Abstract
Pyrazole ligands have become important and accessible building blocks in supramolecular chemistry because of their special coordination properties, which promote the self-assembly of complex molecular structures. Pyrazole ligands can self-assemble into supramolecular structures such as metal–organic frameworks, coordination polymers, and nanostructured materials through noncovalent interactions such as hydrogen bonding, π–π stacking, and metal coordination. These assemblies demonstrate exceptional capabilities with applications ranging from catalysis to sensing, drug delivery, and material science. Despite having great potential, pyrazole ligands face significant complications, such as low stability under different environmental conditions, low synthesis scalability, and low selectivity and sensitivity in real-world applications. To overcome these limitations, future research should focus on improving the design and functionality of pyrazole ligands using advanced synthetic methods, computational modeling, and integration into hybrid materials. The ongoing investigation of pyrazole ligands has the potential to reveal novel possibilities for the development of cutting-edge materials and technologies, underscoring their crucial contribution to the progress of supramolecular chemistry. This review highlights the significance of pyrazole ligands in self-assembly, their applications, the challenges faced, and future directions for research in this area.


1. Introduction
The concept of self-assembly, which is closely tied to the development of molecular biology and chemistry, originated from groundbreaking studies in the 1950s. This era marked a breakthrough in understanding biological molecules, notably lipid bilayer formation, where amphiphilic molecules self-assemble in aqueous environments, forming cellular membranes and underscoring the self-assembly in biology. , In synthetic systems, hydrogen bonding facilitates the formation of robust and adaptable structures, showcasing its utility in both natural and energized environments. Protein folding is another fundamental example of self-assembly, which is vital in biology and supramolecular chemistry and their applications across materials science and technology. Beyond biology, self-assembly is a cornerstone of supramolecular chemistry, which investigates molecule interactions, leading to organized structures. Unlike covalent chemistry, supramolecular chemistry is governed by noncovalent interactions such as hydrogen bonding, van der Waals forces, π–π interactions, and metal coordinations. − Nanoparticles also self-assemble through noncovalent interactions, forming larger structures with unique optical, electrical, and catalytic properties that are essential for biological and synthetic applications. , Block copolymers, for example, self-assemble into nanostructures like spheres and cylinders, which find application in drug delivery, lithography, and photonic materials. , Similarly, self-assembled monolayers on metal surfaces are vital in sensors, catalysis, and nanoelectronics, as they create tailored surfaces with specific chemical and physical properties for high-performance systems. , The spatial organization and interaction of particles in these assemblies are often directed by ligands or templates, which enhance the stability and functionality. For example, lipid bilayers, molecular solids, and conjugated polymers. , Metallic coordination further broadens the scope of self-assembly, enabling the formation of supramolecular structures like metal–organic frameworks (MOFs) and coordination polymers used in gas storage, separation, and catalysis. The self-assembly of supramolecular compounds extends to drug delivery, catalysis, and sensing through the use of host–guest systems, coordination complexes, and amphiphilic molecules to form micelles and bilayers. , Ligands play a pivotal role in supramolecular chemistry by facilitating the construction of complex structures via noncovalent interactions. Cyclodextrins, calixarenes, and pyrazole are prominent examples of ligands known for their supramolecular capabilities. Pyrazole ligands, in particular, possess unique properties that make them highly suitable for a wide range of applications in supramolecular chemistry. Pyrazole is a versatile ligand in supramolecular chemistry due to its unique ability to coordinate with metal ions, forming stable complexes that enable the construction of metal–organic frameworks, coordination polymers, and host–guest systems. Their versatility allows for the adjustment of electrical properties and the creation of stable structures, enabling their use in catalysis, drug delivery, and material science. Its ability to participate in π–π stacking and hydrogen bonding enhances its effectiveness in designing sensors and catalysts, while its coordination versatility supports dynamic self-assembled systems for environmental detection and therapeutic delivery. The distinctive characteristics and coordination flexibility of pyrazole ligands underline their potential in developing novel materials and technologies across diverse scientific fields.
This adaptability highlights the far-reaching promise of supramolecular chemistry in addressing challenges and advancing innovations in contemporary science.
1.1. Review of Related Literature
Malcolm A. Halcrow provided a comprehensive analysis of the metal coordination modes of pyrazoles and their anions, highlighting their versatility and applications. Pyrazolide anions exhibit 20 distinct coordination modes, support metal cluster compounds, and have applications in luminescence, charge–transfer complexes, liquid crystals, supramolecular materials, and MOFs. These materials have applications in gas adsorption, catalysis, and sensing. Figure illustrates the various coordination modes of azole and azolate ligands with metal centers, which are facilitated by pyrazolide anions, as well as the complicated coordination of multidentate and multimetal ligands (bottom rows), where the amounts of contiguous atoms participating in coordination are indicated by hapticity, demonstrating the sophisticated structural possibilities afforded by these ligands. In 2024, Ahmad and colleagues successfully produced a new compound called ethyl 2-(5-(4-methoxypheny1)-3-pheny1–4,5-dihydro-1H-pyrazol-1-y1)thiazole-4-carboxylate (5). The results indicate that C–H···O and C–H···N hydrogen bonds connect the molecules in the crystal packing. In addition to these hydrogen bonds, the centroid-to-centroid distance of 4.1780(5) Å controls the π···π stacking interactions between neighboring rings and thus controls that supramolecular assembly, showcasing the intricate interplay of noncovalent interactions in the crystal structure. Bala et al. reported the synthesis and characterization of metal-directed architectures with a new asymmetric, semirigid bis-chelating pyridine-pyrazole ligand (H2APPC). This ligand facilitated the formation of a variety of multinuclear complexes with transition metals, including a cadmium [3 × 3] grid with four distinct coordination geometries, a hexanuclear cage with nickel, a unique copper structure with an in situ generated ligand, a mononuclear complex with zinc, and a [3 × 3] grid with manganese. These findings highlight the adaptability of pyrazole ligands in constructing diverse supramolecular frameworks with complex geometries. Tigreros et al. demonstrated that chemosensors based on pyrazole derivatives have exceptional photophysical properties and synthetic adaptability compared to other broadly used scaffolds; these properties make pyrazole derivatives particularly attractive for the development of advanced sensing systems. Similarly, Nandurkar et al. synthesized a series of 2-(5-aryl-1-phenyl-1H-pyrazol-3-yl)-4-aryl thiazoles by cyclocondensation of 5-aryl-1-phenyl-1H-pyrazole-3-carbothioamide with substituted phenacyl bromide. The resulting compounds demonstrated notable antibacterial and antifungal properties. Additionally, some compounds showed similar effectiveness to the reference drug Ravuconazole, indicating their potential as primary agents for the treatment of microbial infections. In another significant study, Rasgania et al. successfully synthesized novel 5-phenyl-3-(thiophen-2-yl)-4, 5-dihydro-1H-pyrazol-1-yl) (pyridin-4-yl)methanones in 2024. The resulting compounds showed strong antimycobacterial activity; especially analogue 4i, containing a 2,4-dimethoxyphenyl substituent with a minimum inhibitory concentration of 6.35 μM, has the highest anti-TB action, while analogue 4f, containing a 2-nitrophenyl substituent, shows the highest radical quenching with an IC50 of 96.19 μM. These results underscore the potential of pyrazole ligands as therapeutic agents with broad-spectrum activity against various pathogens and free radicals. Săcărescu et al. described the importance of pyrazoline-based fluorescent sensors due to their selectivity, reliability, and low detection limits for pollution caused by toxic metals (e.g., Zn, Cu, Fe, Ni, Hg, Al, and Ca), multi-ionic mixtures, sulfites, glutathione, and pH. These sensors reveal significant potential in environmental and biological applications. Zhou et al. reported the synthesis of dipalladium (II, II)-based metallacages and metallacapsules via π···π interactions and a metal-coordination-driven hierarchical self-assembly method. The π···π and other weak intermolecular interactions extend the amide-functionalized multipyrazole ligands and dipalladium (II, II) corners into 2-D supramolecular networks, emphasizing their structural and functional diversity. Saha et al. (2023) reported the synthesis of multifunctional Co-MOF and Ni-MOF using bispyrazole ligands and 2-sulphonoterephthalic acid. These MOFs demonstrated exceptional electrical and magnetic characteristics, including high thermal, chemical, and physical stability; substantial conductivity; and ideality factors, making them promising candidates for advanced materials and energy applications. Expanding the utility of the pyrazole-based system, Wang et al. reported the synthesis of pyrazole-based supramolecular [M6L2] metallocages via a self-assembly approach. The synthesized metallocages act as turn-on fluorescence sensors for SO2 and HSO3 – through a disassembly mechanism, making them highly suitable for environmental and biological sensing applications. Wen et al. successfully synthesized multiresponsive (mechanochromism, thermochromism, and metal ion fluorescence switch) smart fluorophores from pyrazole-functionalized tetraphenylethene. The results indicate that the origin of various stimuli-responsive phenomena is due to the increasing number of pyrazolyls, which progressively change the molecular packing of the compound from the C–H stacked compact mode to the X-crossing stacked loose mode. In a related study, Yadav et al. successfully synthesized six novel energetic coordination compounds (ECCs) using [5-(4-nitro-1H-pyrazol-3-yl] in 2023. These compounds form dimeric complexes with remarkable properties such as high densities, exceptional thermal stability, low sensitivity to impact and friction, and promising performance as safe, high-energy-dense materials, positioning them as viable alternatives for industrial applications. Iguarbe et al. investigated the self-assembly behavior of pyrazole-derived platforms grafted onto poly(benzyl ether) dendrons. Liquid crystals and supergels with enhanced photoluminescence in the aggregated states are obtained by supramolecular organization of nondiscoid entities. NMR, IR, and X-ray diffraction (XRD) analyses revealed that 3,5-dimethyl-4-phenyl-1H-pyrazole promotes columnar arrangements of wedge- or cone-shaped dendrons into supramolecular polymers via hydrogen bonding, with variations in mesomorphic and luminescent properties depending on the type of imide or ether linker used. Ghorbanpour et al. reviewed the anticancer application of pyrazole-based copper complex alternatives to approved drugs with lower side effects. The study aimed to evaluate the mechanistic action at the molecular level and find correlations between the chemical structures of these complexes and the biological activity. This study emphasizes various mechanisms, such as DNA inhibition and apoptosis, that may serve as viable alternatives to current medications while exhibiting reduced adverse effects. Gómez et al. reported that the synthesized mononuclear and diplatinum (PtII–PtII) complexes based on 2-phenylbenzothiazole and 2-(4-dimethylaminophenyl) benzothiazole have phosphorescence properties and potential application toward photocatalysis. Results showed that the ligands influenced the properties of the synthesized complexes. Changing the ligands alters the fluorescence and phosphorescence, reversible photoinduced phosphorescence, and promotes efficient O2 generation for photocatalysis. Mandal et al. reported the synthesis of two novel distorted octahedral cationic complexes, e.g., [Co(MPAFA)3](ClO4)2 and [Ni(MPAFA)3] (NO3)2·2H2O. The synthesized complexes exhibit coordination through pyrazolyl tertiary and azomethine nitrogen atoms and display π–π stacking, hydrogen bonding, fluorescence, and DNA groove binding with calf thymus DNA. Hamodin et al. investigated the biological applications of novel pyrazole derivatives with improved amorphous, porous structure and reduced thermal activation energy. Song et al. reported the synthesis of hydrogen-bonded organic frameworks composed of molecular tectons with complementary hydrogen-bonding patterns with high chemical and thermal stability.
