) and Webb et al. (1997); gSato et al. (2000) and Seals et al. (2000).
Intense research has recently been devoted to study the proteins involved in traffic through the trans-Golgi network (TGN) and early endosomes. These two compartments constitute the crossroads of the secretory and endocytic pathways, and are in constant communication between each other, as well as with Golgi cisternae, with the plasma membrane and with late endosomes (Pelham, 1999). The yeast syntaxin Tlg2p plays a critical role in membrane traffic at the TGN and early endosomes. Tlg2p is localized in these two compartments, and cells lacking Tlg2p exhibit defects in endocytosis, in sorting of vacuolar proteins such as carboxypeptidase Y, and in retrieval of TGN-resident proteins such as Kex2p (Abeliovich et al., 1998; Holthuis et al., 1998). In addition, Tlg2p is required for normal endosome biogenesis (Séron et al., 1998), for efficient localization of casein kinases to the plasma membrane (Panek et al., 2000), for import of aminopeptidase I from the cytosol to the vacuole (Abeliovich et al., 1999), for recycling of the SNARE Snc1p through early endosomes (Lewis et al., 2000) and for TGN homotypic fusion (Brickner et al., 2001). The SNAREs Tlg1p and Vti1p are also required for some of these traffic events, and both co-immunoprecipitate with Tlg2p, suggesting that they participate in a common SNARE complex (Holthuis et al., 1998; Coe et al., 1999). Similarly, Arabidopsis thaliana homologs of Tlg2p, Tlg1p and Vti1p, as well as their putative mammalian homologs (syntaxin 16, syntaxin 6 and Vti1a), have been implicated in TGN transport (Bassham et al., 2000; Mallard et al., 2002). The yeast Tlg SNAREs also co-immunoprecipitate with the v-SNARE Snc1p or the closely related Snc2p (Abeliovich et al., 1998; Holthuis et al., 1998). While these v-SNAREs mediate exocytosis, they also function in endocytosis (Gurunathan et al., 2000). Indeed, the notion that Snc1p or Snc2p can form a functional core complex with Tlg2p/Tlg1p/Vti1p acting as the t-SNAREs was recently supported by experiments with recombinant proteins reconstituted into liposomes (Paumet et al., 2001).
Tlg2p interacts physically and functionally with Vps45p, the SM protein involved in TGN/early endosomal transport (Nichols et al., 1998; Abeliovich et al., 1999; Brickner et al., 2001; Bryant and James, 2001) (Table I). Interestingly, Vps45p also functions in late-endosomal transport, which requires the syntaxin Pep12p (Burd et al., 1997; Webb et al., 1997), but does not bind directly to Pep12p (Nichols et al., 1998; Bryant and James, 2001). Understanding why Vps45p binds to Tlg2p but not to Pep12p is hindered by the lack of structural information on these proteins and the absence of a clear sequence homology among the N-terminal regions of syntaxins that function in different cellular compartments. In addition, Vps45p has been generally assumed to bind to Tlg2p folded into a closed conformation based on the neuronal model, but this assumption has not been tested experimentally. To shed light on these questions, we have analyzed the structure of Tlg2p and Pep12p, as well as the mode of interaction between Tlg2p and Vps45p. Our results show that both Tlg2p and Pep12p have domain structures characteristic of syntaxins, but they do not adopt a closed conformation. We also find that Tlg2p binds to Vps45p via an N-terminal peptide motif that is absent in Pep12p but resembles the peptide motifs that mediate binding of Ufe1p and Sed5p to Sly1p. The Tlg2p/Vps45p binding mode is conserved in the mammalian syntaxin 16 and mVps45p, supporting the notion that these are true homologs of the yeast Tlg2p and Vps45p. These results suggest that interactions between syntaxin N-terminal peptide motifs and SM proteins constitute the most widespread mode of SNARE/SM protein coupling in intracellular membrane traffic.
Results
Domain structure of Tlg2p
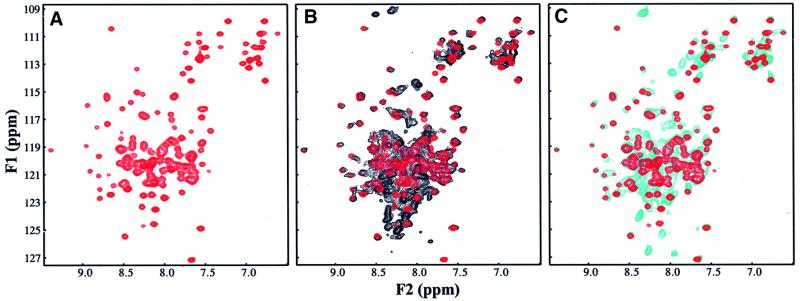
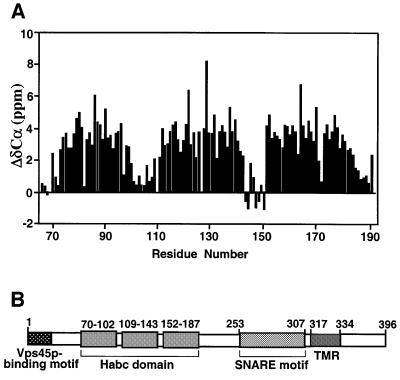
In order to have a framework to rationalize the biochemical and functional properties of Tlg2p, we first studied its domain structure by sequence analysis and nuclear magnetic resonance (NMR) spectroscopy. Tlg2p is a 396 amino acid protein and its SNARE motif can be localized with certainty to residues 253–307 based on the sequence homology among the SNARE motifs from the syntaxin family. The SNARE motif is followed by a transmembrane region and a 63 residue C-terminal sequence that is unusual in the syntaxin family and is not essential for Tlg2p function (Abeliovich et al., 1998). In the 250 residue N-terminal region, Tlg2p does not exhibit a clear sequence homology with the N-terminal regions of syntaxins whose structures have been analyzed previously, all of which contain an Habc domain [syntaxin 1 (Fernandez et al., 1998), Sso1p (Munson et al., 2000), Vam3p (Dulubova et al., 2001) and Sed5p (Yamaguchi et al., 2002)]. To determine whether Tlg2p also contains an Habc domain and whether there are intramolecular interactions between different domains of Tlg2p, we expressed several recombinant fragments covering its entire cytoplasmic region and analyzed them using 1H-15N heteronuclear single quantum correlation (HSQC) spectra. These spectra constitute protein fingerprints that contain one cross-peak for each residue of a protein and the cross-peak dispersion reveals whether a protein is properly folded, while perturbations in the cross-peaks report on intra- or intermolecular interactions. These studies led us to identify a minimal Tlg2p fragment (residues 65–192) with a well dispersed 1H-15N HSQC spectrum characteristic of a well-folded domain (Figure 1A). We then assigned the backbone resonances of this fragment to analyze whether it forms a three-helix bundle. Comparison between the observed Cα carbon chemical shifts and those expected for a random coil (Figure 2A) indeed revealed three α-helices that span residues 70–102, 109–143 and 152–187 of Tlg2p. This conclusion was further confirmed by analysis of chemical shift indices (Wishart and Sykes, 1994) and of the NOE patterns observed in a 3D 1H-15N NOESY-HSQC spectrum. Hence, these results demonstrate that Tlg2p contains an Habc domain and that this domain is preceded by a 70 residue N-terminal sequence, as summarized in the domain diagram shown in Figure 2B.
Fig. 1. 1H-15N HSQC spectra of Tlg2p fragments. (A) 1H-15N HSQC spectrum of Tlg2p(65–192). (B and C) Superposition of the 1H-15N HSQC spectra of Tlg2p(60–283) (black contours) (B), or Tlg2p(1–192) (cyan contours) (C), with the spectrum of Tlg2p(65–192) (red contours).
Fig. 2. Domain structure of Tlg2p. (A) Definition of the three helical Habc domain of Tlg2p. Differences (ΔδCα) between the Cα chemical shifts observed for residues 65–192 of Tlg2p and those expected for a random coil are plotted against the residue number. Three regions with large positive ΔδCα values indicate the three α-helices. (B) Schematic diagram of the Tlg2p sequence. Residue numbers are shown above the diagram. Hatched boxes correspond to different structural elements in Tlg2p. The approximate limits of the three helices of the Habc domain were assigned based on chemical shift indices (Wishart and Sykes, 1994) deduced from the observed ΔδCα values shown in (A).