1.
Known coordination modes of 1H-pyrazoles (Hpz) and pyrazolide anions ([pz]−). Dalton Transactions 12 (2009): 2059–2073 (copyright).
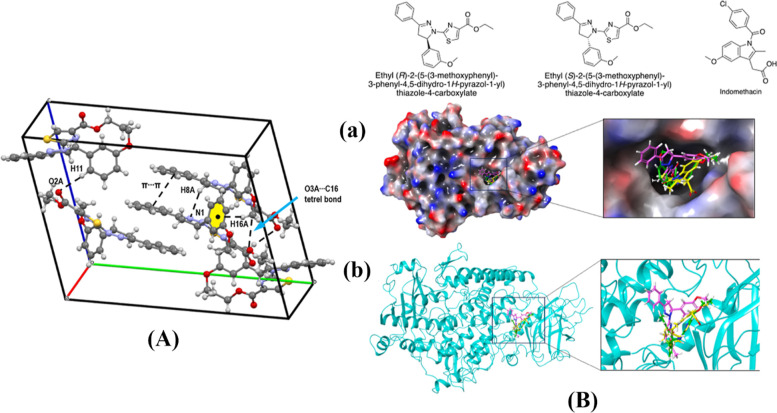
The molecular structures of “ethyl (R)-2-(5-(3-methoxyphenyl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl) thiazole-4-carboxylate” and its enantiomer, as well as “indomethacin, as displayed”, where the pyrazole group contributes to both stabilizing intermolecular force and influencing the binding and docking behavior of the compound on its most favorable confirmations, are shown in Figure .
2.
(A) Packing diagram of 5 viewed parallel to the b-axis displays intermolecular interactions such as C–H···O, C–H···N, C– H···π, π···π, and O···C, which are shown as dashed lines, and (B) an overlay of the docked orientations of its most favorable conformations of compounds ethyl (R)2-(5-(3-methoxyphenyl)–3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)thiazole-4-carboxylate 1(R)-enantiomer (green), 1(S)-enantiomer (pink), and indomethacin (yellow) shown in subfigures (a) and (b). Journal of Molecular Structure 1296 (2024): 136908 (copyright).
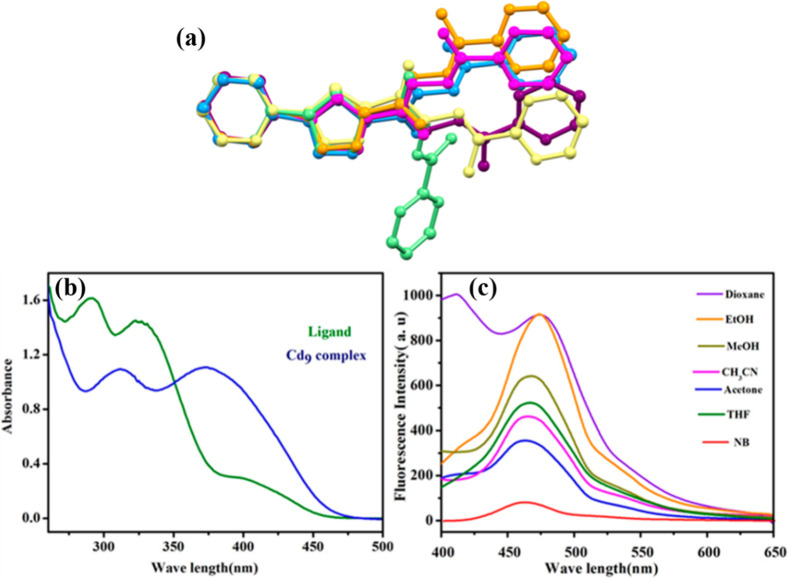
Further exploration of the solvatochromic behavior of the compound H2APPC is represented in Figure a. A vast pool of ligating sites present in a single ligand molecule gives the metal centers sufficient coordination flexibility to produce an optimal shape. Indeed, distinct conformations and varying involvement of coordinating sites for each combination further support the metal–ligand coordination compatibility of the H2APPC molecule. Conversely, Figure c demonstrates the solvatochromic behavior of how the compound’s fluorescence characteristics change by varying solvents.
3.
(a) Coordination and conformational freedom of the flexible H2APPC ligand, (b) UV spectra of the ligand and complex 5, and (c) fluorescence spectra of complex 5 in different solvents. Crystal Growth & Design 20.9 (2020): 5698–5708 (copyright).
Figure a provides a closer look at the spacer, which connects the active unit to the receptor and selectively senses the analyte, causing the analyte to undergo a chemical change or complexation. As a result, the active unit signals the presence of the analyte through changes in optical properties. When molecule 2 reacts with Cu2+ in acetonitrile, it “turns off” absorption at 352 nm and weak fluorescence at 275 nm, while the reaction of molecule 3 with Cu2+ in a mixture of water and dimethyl sulfoxide (DMSO) “turns off” fluorescence at 463 nm, and strong fluorescence is restored when a sulfur-containing species (S2–) is added.
4.
(a) Schematic representation of the colorimetric/fluorescent chemosensor and pyrazole-based probes for Cu2+ chemosensing. Three derivatives of (b) NH-pyrazole, (c) bis-pyrazole 2, and (d) pyrazoline 3 are shown. RSC advances 10.33 (2020): 19693–19712 (copyright).
Figure demonstrates how MOFs with different metal centers (Co and Ni) can be synthesized solvothermally by using the same ligands (i.e., sulfoterephthalic acid and a pyrazole-based ligand), and the pyrazole group in H2MDP serves as a coordinating site for metal centers.
5.
Synthesis of Ni-MOF and Co-MOF using H2MDP and H2STA. Crystal Growth & Design 23.2 (2023): 1104–1118 (copyright).
Figure presents the use of fluorescent sensors for the identification and distinction of different anions and gases. The emission profiles and comparison of intensities across various anion concentrations collectively show that compound 2a [(phenPd)6L2] (NO3)6 exhibits a selective and dose-dependent fluorescence “turn-on” response to HSO3 –, with significantly higher intensity compared to other anions. Figure A shows the fluorescence intensity of compound 1a [(phenPd)6L2] (NO3)6 for the presence of different gases (CO3, HCl, SO3, Ar, N, NH, NO, and CO). Compared with other gases, SO2 exhibits the greatest fluorescence intensity. The compound “1a” exhibits a discernible shift in fluorescence upon exposure to SO, indicating that 1a selectively detects SO2.
6.
(A) (a) Enhancement effect of various gas species on the fluorescence of 1a. (b) Color responses of probe 1a to different gas species under a 365 nm UV lamp. (B) Detection of HSO3 – in real water samples using 2a: (a) deionized water, (b) tap water, (c) seawater, and (d) lake water. Dalton Transactions 52.18 (2023): 6129–6137 (copyright).
Figure shows the fluorescence characteristics of the pyrazole-based cages. Figure A clearly shows that increasing the water percentage (fw) to 90% increases the fluorescence intensity of the chemicals; however, the intensity decreases at fw = 70–80%. The UV light images corroborate the dramatic increase in fluorescence at fw = 90–99%. Compared to the other metal ions, compound 1a demonstrates a significantly higher and more selective fluorescence “turn-on” response for Hg2+. Figure B shows the fluorescence enhancement in the presence of Hg2+ and a dose-dependent increase in intensity.
7.
(A) The fluorescence spectra of 1a (a,b), 2 (c,d), and 4 (e,f) in DMSO/water mixtures with varying water fractions (fw), concentration: 10 μM. Inset: fluorescence images at fw = 0% and 99%. (B) (a) PL spectra of 1a, (b) fluorescence intensity of 1a, (c) changes in the fluorescence spectra of 10 μM 1a after adding Hg2+ ions, and (d) fluorescence intensity at 535 nm as a function of [Hg2+]. Dyes and Pigments 223 (2024): 111954 (copyright).
The compounds 3,4,5-tris(4′-n-decyloxybenzyloxy)-N-[4-(3,5-dimethyl-1H-pyrazol-4-l) phenyl]benzamide, 4–3,4,5-C10-AHPz, and 3,4,5-tris(3′,4′,5′-tri-n-decyloxybenzyloxy)-N-[4-(3,5-dimethyl-1H-pyrazol-4- yl)phenyl]benzamide, 3,4,5–3,4,5-C10-AHPz, were synthesized. In contrast, Figure B displays the liquid crystalline textures, phase transitions, and molecular structures of the samples where pyrazole plays a crucial role in governing the phase behavior, gelation’s properties, thermal transition, and fluorescence characteristics of the materials.
8.
(A) Textures of the mesophases observed by POM on cooling at 98 °C (a) and 90 °C (d). DSC traces show the first cycle at 10 °C/min (black), the second cycle at 10 °C/min (red), and the cycle at 2 °C/min (blue). (b,e) XRD diffractograms at low angles are shown for 20 °C (c) and 125 °C (f). (B) Gels in cyclohexane at 0.5 wt % (a,b) microphotographs under polarized light, (c) TEM image, (d) SEM image, (e) PL intensity variation with temperature, and (f) PL spectral changes during heating to the sol state and a gel photo under UVA light (365 nm). Journal of Molecular Liquids 365 (2022): 120109 (copyright).
Figure a presents two main components: the self-assembly of macrocycles and the [2] catenane system involved pyrazole ligands and the biochemical study of cleaved PARP, the pyrazole complex interacting with enzymes and inducing the biochemical changes. The cleavage of PARP and caspase 3 in a dosage-dependent manner suggests that the pyrazole-based complexes may play a role in inducing apoptosis and other cellular pathways.
9.
(a) Self-assembly of [2] catenane 1 and macrocycle ligands 2–5; and (b) cell viability for AGS cells exposed to varying concentrations of macrocycle 3. Inorganic Chemistry 56.14 (2017): 8430–8438 (copyright).
Figure displays the UV–vis absorption spectra of the pyrazole-based metal complexes following contact with DNA. Every spectrum illustrates the wavelength-dependent changes in absorbance, with arrows highlighting certain wavelengths where notable variations occur, perhaps signifying binding events. The absorbance usually varies with the concentration of DNA, indicating a possible interaction between the complex and DNA. The plots of the binding constants or any comparable quantitative measure, such as absorbance against DNA concentration, are shown as insets inside the spectra. The slope and intercept of these linear plots, used to assess the binding affinity of metal complexes to DNA, may provide information about the type and intensity of the interaction.
10.
Complexes of a pyrazole-containing Schiff base ligand and UV–vis absorption spectra of the pyrazole-based metal complexes upon interaction with DNA. Journal of Coordination Chemistry 77.1-2 (2024): 170–187 (copyright).
Using various approaches, Hamodin et al. synthesized a novel chitosan derivative (DPPS-CH) containing a pyrazole-based heterocyclic molecule for its intended uses. Figure A compares the efficacy of CH, DPPS, and DPPS-CH treatments. The inhibition zones (millimeters) often expanded with increasing doses (25–300 μg/mL), suggesting improved efficacy. Different letters (a, b, and c) above the bars denote statistical significance and indicate significant changes across treatments at the same concentration. Figure B compares the percentage of cell viability between MCF-7 and WI-38 cell lines at different treatment doses (ranging from 31.25 to 1000 μg/mL). The higher IC50 values for WI-38 imply improved efficacy against the MCF-7 cancer cell line, while the lower IC50 values signify increased therapeutic potency.
11.
(A) The antimicrobial activity of CH, DPPS, and DPPS–CH against different pathogenic Gram-positive bacteria, B. subtilis (a) and S. aureus (b); Gram-negative bacteria, P. aeruginosa (c) and E. coli (d); and unicellular fungi C. albicans (e). (B) The cell viability assay using the MTT method of cancerous cell lines (MCF-7) and normal fibroblast cells (WI-38) due to treatment with different concentrations of chitosan (a), DPPS (b), and DPPS-CH (c). International Journal of Biological Macromolecules 243 (2023): 125180 (copyright).
2. Pyrazole Ligands and Their Unique Properties
Pyrazole ligands having five-membered rings and two nitrogen atoms are useful for catalyzing processes, supramolecular assemblies, and coordination molecules. Because of this structure, pyrazole ligands may function as bridging, bidentate, or monodentate ligands, making it easier to build stable chelate complexes with various metal ions. The coordination flexibility of pyrazole ligands allows for electrical adjustment with various ring substituents useful in catalysis and materials research. Modifying the pyrazole ligands can alter the nitrogen electron density, crucial for the creation of catalysts or the inhibition of enzyme activity, as illustrated in Figure , offering tailored reactivity and selectivity for specific chemical processes.
12.
Synthesis of pyrazole-based tetradentate ligands: The contrasting influence of pyrazolyl and pyridyl rings on luminescence. Inorganic Chemistry 47.23 (2008): 11129–11142 (copyright).
The aromatic resilience of the pyrazole ring boosts the strength of coordination complexes, necessary for lasting materials in different chemical environments. Pyrazole ligands also participate in secondary interactions, such as hydrogen bonding and π–π stacking, which make the supramolecular structures even stronger and increase their functional possibilities. Pyrazole ligands have manipulable characteristics, which allow them to optimize metal–ligand interactions to enhance catalytic efficiency. Furthermore, pyrazole-based ligands play a crucial role in creating effective and selective catalysts with adjustable electronic characteristics by augmenting the performance in a wide range of organic processes, such as cross-coupling, hydrogenation, and oxidation. The versatile nature of pyrazole ligands can be demonstrated by their exceptional porosity and surface area. Pyrazole-based MOFs are characterized by adjustable pore widths and exceptional stability and show great potential for gas storage, separation, sensing, and catalysis. This includes the collection of CO2 as well as reactions involving large molecules, as shown in Figure . Specifically, the potential of pyrazole-based supramolecular complexes in drug delivery systems has been investigated to treat the microbial infections. The capacity of pyrazole ligands to form hydrogen bonds enables the precise encapsulation and controlled release of medicinal substances, thereby enhancing the effectiveness and specificity of drug delivery. Pyrazole ligands in nanotechnology facilitate the synthesis of nanomaterials with distinct characteristics, thereby improving their applications in sensing, catalysis, and optoelectronics. These ligands enhance the chemical sensors sensitivity, improve the stability of quantum dots for biological imaging, enable environmental remediation by capturing heavy metals, and contribute to the development of smart materials that respond to stimuli such as temperature, pH, and light. , Furthermore, the interaction of pyrazole ligands with different metals and the formation of structurally stable complexes are currently used in the development of batteries and supercapacitors. The redox characteristics of pyrazole-based metal complexes make them very efficient in energy storage devices, thereby making significant contributions to the advancement of high-performance and sustainable energy systems.
13.
(A) (a) UV–visible diffuse reflectance spectrum with a Tauc plot (inset) for JNU-204, (b) Mott–Schottky plots in 0.5 M Na2SO4 solution, (c) optical band gap schematic of JNU-204, and (d) EIS Nyquist plots for JNU-204 and the PBT linker in 0.1 M tetrabutylammonium hexafluorophosphate in anhydrous acetonitrile. (B) EPR spectra of the JNU-204 sample (15 mg) in the presence of (a) DMPO or (b) TEMPO in acetonitrile (3 mL). Journal of the American Chemical Society 143.50 (2021): 21340–21349 (copyright).