Analysis of intramolecular interactions in the entire cytoplasmic region of Tlg2p was hindered by its high tendency to aggregate, even in the presence of detergents such as CHAPS. However, we were able to obtain good quality 1H-15N HSQC spectra for a soluble fragment that included the Habc domain and the part of the SNARE motif corresponding to the sequence involved in forming the closed conformation in syntaxin 1 (Dulubova et al., 1999; Misura et al., 2000) and Sso1p (Fiebig et al., 1999; Munson et al., 2000) [Tlg2p(60–283)] (Figure 1B, black contours). Comparison with the 1H-15N HSQC spectrum of the Habc domain (red contours) revealed no significant shifts in the Habc-domain cross-peaks. These results resemble those obtained for Vam3p (Dulubova et al., 2001) and contrast with the results obtained for syntaxin 1 and Sso1p, where the adoption of a closed conformation causes large and widespread shifts in the N-terminal cross-peaks (Dulubova et al., 1999; and our unpublished results). In addition, the 1H-15N HSQC spectra of Tlg2p(60–283) exhibited numerous sharp cross-peaks with low 1H chemical shift dispersion, suggesting that the sequences outside the Habc domain are mostly unstructured. Hence, these results show that the Habc domain of Tlg2p does not interact intramolecularly with the SNARE motif to form a closed conformation like that observed in syntaxin 1 and Sso1p. We also acquired a 1H-15N HSQC spectrum of a soluble fragment including the N-terminal sequence and the Habc domain [Tlg2p(1–192)] (Figure 1C, cyan contours). Again, this spectrum did not reveal significant shifts in the cross-peaks of the Habc domain and exhibited multiple sharp cross-peaks with low 1H chemical shift dispersion, suggesting that the N-terminal sequence of Tlg2p does not interact intramolecularly with the Habc domain.
Domain structure of Pep12p
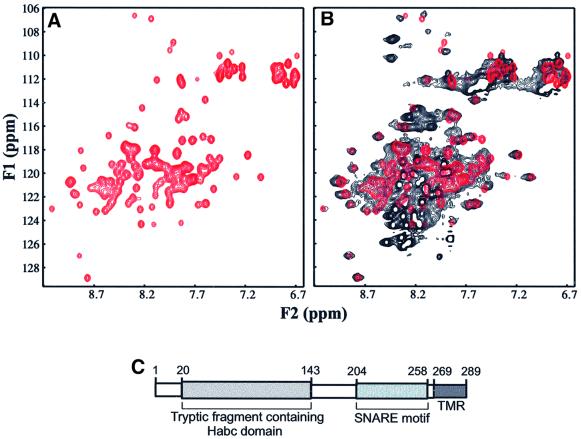
In a parallel analysis of Pep12p, sequence comparisons showed that its SNARE motif is localized in residues 204–258 and also precedes a transmembrane region. In the N-terminal region, we observed a potentially significant similarity between residues 48–136 of Pep12p and the Habc domain of the yeast vacuolar syntaxin Vam3p (residues 25–113) (18% identity, 37% conservation) (data not shown). Limited trypsinolysis of the cytoplasmic region of Pep12p (residues 2–261) confirmed the presence of an autonomously folded, trypsin-resistant N-terminal fragment spanning residues 20–143, as determined by electrospray mass spectrometry and N-terminal sequencing (data not shown). The good chemical shift dispersion observed in the 1H-15N HSQC spectrum of a fragment encompassing this region [Pep12p(17–144)] (Figure 3A) demonstrated the presence of a well folded domain, and the circular dichroism spectrum of this fragment revealed a high α-helical content (data not shown). These results strongly suggest that Pep12p contains an N-terminal Habc domain similar to that of Vam3p (Dulubova et al., 2001). Comparison of the 1H-15N HSQC spectrum of a frag ment containing the cytoplasmic region of Pep12p [Pep12p(17–253)] (Figure 3B, black contours) with that of the N-terminal domain (Figure 3B, red contours) showed that most N-terminal cross-peaks coincide in both spectra and that only a few exhibit slight shifts in the longer fragment. In addition, the 1H-15N HSQC spectrum of Pep12p(17–253) exhibits a large number of sharp cross-peaks with little 1H chemical shift dispersion, which suggests that the SNARE motif is largely unstructured. Hence, these data indicate that Pep12p does not adopt a closed conformation, similarly to Tlg2p (see above) and Vam3p (Dulubova et al., 2001). Overall, these results show that, similarly to Tlg2p, Pep12p has the typical domain organization of syntaxins, but the N-terminal sequence preceding the Habc domain is significantly shorter than that of Tlg2p (compare Figures 2B and 3C).
Fig. 3. 1H-15N HSQC spectra of Pep12p fragments. (A) 1H-15N HSQC spectrum of Pep12p(17–144). (B) Superposition of the 1H-15N HSQC spectrum of Pep12p(17–253) (black contours) with the spectrum of Pep12p(17–144) (red contours). (C) Domain structure of Pep12p.
Tlg2p binds to Vps45p via an evolutionarily conserved N-terminal peptide motif
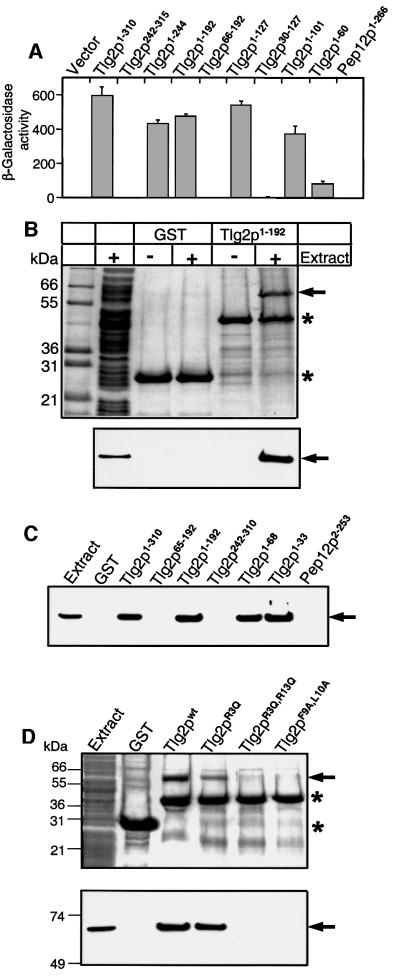
The finding that deletion of the 240 N-terminal amino acid residues of Tlg2p abolished binding to Vps45p in vitro was interpreted as an indication that a closed conformation of Tlg2p is involved in the interaction by analogy with the neuronal syntaxin 1/munc18-1 complex (Bryant and James, 2001). Since our NMR data did not reveal the presence of the closed conformation in Tlg2p, we decided to map precisely the binding site of Vps45p on the Tlg2p sequence using yeast two-hybrid assays with Vps45p as bait, and different fragments of Tlg2p as prey. Robust binding of Vps45p to the full cytoplasmic region of Tlg2p, but not to that of Pep12p, was observed in these assays (Figure 4A). Deletion of the 241 N-terminal residues of Tlg2p abolished binding (Figure 4A), in agreement with previous results (Bryant and James, 2001). However, C-terminal deletions revealed that neither the SNARE motif nor the N-terminal Habc domain of Tlg2p are essential for the interaction. In fact, the Tlg2p fragment corresponding to the N-terminal sequence alone (residues 1–60) still interacted with Vps45p in the yeast two-hybrid assay (Figure 4A). The critical importance of the N-terminal sequence for the Tlg2p/Vps45p interaction was confirmed by the observation that the robust binding exhibited by the Tlg2p(1–192) and Tlg2p(1–127) fragments was abolished when the N-terminal sequence was deleted [yielding Tlg2p(66–192) and Tlg2p(30–127)]. These results indicate that Tlg2p binds to Vps45p via a short N-terminal peptide sequence, by analogy to the results obtained for the interaction of Sly1p with Ufe1p and Sed5p (Yamaguchi et al., 2002), but in sharp contrast to the neuronal syntaxin 1/munc18-1 interaction.