Figure demonstrates that the pyrazole–benzothiadiazole–pyrazole configuration in the JNU-204 MOF functioned as a highly effective photosensitizer. The synthesized photosensitizer absorbs visible light, producing electron–hole pairs and enabling the transfer of these charge carriers to oxygen molecules in photocatalytic aerobic oxidation processes. The remarkable photocatalytic activity of the synthesized material is attributed to its inherent structural stability, advantageous bandgap, and efficient charge separation. Figure presents the electrical characteristics and the ability of pyrazole ligands to form stable complexes with various metal ions. This extensive structural adaptability enables the creation of a diverse array of self-assembled systems ranging from basic coordination complexes to intricate supramolecular structures, each possessing unique characteristics and possible uses. ,
14.
Some pyrazole complexes: (a) [NiCl2(Hpz)4], (b) [TiCl4(Hpz)2], and (c) pyrazolato ligand with carboxylic acids. European Journal of Inorganic Chemistry 2009.33 (2009): 4913–4925 (copyright).
A prominent characteristic of pyrazole ligands is their capacity to assume many coordination modes with metal centers. The versatility of pyrazole coordination enables the formation of many structures, including mononuclear, binuclear, and polynuclear complexes. By coordinating with transition metals, pyrazole ligands can generate mononuclear complexes that act as fundamental components for more intricate assemblies, , as shown in Figure .
15.
Schematic of the self-assembly of pyrazole-based metallacapsules (a) and metallacages (b). Inorganic Chemistry Communications 136 (2022): 109145 (copyright).
Figure illustrates the crucial role of pyrazole and its derivatives in the creation of coordination complexes with Pd ions. The pyrazole nitrogen atoms play a crucial role in binding the metal core, and the particular substituents on the pyrazole ring affect the intricacy and durability of the resultant crystals. Changing the substituents of the ligand or the metal centers involved can easily manifest the intriguing magnetic and electrical characteristics of the binuclear complexes in which the pyrazole ligands bridge two metal centers. In addition to basic coordination complexes, pyrazole ligands are essential for the development of MOFs and coordination polymers.
2.1. Metal–Organic Frameworks (MOFs)
The remarkable structural diversity and unique properties of MOFs synthesized with pyrazole ligands garnered significant interest in the field of supramolecular chemistry. The adoptability of MOFs with pyrazole ligands provides an opportunity to tailor these materials in a broad spectrum of applications. The adaptable nature of MOFs enables the development of various structural and functional characteristics, thereby improving their possible uses. Usually, solvothermal or hydrothermal synthesis methods are used for the chemical synthesis of MOFs using pyrazole ligands in which metal salts and pyrazole-based ligands undergo reactions in a solvent under certain temperature and pressure. The selection of the metal ion, pyrazole ligand, and reaction conditions are essential factors in establishing the structure and characteristics of the resultant MOFs. Pyrazole ligands effectively form coordination complexes with a wide range of metal ions, including transition metals, such as Zn, Cu, Fe, and Co, as well as lanthanides and actinides. The coordination of metal ions influences the geometry of MOFs, whereas the ability to self-assemble under moderate circumstances enhances the environmental friendliness and cost-effectiveness of MOF manufacturing. By selecting certain functional groups, pyrazole ligands can be designed to modify the characteristics of MOFs by greatly affecting ligand binding and the framework structure. The customization of pyrazole ligands with particular functional groups has a substantial impact on the coordination and structure of MOFs. MOFs containing pyrazole ligands exhibit excellent thermal and chemical stability, extensive surface areas, and adjustable porosity, rendering them well suited for catalytic activity and gas preservation applications. Changing the ligand’s structure and synthesis conditions alters the pore size and surface area of the resulting complex, thereby enabling the adsorption and separation of gases such as carbon dioxide, methane, and hydrogen. The wide range of functions shown by pyrazole ligands improves the flexibility in designing MOFs with particular functionalities, as shown in Figure .
16.
Ni-MOF and Co-MOF with a pyrazole-based ligand. Crystal Growth & Design 23.2 (2023): 1104–1118 (copyright).
The inclusion of pyrazoles in the ligand hierarchy may influence the electrical, magnetic, and thermal characteristics of the MOFs. It is clear from Figure that the particular coordination environment created by the pyrazole-based ligand influences the electrical characteristics and temperature-dependent magnetic behaviors. The coordination significantly influences the structural and functional aspects of the MOFs, thereby influencing their characteristics and applications. Including the hydrophilic or hydrophobic groups in the MOFs enhances the efficiency of targeted adsorption and catalysis of the synthesized MOFs for various compounds. Pyrazole-based MOFs are also used as an effective catalyst for a wide range of chemical processes. The adjustable coordination of metal centers in MOFs allows the oxidation of Cu- and Fe-MOFs, whereas Pd- and Ni-MOFs are used for hydrogenation and cross-coupling processes. Certain pyrazole-based MOFs have luminous characteristics, making them appropriate for use in detection and optical electronics. Modifying either the pyrazole ligand or the metallic center allows for the adjustment of luminescence, thereby facilitating the identification of certain molecules or ions by altering the emission spectra. Furthermore, the mechanical strength of pyrazole-based MOFs is crucial for applications that require the preservation of their structural integrity when subjected to stress. MOFs have the ability to endure mechanical stresses without substantial deterioration, which is a crucial factor for their successful use in many industrial processes.
Figure illustrates the significance of the pyrazole moiety in ligands for the synthesis and structural development of MOFs. It also shows the structural modifications of the pyrazole ring through the introduction of nitro and carboxylate groups, which determine their subsequent characteristics. The nitrogen atoms in the pyrazole ring serve as fundamental sites for coordination with the metal ions, resulting in the creation of stable and possibly functional frameworks. The crystal size, shape, and porosity of MOFs modified by self-assembly via solvothermal methods or hydrothermal conditions depend on the solvent, temperature, pressure, and time. MOFs synthesized using pyrazole ligands have a large surface area and high porosity and are important for various applications, particularly in catalysis. The extensive surface area of MOFs provides more active sites for catalytic processes, thereby improving efficiency and reaction rates, which is particularly advantageous in industrial operations that require exceptional catalytic efficiency. Furthermore, the capacity to alter the chemical surroundings within MOFs by the incorporation of pyrazole ligands allows the creation of materials with precise interactions. Pyrazole-based MOFs, composed of functional groups that specifically interact with target molecules, have exceptional sensitivity and selectivity in the detection of chemicals, as illustrated in Figure .
17.
(A) Synthesis of ligands NPTA (nitropyrazole tricarboxylic acid) and NPTN (nitropyrazole trinitro) and energetic coordination compounds 1–3. (B) (a) Molecular structure of 2. (b) Intermolecular H···O interactions induced by the noncoordinated nitrate group. (c) Packing diagram of 2 along the b-axis. (d) Layer-by-layer stacking between the dimeric molecules. Dalton Transactions 52.35 (2023): 12271–12281 (copyright).
18.
(A) Synthesis of Cd (II) complexes with the pyrazole ligand and (B) shows the fluorescence sensor for the acetone molecule and other cooperative organic solvents. Inorganic Chemistry Communications 96 (2018): 16–19 (copyright).
Figure illustrates how the pyrazole-based ligand in the cadmium complex acts as a fluorescence sensor for acetone by producing notable quenching in its presence. The decline in fluorescence intensity as acetone concentrations increase demonstrates the efficacy of the synthesized sensor, thereby establishing its potential as a sensitive instrument for acetone detection in diverse settings. Overall, the self-assembly of MOFs using pyrazole ligands is a breakthrough in materials research. This methodology integrates meticulous structural accuracy, adaptable functionality, pragmatic use, and yielding materials exceptionally well suited for a wide range of applications.
2.2. Supramolecular Complexes
Pyrazole ligands play a crucial role in the creation of many supramolecular structures, including cages, capsules, and networks, each possessing distinct characteristics and possible uses. These architectures are constructed based on the capacity of pyrazole ligands to form coordination bonds with metal ions and engage in various noncovalent interactions. The formation of supramolecular cages by pyrazole ligands is a fascinating structure within this class. Construction of these cages usually involves the coordination of pyrazole ligands with metal ions into a closed three-dimensional cavity. The enclosed regions inside these cages can confine guest molecules, which may vary in size from tiny chemical compounds to larger macromolecules, , as seen in Figure .
19.

Analysis of the photoswitching and thermal characteristics of arylazopyrazole-based metal–organic framework (MOF) host–guest complexes. Crystal Growth & Design 23.10 (2023): 7044–7052 (copyright).
This encapsulation capacity is advantageous for applications such as medication delivery, as the cage may safeguard the drug molecule and facilitate its release at the intended target. Customizing pyrazole ligands or metal centers to preferentially bind guest molecules depending on their size, shape, and functional groups allows precise adjustment of the selectivity of these cages. Cyclophanes composed of pyrazole ligands are analogous to capsules, but they often include dynamic assemblies capable of opening and closing in response to external stresses. Generally, the spontaneous arrangement of pyrazole ligands and metal ions forms hollow, spherical, or ellipsoidal structures.