Fig. 4. Tlg2p binds to Vps45p via a short evolutionarily conserved N-terminal sequence. (A) Yeast two-hybrid assay. Yeast cells were co-transfected with pLexN-Vps45p and the indicated pVP16-Tlg2p or pVP16-Pep12p prey clones (numbers indicate amino acid residues included in the constructs). The relative β-galactosidase activity was calculated as 1000 × OD420/[min × vol. yeast (ml) × OD600]. Data shown are means ± SE from quadruple determinations. (B–D) GST-pulldown assays. In (B), glutathione–Sepharose beads containing GST alone or the GST–Tlg2p(1–192) fragment were incubated with bacterial extracts prepared from cells expressing T7-tagged Vps45p. After extensive washes, the beads were separated on SDS–PAGE and visualized by Coomassie Blue-staining (upper panel) or by immunoblotting with T7·Tag antibody (lower panel). In (C), GST alone, GST– Pep12p(2–253) and different GST–Tlg2p fragments were similarly used in pulldown experiments and analyzed by immunoblotting with anti-T7·Tag monoclonal antibody. In (D), a similar procedure was applied to GST alone, or to GST–Tlg2p(1–192) fragments (either wild type or with the indicated mutations). The upper panel shows Coomassie Blue-stained PAGE and the lower panel shows the corresponding immunoblot with anti-T7·Tag monoclonal antibody. In (B–D), numbers on the left indicate the positions of molecular mass markers. Asterisks show the positions of GST and GST-fusion proteins and the arrows indicate Vps45p.
To corroborate our findings using an independent method, we expressed recombinant T7-tagged Vps45p in bacteria and analyzed its binding to immobilized glutathione S-transferase (GST)-fusion proteins containing the cytoplasmic region of Pep12p or different Tlg2p fragments. Vps45p bound strongly to Tlg2p(1–192), since a major band at ∼65 kDa was readily observed on a Coomassie Brilliant Blue-stained polyacrylamide gel even after stringent washes of the resin (Figure 4B). The identity of this band as Vps45p was established by immunoblotting. Further analysis with a range of Tlg2p fragments fully confirmed the results obtained with the yeast two-hybrid system, showing that the first 33 N-terminal residues of Tlg2p are sufficient for Vps45p binding (Figure 4C). Note that slight discrepancies in the binding strength observed in the two assays could arise from their different natures. For instance, the decreased binding observed in the yeast two-hybrid experiments for the Tlg2p(1–60) fragment (Figure 4A) may be due to instability of this small fragment in vivo. Again, no interaction of Vps45p with the cytoplasmic region of Pep12p was observed in the GST-pulldown assays (Figure 4C).
To explore potential structural determinants of the N-terminal motif of Tlg2p that could be critical for binding to Vps45p, we aligned the 33 N-terminal residues of Tlg2p with the N-terminus of its putative mammalian homolog, syntaxin 16 (see below), and also compared them with the N-terminus of Pep12p:
Tlg2p –MFRDRTNLFLSYRRTFPHNITFSSGKAPLGDDQ
Syntaxin 16 MATRRLTDAFLLLRNNSIQNRQLLA-EQELDELA
Pep12p –MSEDEFFGGDNEGVWNGSRFSDSPEFQTLKEEV
Seven identical residues are observed over the N-terminal 33 residues of Tlg2p and syntaxin 16, with five clustered in the first 13 amino acids. None of these five residues are present in Pep12p. To test the func tional importance of the conserved residues of Tlg2p, we constructed a series of point mutants in the GST– Tlg2p(1–192) fragment, and examined their ability to pull down Vps45p. The R3Q,R13Q and F9A,L10A double mutations completely abolished Vps45p binding, while the single R3Q mutation slightly impaired the interaction (Figure 4D). These results confirmed the critical importance of the N-terminal peptide motif of Tlg2p for binding to Vps45p and suggested that the binding mode is conserved through evolution.
Characterization of syntaxin 16, the putative mammalian Tlg2p ortholog
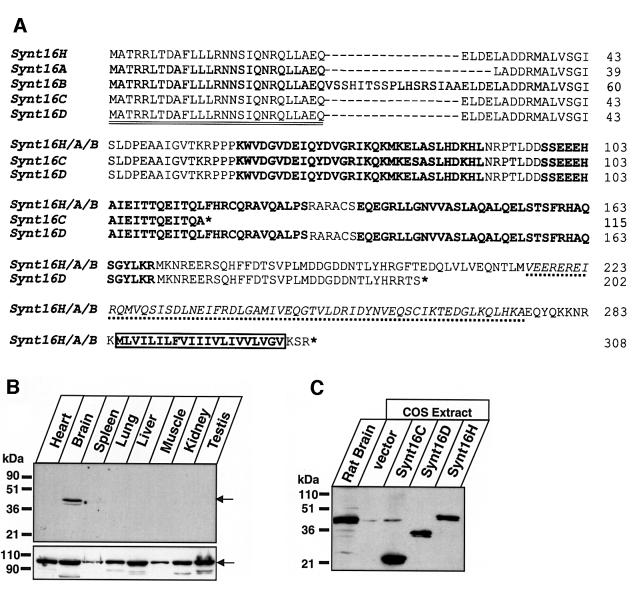
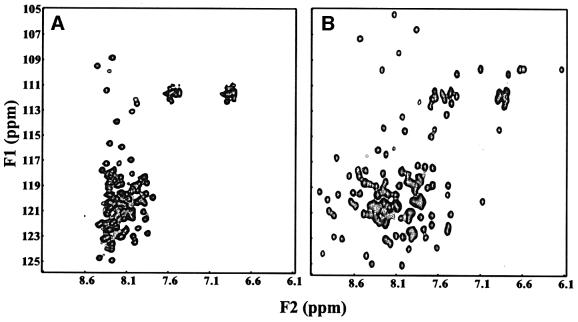
Syntaxin 16 and mVps45 were proposed as vertebrate homologs of Tlg2p and Vps45p, respectively (Tellam et al., 1997; Simonsen et al., 1998; Tang et al., 1998). We recently showed in yeast two-hybrid assays that syntaxin 16 and mVps45 bind directly to each other (Yamaguchi et al., 2002). Furthermore, sequence alignments suggest that syntaxin 16 has a similar domain structure to Tlg2p, with a well conserved N-terminal peptide motif and Habc domain (Figure 5A and data not shown). Interestingly, syntaxin 16 mRNA is expressed in multiple splice variants, some of which predict the synthesis of truncated proteins that lack the SNARE motif and transmembrane region (Figure 5A) (see also Simonsen et al., 1998; Tang et al., 1998). One of the most abundant splice variants, called syntaxin 16C, is truncated in the middle of the predicted Habc domain (Figure 5A), suggesting that this variant is unlikely to be folded. To verify these predictions, we acquired 1H-15N HSQC spectra of recombinant syntaxin 16C. This spectrum exhibited sharp cross-peaks and poor 1H chemical shift dispersion, showing that syntaxin 16C is indeed largely unfolded (Figure 6A). In contrast, the 1H-15N HSQC spectrum of a syntaxin 16 fragment corresponding to the predicted Habc domain of a longer splice variant (syntaxin 16H, residues 59–183) exhibited well dispersed cross-peaks, consistent with the expectation that it forms a folded three-helix bundle (Figure 6B). These results indicate that syntaxin 16H indeed has a domain structure analogous to that of Tlg2p, and demonstrate that syntaxin 16C represents a truncated version of syntaxin 16 where the only intact functional region is the N-terminal peptide sequence predicted to bind to Vps45.