The ability of capsules to confine guest molecules, therefore offering protection and regulated release, renders them well-suited for use in medication administration and molecular recognition applications. The inherent dynamism of these capsules enables them to react to variations in pH, temperature, or the presence of certain ions or molecules, thereby facilitating the development of intelligent delivery systems that release their contents specifically in response to designated stimuli. Pyrazole ligand-based compounds also explained the basic coordination geometry of the complexes. Figure presents pyrazole ligand-based one-dimensional chains, two-dimensional sheets, and three-dimensional supramolecular frameworks.
20.
Self-assembled pyrazole-based (zero-dimensional to three-dimensional) complexes. Crystal Growth & Design 17.6 (2017): 2975–2986 (copyright).
Coordination of pyrazole ligands with metal ions into one- or more-dimensional chains displays fascinating magnetic and electrical characteristics. The formation of two-dimensional sheets occurs when pyrazole ligands bridge with metal centers in a planar configuration, resulting in extended layered structure creations. Pyrazole ligands facilitate the creation of supramolecular networks with high-surface-area sheets with adjustable porosity for gas storage, separation, and catalysis. The three-dimensional MOFs, configured with adjustable surface areas and porosity using pyrazole ligands and synthesis conditions, are suitable for gas storage, segregation, catalytic processes, and sensing applications. Pyrazole ligands in MOFs can provide sites for binding or interacting with target molecules, thus modifying the electrical characteristics or structural arrangement of the MOFs and making them highly efficient for sensing applications. Robust metal–ligand interactions improve the stability of these frameworks, whereas their capacity to respond to stimuli such as temperature or pH allows for tailored material characteristics. For example, photoresponsive pyrazole ligands may change their structure when exposed to light, allowing for the creation of photoswitchable materials suitable for use in sensing and other optical electronics.
2.2.1. Polymers and Gels
Pyrazole ligands with various coordination chemistries and the ability to form stable complexes with various metal ions are crucial in the development of self-assembly systems that are polymeric and gel-based, as shown in Figure . Pyrazole ligands with diverse coordination chemistries are crucial for forming stable complexes with various metal ions such as Ag+, Fe, Co, and Ni.
21.
Explanation of the tpt ligand (2,4,6-tri[pyrazol-1-yl]-1,3,5-triazine) with the precursor complexes [M(tpt)2]2+ (M = Mn, Fe, Co, Ni, Cu). ACS omega 3.12 (2018): 18466–18474 (copyright).
These complexes are pivotal in the creation of coordination complexes. By coordinating with multiple metal centers, pyrazole facilitates the formation of extensive networks that significantly influence the physical characteristics of the resultant complexes, including their colors, stability, and mechanical properties. One of the key applications of pyrazole-based coordination polymers lies in their use as functional materials in electronics and sensors. For example, one-dimensional coordination polymers exhibit exceptional magnetic and electrical characteristics, while two-dimensional coordination polymers with their layered structures are well-suited for interaction with guest molecules. The performance of these systems can be enhanced by enhancing the ratio of pyrazole ligands or metal centers, which optimizes the materials properties for specific tasks. In addition to their use in coordination polymers, pyrazole ligands are crucial for the advancement of metallogels, which are self-assembled structures that react to environmental stimuli like temperature, pH, and light. , The reversible sol–gel transitions of pyrazole-based metallogels render them suitable for drug delivery systems capable of releasing therapeutic compounds in response to specified stimuli. Furthermore, the integration of functional groups into the pyrazole ligands might augment the gels, imparting characteristics such as antibacterial capabilities or the capacity to engage with particular proteins, so broadening their potential uses. , Ultimately, pyrazole ligands play a vital role in creating adaptable polymeric and gel-based systems that exhibit stable coordination of metal ions and responsive properties, as depicted in Figure .
22.
(A) Pyrazole-based crystal structures of metal-coordination complexes and (B) explains colorimetric ammonia gas sensing through gel-to-gel transformation. Crystal Growth & Design 14.5 (2014): 2366–2374 (copyright).
Figure illustrates that the impact of pyrazole encompasses the creation, stability, and functionality of coordination complexes, affecting their electrical and magnetic properties. Furthermore, pyrazole ligands enhance colorimetric detection of ammonia gas, demonstrating their multifunctional capabilities in sensor technologies.
3. Utilizations of Pyrazole Ligands in Self-Assembled Systems
Owing to their distinctive coordination chemistry and adaptable bonding, pyrazole ligands play a crucial role in the development of self-assembled systems. They have wide-ranging applications in catalysis, drug delivery, sensing, gas storage, and materials research.
3.1. Catalysis
Using pyrazole-based self-assembled structures in catalysis is notable progress in chemistry, providing a novel means to improve the effectiveness, specificity, and durability of catalytic processes. , The distinct technical features of pyrazole ligands, including their adjustable electrical and steric properties, are crucial for maximizing the catalytic efficiency in a wide range of chemical processes. An inherent benefit of pyrazole-based catalysts is their capacity to maintain the transition metal centers, which frequently serve as crucial elements in catalytic cycles, as seen in Figure .
23.
Peroxidative (by H2O2) oxidation of cyclohexane to cyclohexanol and cyclohexanone is catalyzed by [FeCl2[ k3-HC(pz)3]]. Coordination Chemistry Reviews 265 (2014): 74–88 (copyright).
Figure shows that the pyrazole moiety in [FeCl2[κ3-HC(pz)3]] is essential for the stability, reactivity, and selectivity of the catalytic mechanism. By collaborating with the iron center, it stabilizes the metal catalyst, activates the oxidant (H2O2), and possibly stabilizes chemical intermediates, thus promoting the effective oxidation of cyclohexane to cyclohexanol and cyclohexanone. The coordination of pyrazole ligands displays that all of the nitrogen atoms in the pyrazole ring donate electron density to the metal center, enhancing its reactivity and facilitating various catalytic reactions. The feature has been particularly valuable in palladium-catalyzed cross-coupling reactions, such as the Suzuki–Miyaura and Heck reactions, which have effectively used pyrazole ligands. These reactions are essential in organic synthesis for the formation of carbon–carbon bonds. The incorporation of pyrazole ligands enhances the stability and activity of the catalyst, resulting in an increased yield and refined selectivity. , Specifically, in complexes of metal-like Ni and Cu complexes, pyrazole ligands imitate enzyme active sites and operate as catalysts in organic synthesis reactions. Their adjustable electronic characteristics improve the catalytic efficiency and selectivity in processes such as aziridination, cyclopropanation, and oxidation. , For example, in Cu complexes, the capacity of Cu to undergo oxidation state changes in the presence of pyrazole ligands promotes the activation of molecular oxygen and other oxidants. This activation is key to conducting oxidative transformations, such as the selective oxidation of alcohols to aldehydes and ketones. The stability provided by pyrazole ligands ensures the robustness and prolonged activity of the catalyst, making the process more sustainable and economically feasible, as shown in Figure .
24.
(a) Structure of the prepared monoalkylated pyrazoles L1–L4, (b) oxidation of catechol to o-quinone, while (c) represents the correlation between reaction rate and catechol concentrations catalyzed by various pyrazole complexes. Arabian Journal for Science and Engineering 47.1 (2022): 269–279 (copyright).
Pyrazole ligand-based MOFs are particularly well suited for heterogeneous catalysts in gas-phase reactions, such as volatile organic compound (VOC) oxidation and NOx reduction. Additionally, pyrazole ligands accelerate the reactive nature of the metal centers, resulting in highly effective catalytic processes with minimal byproduct production. This characteristic is especially significant in environmental applications and liquid-phase organic transformations. Moreover, pyrazole ligand-based MOFs have shown exceptional performance in catalytic processes, such as Knoevenagel condensation and Friedel–Crafts acylation. Their adjustable pyrazole ligands and sturdy structures allow for many reuses, thereby improving the sustainable synthesis of medicines and agrochemicals. , Beyond their function in conventional catalysis, pyrazole-based metal–organic frameworks (MOFs) incorporating photoactive metals such as nickel (Ni) and zinc (Zn) demonstrate efficacy in photocatalysis. In these complexes, the pyrazole moiety interacts with the nickel core to stabilize the metal complex, so ensuring the requisite conditions for catalytic activity, as shown in Figure . The electrical properties of the pyrazole ligand affect the reactivity of the nickel center, making it an effective catalyst for processes including pollutant degradation and hydrogen generation. Overall, the adaptability of pyrazole ligands in homogeneous and heterogeneous catalysis is apparent across several applications, such as organic synthesis, environmental remediation, and renewable energy. Pyrazole-based systems boost catalytic activity and stability, rendering them essential in many industrial processes, with considerable promise for future advancements in sustainable and efficient chemical transformations. Table shows the list of pyrazole- and polypyrazole-based ligands reported in this study.
25.