Fig. 5. Characterization of syntaxin 16. (A) Proteins encoded by various splice variants of human syntaxin 16. Three different full-length syntaxin 16 variants were reported in the literature: syntaxin 16H (synt16H) (Tang et al., 1998), syntaxin 16A (synt16A) and syntaxin 16B (synt16B) (Simonsen et al., 1998). Note that all three published syntaxin 16 sequences probably contain an internal frameshift error (corrected in our translation here, residues from Ala125 to Leu148 according to synt16H numbering) since the cDNAs we sequenced and the conceptual sequence deduced from the genome product (accession no. XM_030663) agree completely. The Synt16H, -A and -B sequences differ in an alternatively spliced region located between the N-terminal peptide that binds to mVps45 (see below) and the Habc domain. The truncated group consists of a splice variant called syntaxin 16C (Simonsen et al., 1998) that contains a stop codon in the middle of the predicted Hb helix, and a new splice variant called syntaxin 16D (image clone 4413533; accession no. BG035083) that contains a stop codon after the Habc domain but before the SNARE motif. The N-terminal variations that distinguish between Synt16H, -A and -B are probably also encoded by the mRNAs of the truncated syntaxin 16 variants. In the alignment, asterisks indicate positions of stop codons and dashes represent gaps. The double-underlined sequence corresponds to the N-terminal peptide sequence that binds to mVps45. Sequences in bold represent predicted α-helices of the Habc domain (based on sequence comparison with Tlg2p), the italicized sequence underlined with a dotted line represents the SNARE motif, and the box demarks the transmembrane region. Numbers on the right correspond to syntaxin 16H splice variant. (B) Syntaxin 16 tissue distribution. Equal amounts of homogenates from the indicated mouse tissues were analyzed by immuno blotting with antibodies to syntaxin 16 (upper panel) and VCP (vasolin-containing protein, lower panel). Arrows indicate the position of syntaxin 16 (upper panel) and VCP (lower panel). (C) Expression of various splice variants of syntaxin 16 in a mammalian cell line. Mouse brain homogenate and extracts of COS-1 cells expressing the full-length human syntaxin 16C, syntaxin 16D and the entire cytoplasmic region of syntaxin 16H (residues 1–284) were analyzed by immunoblotting with antibodies to syntaxin 16. Numbers on the left indicate positions of molecular weight markers.
Fig. 6. 1H-15N HSQC spectra of syntaxin 16 fragments. (A) 1H-15N HSQC spectrum of the full-length syntaxin 16C. (B) 1H-15N HSQC spectrum of the syntaxin 16H fragment corresponding to the predicted Habc domain (residues 59–183).
Although northern blot analysis suggested that syntaxin 16 is uniformly expressed in all tissues (Simonsen et al., 1998; Tang et al., 1998), and polymerase chain reaction (PCR) experiments and expressed sequence tag (EST) clone sequences confirmed the existence of multiple splice variants (data not shown), the endogenous proteins have not been characterized. Thus, we raised an antibody to the shortest splice variant, syntaxin 16C, and tested total proteins from various mouse tissues for immunoreactivity. A single immunoreactive band corresponding to the full-length syntaxin 16 was observed in brain (Figure 5B). The same band was observed with much longer exposure times in all tissues tested (data not shown), but the expression levels were >10-fold lower than in brain. No major cross-reactive bands other than the full-length protein were observed in any tissue. To ensure that the antibody used in these experiments would in fact have recognized the proteins encoded by the truncated splice variants, we overexpressed the proteins in COS cells, and analysed them by immunoblotting in parallel with brain proteins (Figure 5C). These experiments confirmed that the antibodies used react with the truncated syntaxin 16 variants, suggesting that these variants lacking SNARE motifs are normally not present at detectable steady-state levels in brain.
An N-terminal peptide-based mechanism also mediates binding of syntaxin 16 to mVps45p
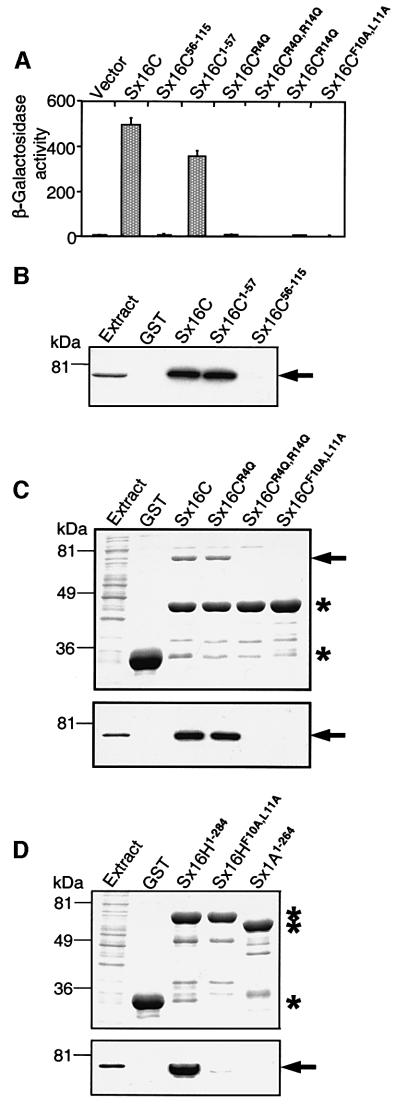
The similarity of the N-terminal sequences of Tlg2p and syntaxin 16 (see above) indicates that syntaxin 16 could bind to mVps45p by the same mechanism observed for the Tlg2p/Vps45p interaction. To test this proposal, we again used a combination of the yeast two-hybrid and GST-pulldown assays. Strong β-galactosidase activity was observed when the entire syntaxin 16C sequence or its N-terminal 57 residues were used as a prey (Figure 7A), revealing that the interaction with mVps45 involves the very N-terminal sequence of syntaxin 16. This interaction was abolished by removal of the N-terminal 55 residues of syntaxin 16C, or by mutations of the same conserved residues that were found to be critical for the Tlg2p/Vps45p interaction (Figure 7A). Note that these residues are present in all splice variants of syntaxin 16 (Figure 5A). GST-pulldown experiments, performed with mVps45 expressed both in mammalian and bacterial cells, also demonstrated a strong syntaxin 16C/mVps45 interaction that was detectable on Coomassie Blue-stained polyacrylamide gels and was confirmed by immunoblotting (Figure 7B and C). Furthermore, binding of syntaxin 16C to mVps45 in this assay also required the N-terminal 57 residues (Figure 7B) and was abolished by the double R4Q,R14Q and F10A,L11A mutations in the conserved N-terminal sequence motif (Figure 7C). The single point mutation R4Q had a more severe effect on binding in the yeast two-hybrid assay that in the GST-pulldown assay (Figure 7A and C), perhaps because of the different nature of the two methods. Since syntaxin 16C represents a truncated version of syntaxin 16H lacking any folded domain, we performed additional GST-pulldown assays to confirm that full-length syntaxin 16 follows the same binding paradigm. These experiments showed that the entire cytoplasmic region of syntaxin 16H (residues 1–284) also binds strongly to mVps45 and that the interaction is again abolished by the F10A,L11A mutation (Figure 7D).
Fig. 7. Mammalian syntaxin 16C binds to mVps45p via an evolutionarily conserved N-terminal peptide motif. (A) Yeast two-hybrid assay. Yeast cells were co-transfected with pLexN-mVps45 and the indicated pVP16-syntaxin 16C prey clones (numbers indicate amino acid residues included in the constructs or mutated in the context of full-length syntaxin 16C). The relative β-galactosidase activity was calculated as described in the legend to Figure 4. Data shown are means ± SE from quadruple determinations. (B–D) GST-pulldown assays. In (B), glutathione–Sepharose beads containing GST alone or GST–syntaxin 16C fragments were incubated with extract of COS-1 cells expressing myc-tagged mVps45. After washing, the beads were analyzed by SDS–PAGE and visualized by immunoblotting with anti-myc·tag monoclonal antibody. In (C) and (D), GST alone, GST–syntaxin 1A (used as a negative control) and different GST–syntaxin 16 constructs were incubated with bacterial extract expressing T7-tagged mVps45. The beads were processed as in (B) and gels were visualized by Coomassie Blue staining (upper panels) or by immunoblotting with anti-T7·Tag monoclonal antibody (lower panels). Note that the recombinant mVps45 co-migrates with GST–syntaxin 16H1–284 and cannot be seen on the Coomassie Blue-stained gel (D, upper panel). Numbers on the left indicate the positions of molecular mass markers. Asterisks indicate the positions of GST and GST-fusion proteins and the arrows indicate mVps45.
The interaction between Tlg2p/syntaxin 16 and Vps45 is highly specific
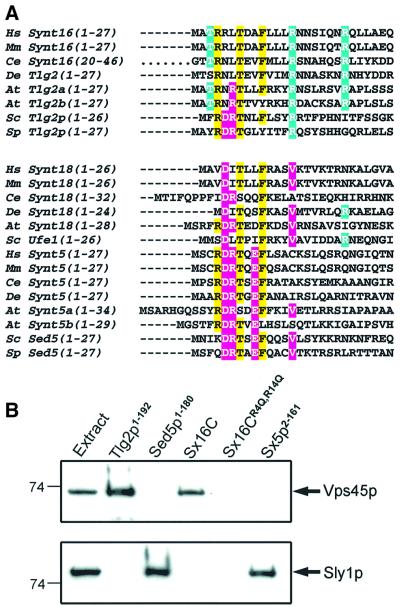
Overall, our results demonstrate that Tlg2p and syntaxin 16 bind to Vps45p/mVps45 via an evolutionarily conserved peptide motif at the N-terminus of their sequence. Interestingly, two other yeast syntaxins, Sed5p and Ufe1p, bind to the SM protein Sly1p via N-terminal peptide motifs, and this binding mode is conserved in their mammalian counterparts, syntaxin 5, syntaxin 18 and mammalian Sly1 (Yamaguchi et al., 2002). Comparison of the N-terminal sequences of these syntaxins from yeast to man (Figure 8A) shows that their N-terminal peptide motifs exhibit some clear differences but also share some similarities, and a TXXF motif appears to be almost absolutely conserved in all of them. A key question raised by this observation is to what extent these syntaxin/SM protein interactions are specific. While yeast two-hybrid assays already indicated that these interactions are specific both in yeast and mammals (Yamaguchi et al., 2002), the similarity between some of these peptide motifs, particularly those of Tlg2p and Sed5p, led us to test the specificity of their interactions with Vps45p and Sly1p further, using an independent method. GST-pulldown experiments revealed that the specificity is in fact striking (Figure 8B). Thus, neither Sed5p nor syntaxin 5 bound to Vps45p, but both bound strongly to Sly1p. On the other hand, Tlg2p and syntaxin 16 did not bind to Sly1p, but both interacted efficiently with Vps45p, and the binding observed for syntaxin 16 was abolished by the R4Q,R14Q mutation. Note that the interactions of the mammalian syntaxin 5 and syntaxin 16 with the corresponding SM proteins from yeast is remarkably strong since binding was observed even after washing the resin with 0.9 M NaCl (data not shown). In addition, pulldown experiments with GST–Tlg2p(1–68) revealed strong binding to mVps45, but no binding to GST–Tlg2p(65–192) was observed (data not shown). All these results emphasize the importance of the residues selectively conserved in Tlg2p homologs for Vps45 binding, and of those selectively conserved in Sed5p homologs for Sly1p binding. The nature of the residue in the position corresponding to R13 of Tlg2p appears to be particularly critical for binding to the corresponding SM protein, but additional mutagenesis experiments will be required to define further the determinants of specificity. The cross-reactivity of mammalian syntaxin 16 and mVps45 with the yeast Vps45p and Tlg2p, respectively, also reinforces the notion that they are true homologs of these proteins.
Fig. 8. Specificity of syntaxin/SM protein interactions. (A) Sequence alignment of syntaxin N-terminal peptide motifs involved in binding to SM proteins. The aligned sequences have been divided in two classes: Tlg2/syntaxin 16 homologs, which bind to Vps45 (on the top), and Ufe1/syntaxin 18 and Sed5/syntaxin 5 homologs, which bind to Sly1 (on the bottom). Identical amino acid residues common to both classes and present in >50% of the sequences are shown on the yellow background. Class-specific residues present in >50% of the sequences from the given class are shown in white on either a blue (Vps45-binding syntaxins) or a pink (Sly1-binding syntaxins) background. Hs, Homo sapiens; Mm, Mus musculus; Ce, Caenorhabditis elegans; Dm, Drosophila melanogaster; At, Arabidopsis thaliana; Sc, Schizosaccharo myces cerevisiae; Sp, Schizosaccharomyces pombe. (B) Binding of Vps45p and Sly1p to different syntaxins. GST-pulldown assays with T7-tagged Vps45p (upper panel) or T7-tagged Sly1p (lower panel) were performed as described in Materials and methods, except that resins were washed with binding buffer containing 400 mM NaCl. Gels were analyzed by immunoblotting with anti-T7·Tag monoclonal antibody. Numbers on the left show the positions of molecular mass markers.
Discussion
In this study, we have shown that Tlg2p, Pep12p and syntaxin 16 have a domain organization typical of proteins from the syntaxin family, but that Tlg2p and syntaxin 16 share an N-terminal peptide motif that is not present in Pep12p. This motif is responsible for binding to Vps45, explaining why this SM protein does not interact directly with Pep12p, and is similar to those involved in binding of Sed5p, Ufe1p and their mammalian homologs to Sly1 (Yamaguchi et al., 2002). However, these syntaxin/SM protein interactions are highly specific both in yeast and in mammals. These results indicate that SM protein/SNARE coupling in TGN and early endosomal membrane fusion occurs via an unanticipated mechanism that is drastically different from the neuronal exocytotic system but resembles the mechanisms acting in fusion at the ER and the Golgi. This is the only mode of interaction between these two families of proteins that has been shown to be common to more than one cellular compartment and to be conserved through evolution.
The notion that SM proteins generally function by binding to a syntaxin closed conformation was enigmatic since, biochemically, this interaction competes with core complex formation (Yang et al., 2000) whereas, functionally, SM proteins are essential for membrane fusion (Verhage et al., 2000). In contrast, the binding mode based on an N-terminal peptide motif should be fully compatible with core complex formation (Figure 9A). In support of this proposal, Vps45p co-immunoprecipitates with Tlg2p, Tlg1p and Vti1p (Nichols et al., 1998; Coe et al., 1999), and removal of the SNARE motif does not affect the Tlg2p/Vps45p interaction (Figure 4). In addition, Sly1p has been recently shown to bind to Sed5p assembled into core complexes (Peng and Gallwitz, 2002). These observations imply that SNAREs and SM proteins can perform active roles in membrane fusion while forming part of the same protein complex. In fact, the peptide motif may recruit the SM protein to the sites of membrane fusion, and it is possible that the peptide motif is sometimes provided by a SNARE that is not a syntaxin. Such a mechanism could explain the ability of Sec1p to bind to the core complex formed by exocytotic yeast SNAREs (Carr et al., 1999). Whether or not these possibilities turn out to be true, it now seems clear that the primary function of SM proteins that makes them essential factors in intracellular membrane traffic is not to fix a syntaxin into a close conformation, and must involve a conserved property that may only be indirectly related to their ability to bind to syntaxins.
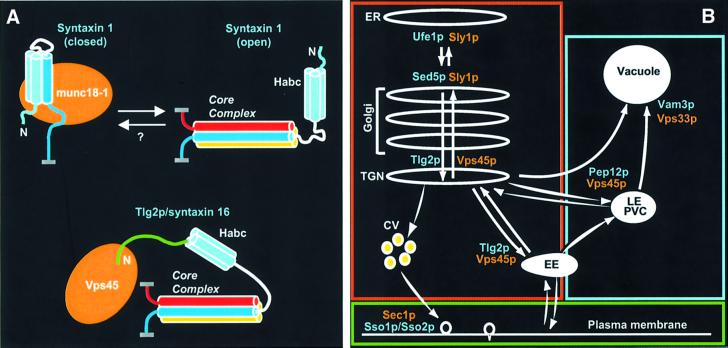
Fig. 9. Syntaxin–SM protein coupling in membrane traffic. (A) Two different modes of direct syntaxin/SM protein interactions currently known. In the upper scheme, the neuronal syntaxin 1 interacts with munc18-1 in a closed conformation (left), while core complex formation requires an open conformation of syntaxin 1 (right). Syntaxin 1 is colored in light blue (N-terminal Habc domain), white (linker region) and blue (SNARE motif), while munc18-1 is colored in orange, and other SNARE motifs involved in the core complex are colored in yellow and red (for the vesicular SNARE). ‘N’ indicates the N-terminus of syntaxin 1, helices are represented by cylinders and the attachment points to the corresponding membranes are represented by thick gray lines. In the closed conformation, only part of the SNARE motif binds to the Habc domain and has a defined structure (Dulubova et al., 1999; Misura et al., 2000). It is currently unknown if, in vivo, there is a true equilibrium between the two states of syntaxin 1 (hence the ‘?’). The lower scheme illustrates the N-terminal peptide-based interaction between the TGN/endosomal Tlg2p/syntaxin 16 and Vps45, which is analogous to the interaction of the ER Ufe1p/syntaxin 18 and the Golgi Sed5p/syntaxin 5 with Sly1p. The coloring scheme is the same, except that the N-terminal peptide motif of Tlg2p/syntaxin 16 is colored in green. The diagram emphasizes the fact that this mode of interaction is compatible with core complex formation and allows a simultaneous active role for the core complex and the SM protein in membrane fusion. (B) Summary of membrane traffic pathways in yeast. The syntaxins (blue labels) and SM proteins (orange labels) involved in each pathway are indicated. The red box encloses all the membrane compartments where syntaxin/SM protein coupling occurs by an N-terminal peptide-based mechanism. In the late endosome/prevacuolar compartment (PVC) and the vacuole (included in the blue box), syntaxins appear to interact indirectly with SM proteins incorporated into large protein complexes. A different mode of syntaxin/SM protein coupling may operate in the plasma membrane (green box). CV, constitutive secretory vesicles; EE, early endosome; ER, endoplasmic reticulum; LE, late endosome; TGN, trans-Golgi network. Adapted from Südhof and Scheller (2001).