(A) Synthesis of pyrazolyl-pyrimidine nickel skeleton (POP–PP-Ni) and crystal structure and (B) catalytic experiments on POP–PP-Ni. Chemical Engineering Journal 474 (2023): 145642 (copyright).
1. Name and Applications of Pyrazole- and Polypyrazole-Based Ligands Reported in the Present Study.
3.2. Sensing and Detection
Pyrazole groups augment sensor and detection systems by coordinating with metal centers to create stable MOFs or coordination polymers, facilitating the detection of diverse analytes, including gases, metal ions, and biomolecules. , Figure presents the importance of pyrazole-based compounds in chemical sensing and detection owing to their distinctive structural and electrical characteristics.
26.
(A) Probable quenching mechanism of the Al–H2DPC (E)-N′-(2,3-dihydroxybenzylidene)-3-(pyridin-2-yl)-1H-pyrazole-5-carbohydrazide complex by picric acid. (B) Operation of the INHIBIT logic gate (a), pictorial representation of the logic gate (b), and visual color outputs. (c) Truth table corresponding to the INHIBIT logic gate. RSC advances 11.17 (2021): 10094–10109 (copyright).
These ligands play a pivotal role in various applications including monitoring the environment, medical diagnosis, and manufacturing processes, owing to their extensive utility and significance. A crucial application of these ligands lies in the accurate detection of metal ions, which is essential in many domains, such as environmental surveillance and diagnosis in medicine. For instance, the formation of complexes in between pyrazole ligands and metal ions alters the photonic and electrochemical properties of the synthesized complex shown in Figure .
27.
(A) Fluorescence quenching efficiency of aggregates of 1, [tris(4-Ph-pz); (C42H42N6)] (a), and 2, [(PA)2 (C60H47Cl3N15O21)] (b), with various nitroaromatic compounds. (B) Comparison plot of fluorescence quenching performance for compounds 1 and 2 with PA vapors upon exposure at different time periods (0 to 30 min). New Journal of Chemistry 43.19 (2019): 7251–7258 (copyright).
Figure demonstrates how pyrazole-derived ligands facilitate the sensitive and selective detection of copper ions (Cu2+) in aqueous environments, essential for evaluating water quality and pollution levels.
28.
(A) Schematic representation of pyrazole-based imine hybrids. (B) (a) Job’s plot of AS1 with Cu2+ ions and (b) competitive studies of AS1 with various metal ions. (C) (a) Interference study of AS2 with various metal ions and (b) Job’s plot of probe AS2 with Cu2+ ions. (D) (a) Competitive experiment of AS3 and (b) Job’s plot of AS3 with Cu2+ ions. Results in Chemistry 4 (2022): 100501 (copyright).
Pyrazole serves as the central functional group in AS1, AS2, and AS3, where its nitrogen atoms act as donor sites for metal binding, ensuring high affinity and specificity for Cu2+ ions. Moreover, pyrazole-based ligands containing a thiol group demonstrate rapid and selective detection capabilities for hazardous metals like mercury ions (Hg2+), as mentioned in Figure , which are critical for environmental and public health monitoring.
29.
Probes for Hg2+ sensing based on pyrazoles (a) 13 and (b) 14. (c) Structural relationship between pyrazole-based probes for Hg2+ sensing. RSC advances 10.33 (2020): 19693–19712 (copyright).
Furthermore, pyrazole-based compounds also show potential for detecting biomolecules such as glucose, which is crucial for industrial and environmental safety. , Pyrazole-based compounds integrated into MOFs and coordination polymers are particularly adept at detecting volatile organic compounds (VOCs) due to their large surface area and tunable pore environments, which modify their photoluminescent or electrical properties for detection. −
3.3. Drug Delivery
Pyrazole-based assemblies utilize the chemical properties and structural flexibility of pyrazole ligands to synthesize stable complexes with metal ions, enhancing the transport and release, offering innovative solutions for improving the bioavailability and efficiency of therapeutic agents, − as illustrated in Figure . As shown in Figure A, the three-dimensional structure interaction between pyrazole-containing compounds and proteins demonstrated the molecular binding interactions at a detailed level. Additionally, Figure B shows bar graphs that indicate the percentage inhibition of cancer cell proliferation at different concentrations of pyrazole derivatives. The results clearly demonstrate that the higher the concentration of the pyrazole, the greater the inhibition of cancer cell growth, supporting the potential for pyrazole compounds in cancer treatment. The ability to modify the pyrazole ring structure with various substituents offers the flexibility to customize the physiochemical properties of the compound, making it adaptable for specific therapeutic applications.
30.
(A) The docking pose of C1 [Cu4(L)2 (μ-pz)2] (L = N,N′-bis(salicylidene)-1,3-diamino-2-propanol; pz = pyrazolate) (a) and C2 [Cu2(L′)(μ-pz)] (L′ = 2,6-bis(2-hydroxyphenylimino)-4-methylphenol) (b) with BSA (DNA/protein). (B) The growth inhibitory effect of compounds on various cancer cell lines using the MTT assay. Cells were treated with C1 for 24 (a) and 48 (b) h and with C2 for 24 (c) and 48 (d) h. Inorganica Chimica Acta 509 (2020): 119674 (copyright).
Furthermore, MOFs derived from pyrazole assemblies are crystalline materials with tunable porosity and stability, ideal for enhancing drug delivery by encapsulating and protecting therapeutic agents to improve bioavailability and effectiveness. , Nanoparticles derived from pyrazole assemblies represent a notable breakthrough in drug delivery. They encapsulate drugs within pyrazole-based MOF complexes to safeguard them from degradation and to improve their solubility. To guide the drug-loaded nanoparticles to specific cells or tissues, targeting moieties, such as antibodies or peptides, modify the surface of these nanoparticles. This facilitates the concentration of the drug at the intended site, thereby enhancing therapeutic results and minimizing negative effects.
The regulated release of drugs can also be achieved by engineering pyrazole-based nanoparticles that react to external stimuli. , For instance, magnetic pyrazole-based nanoparticles can be directed to the desired location using an external magnetic field, while changes in temperature or pH can trigger release of the encapsulated medication, , as shown in Figure . The degree of control over the administration and release of drugs significantly improves the accuracy and effectiveness of medicinal interventions, especially in cancer treatment, where it is essential to minimize harm to healthy tissue. In addition, pyrazole-based assemblies augment the stability and drug-carrying capability of liposomes and micelles, thereby safeguarding enclosed pharmaceuticals and enhancing their bioavailability. The integration of pyrazole-based molecules into these carriers can make them more stable, control the release of medications, and achieve targeted delivery. , Figure shows that the versatile nature of pyrazole chemistry enables the precise adjustment of liposome and micelle characteristics to enhance drug delivery for certain therapeutic purposes.
31.
(a) Acidic conditions induce assembly of the platinum(II) complexes through Pt–Pt interactions, (b) emission spectra of compound 2 recorded at varying pH levels highlight its pH-dependent luminescent behavior, (c) cationic Pt (II) complexes assemble along the phosphate backbone of single-stranded DNA (ssDNA), and (d) emission spectra of compound 1a demonstrate its interaction with ssDNA across a pH range from 8.3 to 4.2. Chemical Science 6.7 (2015): 3823–3830 (copyright).
32.
(A) Chemical structures of 1-(S-benzyldithiocarbazate)-3-methyl-5-phenyl-pyrazole (DTC) and Pluronic F127 block copolymers and (B) molecular docking of DTC and proteins of the iNOS expression stimulation pathway: (a) DTC (in red) docked in the CD14 receptor (b), the TLR4 and MD2 complex represented in cartoon, and the DTC molecule (in red) docked with the MD2 protein. (c) IKK1 complex represented in cartoons and DTC molecules in their regions of interaction. Analysis of the IKK1 complex–DTC interaction revealed the participation of A, B, C, D, E, F, G, H, I, J, K, and L chains. (d) Summary of possible activation of the DTC-induced NF-κB pathway. Colloid and Interface Science Communications 41 (2021): 100378 (copyright).
Pyrazole derivatives, such as DTC, selectively bind to specific proteins implicated in the NF-κB signaling cascade. These interactions exert inhibitory effects on pathway activation, thereby limiting inflammation and decreasing the transcription of more than 500 genes that are concerned with the immune response. The red-highlighted regions in the protein models shown by panels A, B, and C in Figure B indicate where pyrazole derivatives bind. Through occupation of these sites, pyrazole chemicals inhibit the enzymatic activity or interaction of crucial proteins necessary for the promotion of these pathways. Through the inhibition of crucial inflammatory processes, pyrazole compounds show promising therapeutic promise in disorders characterized by excessive inflammatory conditions, such as autoimmune illnesses and chronic inflammation-related conditions. The pyrazole core plays a vital role in facilitating these actions, making it a useful framework for developing specific anticancer chemical compounds. In addition to their use in conventional systems for drug delivery, pyrazole-based assemblies are investigated for gene delivery, anticancer, and anti-HIV applications. They are formed into stable complexes with nucleic acids to protect them from degradation and enhance their cellular absorption. , Functioning pyrazole ligands provide precise gene delivery and controlled drug release, thereby boosting the efficiency of gene therapy. Additionally, their integration with 3D printing and microfluidics enables the accurate manufacturing and development of sophisticated drug delivery systems that exhibit enhanced targeting and therapeutic effectiveness. −
4. Recent Progress and Breakthroughs
Recent progress in supramolecular chemistry based on pyrazole has greatly enhanced our comprehension and implementation of these adaptable molecules in many scientific and industrial fields. Pyrazole derivatives play a central role in forming stable supramolecular structures through strong hydrogen bonding and metal ion coordination with potential applications in material science, catalysis, and energetic materials, as shown in Figure .
33.

Azopyrazole carboxylic derivatives for potential energetic materials. Crystal Growth & Design 24.15 (2024): 6292–6299 (copyright).
One of the significant advancements in supramolecular chemistry is the exploration of pyrazole derivatives for the formation of nanoporous structures. Research utilizing scanning tunneling microscopy and density functional theory simulations demonstrated that bis-pyrazole derivatives, when sublimated onto noble metal surfaces, can create a hexagonal nanoporous network supported by triple hydrogen bonds. The self-assembly process of pyrazole derivatives played a vital role, since the robust bonding mechanisms guarantee the structural integrity of these nascent networks. The development of nanoporous structures facilitates their use across several fields, including catalysis and material design, as shown in Figure .
34.
Pyrazole ligands in coordination compounds and metal organic frameworks. Inorganica Chimica Acta 560 (2024): 121789 (copyright).
Pyrazole-based self-assembled complexes have shown exceptional catalytic efficiency. Their distinctive electrical characteristics and structural stability enhance their catalytic activity. Coordinating pyrazole ligands with transition metals has been used to fabricate catalysts that exhibit exceptional activity and selectivity for organic transformations such as cross-coupling reactions and hydrogenation activities. The advancements in this field have significant implications for industrial catalysis by enhancing the efficiency and environmental sustainability of the processes. Figure demonstrates that the large surface area and specific bonds of pyrazole-based nanoporous networks render them very suitable for sensing and detection applications.
35.
Pyrazole moieties as organic chemosensors for the detection of cations and anions. Inorganica Chimica Acta (2024): 122118 (copyright).
The aforementioned structures have been successfully integrated into sensors that exhibit exceptional sensitivity and selectivity for detecting a diverse array of chemical and biological analytes. The development of pyrazole-based sensors for the detection of contaminants such as heavy metals, explosives, and biomarkers has shown promise in environmental surveillance and security applications. , Within the field of medication delivery, pyrazole ligands are viable alternatives. As shown in Figure , their capacity to form stable complexes with metals and organic molecules enables the encapsulation and regulated release of medicinal substances. ,,
36.
Examples of drugs and agrochemicals that contain pyrazole motifs. Organic & Biomolecular Chemistry 20.45 (2022): 8787–8817 (copyright).
Another important area of innovation is the design of energetic coordination compounds using nitrogen-rich pyrazole and triazole ligands. Elastic colloidal composites (ECCs) are well appreciated for their exceptional thermal stability and resistance to mechanical stresses. These characteristics are crucial for the development of high-energy-density materials utilized in the military and civilian sectors. According to single-crystal X-ray diffraction investigations, the synthesized ECCs form dimeric structures and exhibit robust hydrogen-bonding interactions, which enhance their stability and resistance to external stimuli. Pyrazole-based electrochemical covalent catalysts effectively address several significant obstacles in the progress of energy materials such as trinitrotoluene and hexanitrostilbene. Pyrazole ligands in green chemistry can improve sustainable catalysis and material synthesis using renewable resources and minimize environmental effects. Through the integration of advanced computational methods such as artificial intelligence and machine learning with experimental techniques, the behaviors of new ligand assemblies can be optimized and predicted. As ongoing studies probe the complete capabilities of pyrazole-based complexes, these compounds will probably assume a progressively significant position in developing novel technologies and materials, thereby stimulating innovation in many scientific fields.
5. Limitations
Analysis of Coordination Mode Complexity: The conjugation mechanisms shown by pyrazole ligands may introduce complexities in the creation and regulation of intended supramolecular structures. The intricate nature of this phenomenon may lead to less foreseeable results and less ability to replicate the experimental procedure
Stability Issues: Self-assembled structures are mainly dependent on noncovalent factors such as pH, temperature, and organic solvents. The integral instability of these systems limits their use, especially in challenging environments.
Limited Selectivity and Sensitivity: The ability to select and effectiveness of pyrazole-based sensors in sensing applications might be impeded by interactions with other species. This may compromise the reliability of these systems when dealing with intricate biological and environmental samples.
The Scalability of Synthesis: Pyrazole-based assemblies and MOFs might be complicated and not readily inflatable, which presents difficulties for commercial applications
In some pyrazole-based catalysts and sensors, this leads to higher expenses and increases the environmental problems.
6. Challenges
Tuning Functional Properties: Pyrazole ligand design with target functional features requires particular manipulation of electronic and steric effects, which is a complex task that sometimes demands significant experiments.
Integration with Biological Systems: The pharmacological drug delivery and mostly therapeutic applications require meticulous assessments of the biocompatibility and possible toxicity of pyrazole-based assemblies, since unexpected interactions with biological compounds may arise.
Achieving High Porosity and Stability: The balancing problems of porosity and stability with pyrazole-based MOFs continue as achieving high porosity typically results in compromising structural stability, which is crucial for practical applications in gas storage and separation.
Response to Stimuli: The ability of pyrazole-based assemblies to react to external stimuli like pH or light is limited; mainly, these conditions are under dynamic and fluctuating conditions, making it challenging to get a systematic and regulated reaction.
Optimization for Catalysts: The unremitting difficulties and deceits in the development of pyrazole-based catalysts that exhibit high activity while also demonstrating toughness under such conditions that promote deactivation or degradation.
7. Future Work
A future study will endeavor to enhance the selectivity and binding characteristics of pyrazole ligands using sophisticated computational modeling and synthesis methodologies. The objective was to investigate novel substituents and alternations on the pyrazole ring to precisely adjust its electrical, steric, and coordination characteristics; thereby, its catalytic and sensing application effectiveness improves.
Supramolecular Structure Stabilization: Concentrated efforts will be made to enhance the stability of pyrazole-based self-assembled systems under different environmental conditions. This covers the advancement of hybrid materials that integrate pyrazole ligands with stronger frameworks or use covalent contacts to strengthen their structural integrity, rendering them appropriate for industrial and biological uses.
Scalable and Green Synthesis Methods: Future research will prioritize the optimization of the synthesis of pyrazole-based assemblies to enhance their scalability and environmental sustainability. One area of focus is the investigation of green chemistry methodologies, which include the use of nontoxic solvents, sustainable catalysts, and energy-efficient synthesis methods to minimize the environmental impact and decrease manufacturing expenses.
Assimilation into Refine Materials: Further investigation will improve the incorporation of pyrazole ligands into sophisticated materials, including metal–organic frameworks, polymers, and nanocomposites. The objective is to use their distinctive coordination chemistry to create materials with enhanced characteristics, such as energy storage, gas separation, and catalytic applications.
Broadening Roles in Pharmaceutical Delivery and Therapeutics: Advancing the use of pyrazole-based assembled systems for medication delivery is a crucial frontier for future research. The objective is to develop the biocompatibility and targeting functionality of these assemblies for precision medicine.
Innovative Tracking and Detection Technologies: Successive investigation will concentrate on enhancing the sensitivity and selectivity of pyrazole-based sensors for the detection of ions, gases, and biomolecules. This encompasses the advancement of sensors capable of detecting many gases and their combination into electronic equipment to enable immediate monitoring in both ambient and clinical environments.
Computational Study: Predictions indicated that computational tools will have a more significant impact on the design and optimization of pyrazole ligands. These technologies may facilitate the prediction of the behavior of novel ligands, simplify the design process, and expedite the identification of ligands with ideal characteristics for specific applications
8. Conclusion
This review highlights that pyrazole ligands exhibit remarkable versatility as vital aspects in the self-assembly process, contributing to the formation of complex molecular architectures, including MOFs, coordination polymers, and nanostructures. Various applications, including catalysis, drug delivery, material research, and sensing technologies, have shown significant potential for this system.
-
1.
This study highlights the coordination of pyrazole ligands, enabling them to participate in diverse noncovalent interactions such as hydrogen bonding, π–π stacking, and metal coordination functions. These interactions enable the spontaneous arrangement of molecules into precisely specified supramolecular structures.
-
2.
In addition to their potential, obstacles such as restricted stability under various environmental conditions and scaling problems in synthesis continue to exist. Future research is anticipated to concentrate on overcoming these obstacles by enhancing synthetic techniques, integrating computational modeling, and investigating green chemistry methodologies.
-
3.