A recent study showed that Tlg2p becomes unstable and unable to co-immunoprecipitate with Tlg1p and Vti1p in the absence of Vps45p, and that removal of the 230 N-terminal residues of Tlg2p rescued this phenotype (Bryant and James, 2001). Based on the neuronal model, it was proposed that Vps45p facilitates SNARE complex formation by acting on a closed conformation of Tlg2p. However, our data indicate that Tlg2p does not adopt a closed conformation analogous to that of syntaxin 1, and that the Habc domain is not required for Vps45p binding. We cannot rule out the possibility that SNARE complex assembly is hindered by an interaction between the peptide motif and the SNARE motif of Tlg2p, yielding a novel type of closed conformation, and that Vps45p binding releases the inhibition. Alternatively, the results of Bryant and James (2001) may reflect not only binding of Vps45p to the peptide motif of Tlg2p, which explains the stabilization effect, but also the currently unknown function of the Habc domain. Thus, an inhibitory factor might prevent SNARE complex formation by binding to Tlg2p, perhaps via the Habc domain, and Vps45p may release this inhibition. Another recent study showed that lipid vesicles containing reconstituted Tlg2p, Tlg1p and Vti1p (t-SNAREs) could not fuse with vesicles containing Snc2p (v-SNARE), but fusion was observed upon addition of a peptide corresponding to the C-terminal half of the Snc2p SNARE motif (Paumet et al., 2001). These results were also interpreted in terms of a model whereby Tlg2p is locked in an inactive state that requires activation by a factor such as Vps45p, and it was suggested that the Snc2p peptide bypasses this requirement. However, it is unlikely that the inert t-SNARE state could be affected by Vps45p since the recombinant Tlg2p fragment used in this study lacked the 35 N-terminal residues. Instead, it is plausible that the Snc2p peptide released a kinetic trap formed by a t-SNARE four-helix bundle resembling a kinetically trapped state that has been observed for the neuronal syntaxin 1 and SNAP-25 (Misura et al., 2001).
The notion that syntaxin 16 and mVps45p are mammalian homologs of Tlg2p and Vps45p is reinforced by our finding that they share a common binding mechanism and that they cross-react. Unexpectedly, we find that although syntaxin 16 mRNA is expressed at fairly uniform levels in most tissues (Simonsen et al., 1998; Tang et al., 1998), the levels of syntaxin 16 protein expressed are selectively high in brain. These results suggest that the synthesis and/or stability of syntaxin 16 are regulated posttranscriptionally, and that syntaxin 16 has a specific, as yet uncharacterized, brain function. The nature of the multiple splice variants observed in syntaxin 16 mRNAs (Simonsen et al., 1998; Tang et al., 1998) (Figure 5A) gains new meaning from the observation that an N-terminal peptide motif mediates binding to mVps45p, since this is the only functional region of syntaxin 16 that is intact in all variants. Thus, differences in the length of the sequence that follows the peptide motif may modulate the interplay between mVps45p binding and core complex formation. These two events should be uncoupled for the C-terminally truncated forms of syntaxin 16 since they are still capable of mVps45p binding, but cannot form complexes and are not anchored to a membrane. The fact that we could not detect the truncated syntaxin 16 proteins suggests that they are normally not synthesized or are rapidly degraded. These variants may have little biological significance, or they may be subject to posttranscriptional regulation that allows their production in a subset of cells under specialized conditions. We favor the latter hypothesis because the alternative splicing of syntaxin 16 is evolutionarily conserved in mammals, the full-length form of syntaxin 16 also appears to be subject to posttranscriptional regulation, and the nature of the truncated forms is ideally suited for regulatory events that uncouple mVps45 binding from SNARE complex formation.
Seven syntaxins and four SM proteins are found in the yeast genome (Pelham, 1999) (Figure 9B). Together with previous studies of these proteins and some of their homologs in higher eukaryotes, the results described here yield a complex picture whereby the membrane fusion machineries from different cellular compartments and different organisms share some common features but also exhibit critical distinctions. Our data reinforce the notion that all syntaxins contain an N-terminal Habc domain while the closed conformation is not a general property of syntaxins. The discovery that Ufe1p, Sed5p and Tlg2p contain an N-terminal peptide motif that is critical for SM-protein binding reveals another functional domain of syntaxins that is evolutionarily conserved, but is common to only a subset of syntaxins. Interestingly, Sly1p binds to both Ufe1p and Sed5p by an analogous mechanism, while Vps45p interacts functionally with Tlg2p and Pep12p but binds only to Tlg2p by this mechanism. In the yeast prevacuolar compartment and vacuole, syntaxins (Pep12p and Vam3p) and SM proteins (Vps45p and Vps33p) appear to interact indirectly by a different, unknown mechanism that involves incorporation of the SM protein into a large multiprotein complex (Tall et al., 1999; Sato et al., 2000; Seals et al., 2000). The interaction between syntaxin 1 and munc18-1 constitutes a third mode of syntaxin/SM protein coupling, and a different but as yet unclear coupling mode between Sec1p and Sso1p operates in the yeast plasma membrane. A possible conceptual explanation for this diversity is suggested by considering the types of syntaxin/SM protein interactions that occur in different cellular compartments (Figure 9B). The N-terminal peptide-based binding mode appears to represent the simplest and most widespread mechanism of coupling, and operates in membrane traffic events to and from the Golgi. These events involve internal membrane compartments whose fusion machineries may have originated from a common ancestor and are conserved from yeast to humans. Other modes of syntaxin/SM protein coupling may have arisen to meet specific requirements and some may have changed through evolution. Further studies of membrane traffic in yeast and higher eukaryotes will help to expand this view of conservation and divergence in the mechanisms of intracellular membrane fusion, and additional surprises are likely to surface.
Materials and methods
Protein expression and purification
Constructs for expression of the different fragments of Tlg2p, Pep12p, Sed5p, syntaxin 1A, syntaxin 5 and syntaxin 16 were generated by PCR with custom-designed primers, either on total yeast genomic DNA prepared from Saccharomyces cerevisiae strain SEY6210 (for the yeast syntaxins) or human brain cDNA library (for the mammalian syntaxins), subcloned into pGEX-KG (Guan and Dixon, 1991) or pGEX-KT (Hakes and Dixon, 1992) vectors and expressed in bacteria (E.coli BL21) as GST-fusion proteins. Site-directed mutagenesis was performed using either standard PCR techniques, by introducing the mutated sequence via the PCR primer, or by using the QuickChange™ Site-Directed Mutagenisis Kit (Stratagene). To generate uniformly 15N- or 15N-13C- labeled samples for NMR studies, bacteria were grown in minimal media supplemented with 15NH4Cl and with or without [13C6]glucose (CIL, Inc., Andover, MA) as the sole nitrogen and carbon sources. The fusion proteins were affinity-purified on glutathione–Sepharose (Pharmacia), cleaved from the GST moiety with thrombin (Sigma) and purified further by ion-exchange chromatography essentially as described previously (Dulubova et al., 1999).
NMR spectroscopy
NMR data were acquired at 25°C on Varian INOVA500 or INOVA600 spectrometers using H2O/D2O 95:5 (v/v) as the solvent. All Tlg2p and Pep12p 1H-15N HSQC spectra were obtained at 100–200 µM protein concentrations in 20 mM sodium phosphate (pH 7.3) containing 200 mM NaCl and protease inhibitors. Backbone assignments for the Tlg2p(65–192) fragment were obtained using 1H-15N 3D NOESY-HSQC, HNCO, HNCACB and CBCACONH spectra acquired on a 0.7 mM 15N-13C-labeled sample. Syntaxin 16C and syntaxin 16H(59–183) 1H-15N HSQC spectra were obtained at 100 µM protein concentration in 20 mM sodium phosphate (pH 7.3) containing 1 mM EGTA, 1 mM EDTA, 2 mM DTT and Sigma protease inhibitors cocktail.
Yeast two-hybrid assays
Yeast two-hybrid assays were performed using full-length yeast or mouse Vps45 cloned into the pLexN bait vector, and different Tlg2p, Pep12p and human syntaxin 16 cDNA fragments cloned into the pVP16-3 prey vector. All assays were performed in the yeast strain L40 harboring bait and prey vectors, with empty vectors as controls. Quantitation of interactions was carried out using liquid β-galactosidase assays as described (Cao and Südhof, 2001).
GST-pulldown assays
To produce T7-tagged yeast and mammalian Vps45, the corresponding cDNAs were subcloned into the pET-21 bacterial expression vector (Novagen) and transformed into E.coli BL21trxB(DE3). Since both yeast and mammalian Vps45 are largely insoluble when expressed in bacterial cells, to increase solubility the recombinant proteins were induced at a low temperature (16°C) for 20 h with 0.2 mM IPTG. After induction, cells were lysed in PBS containing 1 mM EGTA, 1 mM EDTA, 2 mM DTT and Sigma protease inhibitors cocktail, pre-cleared by incubation for 4 h at 4°C with glutathione–agarose, and Triton X-100 was added to a final 1% concentration. Myc-tagged Vps45 was subcloned into the pCMV5 vector and expressed in COS-1 cells. T7-tagged Sly1p was expressed as described previously (Yamaguchi et al., 2002). Recombinant GST-fusion proteins corresponding to different fragments of Tlg2p, Pep12p, Sed5p, syntaxin 16, syntaxin 5 and syntaxin 1a (as indicated on the figures) or GST alone were affinity purified on glutathione–Sepharose beads (see above). For mammalian Vps45 binding, beads corresponding to ∼15 µg of protein were incubated with 1 ml of bacterial or COS-1 cell extract expressing T7- or myc-tagged mVps45. After 4 h incubation at 4°C, the beads were washed five times with 1 ml of ice-cold binding buffer. For the yeast SM protein binding, beads (corresponding to ∼100 µg of recombinant protein) were incubated with 1.8 ml of the T7-Vps45p or T7-Sly1 containing cellular extract (with a total protein concentration of ∼5 mg/ml). After incubation overnight at 4°C, these beads were exposed to stringent washes [sequentially washed with 3× 5 ml of binding buffer (PBS with 1 mM EDTA, 1 mM EGTA and 2 mM DTT) additionally containing 0, 400 or 900 mM NaCl]. Finally, beads were resuspended in 240 µl of 1× SDS–PAGE loading buffer, 20 µl aliquots were separated on a 10% SDS–polyacrylamide gel along with 5 µl of the corresponding cellular extract and the bands were visualized by staining with Coomassie Blue. Aliquots equivalent to 1/20th part of the samples used for the staining were run separately, transferred onto a nitrocellulose membrane and probed with anti-T7·Tag (Novagen) or myc-tag (Santa Cruz Biotechnology) monoclonal antibodies. Immunoblots were developed using enhanced chemiluminescence (ECL) detection (Amersham).
Generation of antibodies and immunodetection of syntaxin 16 in mouse tissues
Syntaxin 16 antibodies (4398) were raised in rabbits against bacterially expressed recombinant syntaxin 16C. The serum was affinity purified on a GST–syntaxin 16C column using SulfoLink Coupling Gel (Pierce). Homogenates from different mouse tissues were obtained as described previously (Sugita et al., 2001). Aliquots (50 µl) of the extracts were run on a 7.5% SDS–polyacrylamide gel, transferred to nitrocellulose and probed with syntaxin 16 and VSP antibodies followed by ECL detection.
Expression of syntaxin 16 splice variants in mammalian cells
COS-1 cells were transiently transfected with pCMV5 expression vectors encoding human syntaxin 16C, syntaxin 16D or the entire cytoplasmic region of syntaxin 16F (1–284) using Lipofectamine (Invitrogen) according to the manufacturer protocol. After 48 h, cells were harvested in PBS containing 1 mM EDTA, 1 mM DTT, 0.1 mM PMSF, and analyzed on SDS–polyacrylamide gel followed by western blotting with syntaxin 16 antibodies and ECL detection.
Acknowledgments
Acknowledgements
We thank Drs B.Horazdovsky (UT Southwestern Medical Center at Dallas) and D.James (University of Queensland, Australia) for reagents, and I.Leznicki and E.Borowicz for technical assistance. This work was supported by an Established Investigator Award from the American Heart Association and by NIH grant NS37200 (to J.R.).
References
- Abeliovich H., Grote,E., Novick,P. and Ferro-Novick,S. (1998) Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J. Biol. Chem., 273, 11719–11727. [DOI] [PubMed] [Google Scholar]
- Abeliovich H., Darsow,T. and Emr,S.D. (1999) Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE–Sec1p complex composed of Tlg2p and Vps45p. EMBO J., 18, 6005–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham D.C., Sanderfoot,A.A., Kovaleva,V., Zheng,H. and Raikhel,N.V. (2000) AtVPS45 complex formation at the trans-Golgi network. Mol. Biol. Cell, 11, 2251–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M.K. and Scheller,R.H. (1993) The molecular machinery for secretion is conserved from yeast to neurons. Proc. Natl Acad. Sci. USA, 90, 2559–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner J.H., Blanchette,J.M., Sipos,G. and Fuller,R.S. (2001) The Tlg SNARE complex is required for TGN homotypic fusion. J. Cell Biol., 155, 969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant N.J. and James,D.E. (2001) Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation. EMBO J., 20, 3380–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C.G., Peterson,M., Cowles,C.R. and Emr,S.D. (1997) A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol. Biol. Cell, 8, 1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. and Südhof,T.C. (2001) A transcriptively active complex of APP with Fe65 and histone acetyltransferase Tip60. Science, 293, 115–120. [DOI] [PubMed] [Google Scholar]
- Carr C.M., Grote,E., Munson,M., Hughson,F.M. and Novick,P.J. (1999) Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol., 146, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe J.G., Lim,A.C., Xu,J. and Hong,W. (1999) A role for Tlg1p in the transport of proteins within the Golgi apparatus of Saccharomyces cerevisiae. Mol. Biol. Cell, 10, 2407–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I., Sugita,S., Hill,S., Hosaka,M., Fernandez,I., Südhof,T.C. and Rizo,J. (1999) A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J., 18, 4372–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I., Yamaguchi,T., Wang,Y., Südhof,T.C. and Rizo,J. (2001) Vam3p structure reveals conserved and divergent properties of syntaxins. Nat. Struct. Biol., 8, 258–264. [DOI] [PubMed] [Google Scholar]
- Fernandez I., Ubach,J., Dulubova,I., Zhang,X., Südhof,T.C. and Rizo,J. (1998) Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell, 94, 841–849. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S. and Jahn,R. (1994) Vesicle fusion from yeast to man. Nature, 370, 191–193. [DOI] [PubMed] [Google Scholar]
- Fiebig K.M., Rice,L.M., Pollock,E. and Brunger,A.T. (1999) Folding intermediates of SNARE complex assembly. Nat. Struct. Biol., 6, 117–123. [DOI] [PubMed] [Google Scholar]
- Grabowski R. and Gallwitz,D. (1997) High-affinity binding of the yeast cis-Golgi t-SNARE, Sed5p, to wild-type and mutant Sly1p, a modulator of transport vesicle docking. FEBS Lett., 411, 169–172. [DOI] [PubMed] [Google Scholar]
- Guan K.L. and Dixon,J.E. (1991) Eukaryotic proteins expressed in E.coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem., 192, 262–276. [DOI] [PubMed] [Google Scholar]
- Gurunathan S., Chapman-Shimshoni,D., Trajkovic,S. and Gerst,J.E. (2000) Yeast exocytic v-SNAREs confer endocytosis. Mol. Biol. Cell, 11, 3629–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakes D.J. and Dixon,J.E. (1992) New vectors for high level expression of recombinant proteins in bacteria. Anal. Biochem., 202, 293–298. [DOI] [PubMed] [Google Scholar]
- Hanson P.I., Roth,R., Morisaki,H., Jahn,R. and Heuser,J.E. (1997) Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell, 90, 523–535. [DOI] [PubMed] [Google Scholar]
- Harrison S.D., Broadie,K., van de Goor,J. and Rubin,G.M. (1994) Mutations in the Drosophila Rop gene suggest a function in general secretion and synaptic transmission. Neuron, 13, 555–566. [DOI] [PubMed] [Google Scholar]
- Hata Y., Slaughter,C.A. and Südhof,T.C. (1993) Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature, 366, 347–351. [DOI] [PubMed] [Google Scholar]
- Holthuis J.C., Nichols,B.J. and Pelham,H.R. (1998) The syntaxin Tlg1p mediates trafficking of chitin synthase III to polarized growth sites in yeast. Mol. Biol. Cell, 9, 3383–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono R., Hekimi,S., Kamiya,Y., Sassa,T., Murakami,S., Nishiwaki,K., Miwa,J., Taketo,A. and Kodaira,K.I. (1992) The unc-18 gene encodes a novel protein affecting the kinetics of acetylcholine metabolism in the nematode Caenorhabditis elegans. J. Neurochem., 58, 1517–1525. [DOI] [PubMed] [Google Scholar]
- Jahn R. and Südhof,T.C. (1999) Membrane fusion and exocytosis. Annu. Rev. Biochem., 68, 863–911. [DOI] [PubMed] [Google Scholar]
- Kosodo Y., Noda,Y. and Yoda,K. (1998) Protein–protein interactions of the yeast Golgi t-SNARE Sed5 protein distinct from its neural plasma membrane cognate syntaxin 1. Biochem. Biophys. Res. Commun., 250, 212–216. [DOI] [PubMed] [Google Scholar]
- Lewis M.J., Nichols,B.J., Prescianotto-Baschong,C., Riezman,H. and Pelham,H.R. (2000) Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell, 11, 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R.C. and Scheller,R.H. (2000). Mechanisms of synaptic vesicle exocytosis. Annu. Rev. Cell. Dev. Biol., 16, 19–49. [DOI] [PubMed] [Google Scholar]
- Mallard F. et al. (2002) Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol., 156, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misura K.M., Scheller,R.H. and Weis,W.I. (2000) Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature, 404, 355–362. [DOI] [PubMed] [Google Scholar]
- Misura K.M., Gonzalez,L.C.,Jr, May,A.P., Scheller,R.H. and Weis,W.I. (2001) Crystal structure and biophysical properties of a complex between the N-terminal SNARE region of SNAP25 and syntaxin 1a. J. Biol. Chem., 276, 41301–41309. [DOI] [PubMed] [Google Scholar]
- Munson M., Chen,X., Cocina,A.E., Schultz,S.M. and Hughson,F.M. (2000) Interactions within the yeast t-SNARE Sso1p that control SNARE complex assembly. Nat. Struct. Biol., 7, 894–902. [DOI] [PubMed] [Google Scholar]
- Nichols B.J., Holthuis,J.C. and Pelham,H.R. (1998) The Sec1p homologue Vps45p binds to the syntaxin Tlg2p. Eur. J. Cell Biol., 77, 263–268. [DOI] [PubMed] [Google Scholar]
- Ossig R., Dascher,C., Trepte,.H., Schmitt,H.D. and Gallwitz,D. (1991) The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol. Cell. Biol., 11, 2980–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek H.R., Conibear,E., Bryan,J.D., Colvin,R.T., Goshorn,C.D. and Robinson,L.C. (2000) Identification of Rgp1p, a novel Golgi recycling factor, as a protein required for efficient localization of yeast casein kinase 1 to the plasma membrane. J. Cell Sci., 113, 4545–4555. [DOI] [PubMed] [Google Scholar]
- Paumet F., Brugger,B., Parlati,F., McNew,J.A., Sollner,T.H. and Rothman,J.E. (2001) A t-SNARE of the endocytic pathway must be activated for fusion. J. Cell Biol., 155, 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H.R. (1999) SNAREs and the secretory pathway—lessons from yeast. Exp. Cell Res., 247, 1–8. [DOI] [PubMed] [Google Scholar]
- Peng R. and Gallwitz,D. (2002) Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes. J. Cell Biol., 157, 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier M.A., Xiao,W., Macosko,J., Chan,C., Shin,Y.K. and Bennett,M.K. (1998) The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat. Struct. Biol., 5, 765–769. [DOI] [PubMed] [Google Scholar]
- Sato T.K., Rehling,P., Peterson,M.R. and Emr,S.D. (2000) Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell, 6, 661–671. [DOI] [PubMed] [Google Scholar]
- Schekman R. (1992) Genetic and biochemical analysis of vesicular traffic in yeast. Curr. Opin. Cell Biol., 4, 587–592. [DOI] [PubMed] [Google Scholar]
- Seals D.F., Eitzen,G., Margolis,N., Wickner,W.T. and Price,A. (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl Acad. Sci. USA, 97, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séron K. et al. (1998) A yeast t-SNARE involved in endocytosis. Mol. Biol. Cell, 9, 2873–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Bremnes,B., Ronning,E., Aasland,R. and Stenmark,H. (1998) Syntaxin-16, a putative Golgi t-SNARE. Eur. J. Cell Biol., 75, 223–231. [DOI] [PubMed] [Google Scholar]
- Südhof T.C. and Scheller,R.H. (2001) Mechanism and regulation of neurotransmitter release. In Cowan,W.M., Südhof,T.C. and Stevens,C.F. (eds), Synapses. The Johns Hopkins University Press, Baltimore/London, pp. 177–215.
- Sugita S., Han,W., Butz,S., Liu,X., Fernandez-Chacon,R., Lao,Y. and Südhof,T.C. (2001) Synaptotagmin VII as a plasma membrane Ca2+ sensor in exocytosis. Neuron, 30, 459–473. [DOI] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer,D., Jahn,R. and Brunger,A.T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature, 395, 347–353. [DOI] [PubMed] [Google Scholar]
- Tall G.G., Hama,H., DeWald,D.B. and Horazdovsky,B.F. (1999) The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol. Biol. Cell, 10, 1873–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B.L., Low,D.Y., Lee,S.S., Tan,A.E. and Hong,W. (1998) Molecular cloning and localization of human syntaxin 16, a member of the syntaxin family of SNARE proteins. Biochem. Biophys. Res. Commun., 242, 673–679. [DOI] [PubMed] [Google Scholar]
- Tellam J.T., James,D.E., Stevens,T.H. and Piper,R.C. (1997) Identification of a mammalian Golgi Sec1p-like protein, mVps45. J. Biol. Chem., 272, 6187–6193. [DOI] [PubMed] [Google Scholar]
- Verhage M. et al. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science, 287, 864–869. [DOI] [PubMed] [Google Scholar]
- Webb G.C., Hoedt,M., Poole,L.J. and Jones,E.W. (1997) Genetic interactions between a pep7 mutation and the PEP12 and VPS45 genes: evidence for a novel SNARE component in transport between the Saccharomyces cerevisiae Golgi complex and endosome. Genetics, 147, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman,B.V., McNew,J.A., Westermann,B., Gmachl,M., Parlati,F., Sollner,T.H. and Rothman,J.E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell, 92, 759–772. [DOI] [PubMed] [Google Scholar]
- Wishart D.S. and Sykes,B.D. (1994) The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR, 4, 171–180. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Dulubova,I., Min,S.W., Chen,X., Rizo,J. and Südhof,T.C. (2002) Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev. Cell, 2, 295–305. [DOI] [PubMed] [Google Scholar]
- Yang B., Steegmaier,M., Gonzalez,L.C.,Jr, and Scheller,R.H. (2000) nSec1 binds a closed conformation of syntaxin1A. J. Cell Biol., 148, 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]