The ongoing investigation of pyrazole ligands, focused on sophisticated material design, has significant promise for the advancement of cutting-edge technologies, particularly in burgeoning areas such as optoelectronics and smart materials.
Acknowledgments
The authors are grateful to acknowledge the support of the Beijing Natural Science FoundationKey Project of Education Commission.
The authors declare no competing financial interest.
References
- Philp D., Stoddart J. F.. Self-assembly in natural and unnatural systems. Angew Chem. Int. Ed. Engl. 1996;35(11):1154–1196. doi: 10.1002/anie.199611541. [DOI] [Google Scholar]
- Monnard, P.-A. ; Deamer, D. W. . Membrane self-assembly processes: steps toward the first cellular life. In The Minimal Cell: The Biophysics of Cell Compartment and the Origin of Cell Functionality; Springer Science & Business Media, 2010; pp 123–151. [Google Scholar]
- Kumar P., Pillay V., Modi G., E Choonara Y., C du Toit L., Naidoo D.. Self-assembling peptides: implications for patenting in drug delivery and tissue engineering. Recent patents on drug delivery & formulation. 2011;5(1):24–51. doi: 10.2174/187221111794109510. [DOI] [PubMed] [Google Scholar]
- Stupp S. I., Palmer L. C.. Supramolecular chemistry and self-assembly in organic materials design. Chem. Mater. 2014;26(1):507–518. doi: 10.1021/cm403028b. [DOI] [Google Scholar]
- Marsh J. A., Teichmann S. A.. Structure, dynamics, assembly, and evolution of protein complexes. Annu. Rev. Biochem. 2015;84(1):551–575. doi: 10.1146/annurev-biochem-060614-034142. [DOI] [PubMed] [Google Scholar]
- Wilson C. J., Bommarius A. S., Champion J. A., Chernoff Y. O., Lynn D. G., Paravastu A. K., Liang C., Hsieh M.-C., Heemstra J. M.. Biomolecular assemblies: moving from observation to predictive design. Chem. Rev. 2018;118(24):11519–11574. doi: 10.1021/acs.chemrev.8b00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahakoon S. B., Diwakara S. D., Thompson C. M., Smaldone R. A.. Supramolecular design in 2D covalent organic frameworks. Chem. Soc. Rev. 2020;49(5):1344–1356. doi: 10.1039/C9CS00884E. [DOI] [PubMed] [Google Scholar]
- Aykanat A., Meng Z., Benedetto G., Mirica K. A.. Molecular engineering of multifunctional metallophthalocyanine-containing framework materials. Chem. Mater. 2020;32(13):5372–5409. doi: 10.1021/acs.chemmater.9b05289. [DOI] [Google Scholar]
- Zhang X., Dai X., Gao L., Xu D., Wan H., Wang Y., Yan L.-T.. The entropy-controlled strategy in self-assembling systems. Chem. Soc. Rev. 2023;52(19):6806–6837. doi: 10.1039/D3CS00347G. [DOI] [PubMed] [Google Scholar]
- Cai Z., Li Z., Ravaine S., He M., Song Y., Yin Y., Zheng H., Teng J., Zhang A.. From colloidal particles to photonic crystals: advances in self-assembly and their emerging applications. Chem. Soc. Rev. 2021;50(10):5898–5951. doi: 10.1039/D0CS00706D. [DOI] [PubMed] [Google Scholar]
- Gröschel A. H., Müller A. H.. Self-assembly concepts for multicompartment nanostructures. Nanoscale. 2015;7(28):11841–11876. doi: 10.1039/C5NR02448J. [DOI] [PubMed] [Google Scholar]
- Cummins C., Lundy R., Walsh J. J., Ponsinet V., Fleury G., Morris M. A.. Enabling future nanomanufacturing through block copolymer self-assembly: A review. Nano Today. 2020;35:100936. doi: 10.1016/j.nantod.2020.100936. [DOI] [Google Scholar]
- Mandler D., Kraus-Ophir S.. Self-assembled monolayers (SAMs) for electrochemical sensing. J. Solid State Electrochem. 2011;15:1535–1558. doi: 10.1007/s10008-011-1493-6. [DOI] [Google Scholar]
- Ziegler J. M., Andoni I., Choi E. J., Fang L., Flores-Zuleta H., Humphrey N. J., Kim D.-H., Shin J., Youn H., Penner R. M.. Sensors based upon nanowires, nanotubes, and nanoribbons: 2016–2020. Anal. Chem. 2021;93(1):124–166. doi: 10.1021/acs.analchem.0c04476. [DOI] [PubMed] [Google Scholar]
- Scarabelli L., Vila-Liarte D., Mihi A., Liz-Marzán L. M.. Templated colloidal self-assembly for lattice plasmon engineering. Acc. Mater. Res. 2021;2(9):816–827. doi: 10.1021/accountsmr.1c00106. [DOI] [Google Scholar]
- Talukdar D., Kumar J. M., Gole B.. Self-assembled macrocycles: design strategies and emerging functions. Cryst. Growth Des. 2023;23(11):7582–7611. doi: 10.1021/acs.cgd.3c00677. [DOI] [Google Scholar]
- Pastore V. J., Cook T. R.. Coordination-driven self-assembly in polymer–inorganic hybrid materials. Chem. Mater. 2020;32(9):3680–3700. doi: 10.1021/acs.chemmater.0c00851. [DOI] [Google Scholar]
- Sayed M., Pal H.. An overview from simple host–guest systems to progressively complex supramolecular assemblies. Phys. Chem. Chem. Phys. 2021;23(46):26085–26107. doi: 10.1039/D1CP03556H. [DOI] [PubMed] [Google Scholar]
- Gong X.-F., Li Y.-J., Wang D., Cao H., Yang Z., Wang H., Wang L.. Process-biomimetic macromolecular materials for in vivo applications. Prog. Mater. Sci. 2023;131:101015. doi: 10.1016/j.pmatsci.2022.101015. [DOI] [Google Scholar]
- Han X.-N., Han Y., Chen C.-F.. Recent advances in the synthesis and applications of macrocyclic arenes. Chem. Soc. Rev. 2023;52(9):3265–3298. doi: 10.1039/D3CS00002H. [DOI] [PubMed] [Google Scholar]
- Vinay B., Karthik C., Hema M., Rajabathar J. R., Kumar A. U., Ramakrishna B., Lokanath N.. Non-covalent interactions orchestrated distinct molecular arrangements along different axes within the novel mixed ligand thorium metal complex: Experimental and theoretical insights. J. Mol. Struct. 2024;1303:137486. doi: 10.1016/j.molstruc.2024.137486. [DOI] [Google Scholar]
- Perez J., Riera L.. Pyrazole complexes and supramolecular chemistry. Eur. J. Inorg. Chem. 2009;2009(33):4913–4925. doi: 10.1002/ejic.200900694. [DOI] [Google Scholar]
- Parshad M., Kumar D., Verma V.. A mini review on applications of pyrazole ligands in coordination compounds and metal organic frameworks. Inorg. Chim. Acta. 2024;560:121789. doi: 10.1016/j.ica.2023.121789. [DOI] [Google Scholar]
- Saha S., Das M., Das K. S., Datta R., Bala S., Liu J.-L., Ray P. P., Mondal R.. Magnetic and electric properties of pyrazole-based metal–organic frameworks grafted with a sulfonic moiety. Cryst. Growth Des. 2023;23(2):1104–1118. doi: 10.1021/acs.cgd.2c01251. [DOI] [Google Scholar]
- Lin R.-B., He Y., Li P., Wang H., Zhou W., Chen B.. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 2019;48(5):1362–1389. doi: 10.1039/C8CS00155C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halcrow M. A.. Pyrazoles and pyrazolidesflexible synthons in self-assembly. Dalton Trans. 2009;(12):2059–2073. doi: 10.1039/b815577a. [DOI] [PubMed] [Google Scholar]
- Ahmed M. N., Andleeb H., Fahim A. M., Madni M., Khan S. W., Kaboudin B., Ibrahim M. A., Sidhom P. A., Gil D. M.. Supramolecular assembly involving weak hydrogen bonds, anti-parallel π··· π stacking and O··· C tetrel bonding interactions and LOX studies in a 1H-pyrazol-1-yl) thiazole-4-carboxylate derivative: An experimental and theoretical study. J. Mol. Struct. 2024;1296:136908. doi: 10.1016/j.molstruc.2023.136908. [DOI] [Google Scholar]
- Bala S., De A., Adhikary A., Saha S., Akhtar S., Das K. S., Tong M.-L., Mondal R.. Influence of semirigidity and diverse binding modes of an asymmetric pyridine-pyrazole based bis-chelating ligand in controlling molecular architectures and their properties. Cryst. Growth Des. 2020;20(9):5698–5708. doi: 10.1021/acs.cgd.9b01496. [DOI] [Google Scholar]
- Tigreros A., Portilla J.. Recent progress in chemosensors based on pyrazole derivatives. RSC Adv. 2020;10(33):19693–19712. doi: 10.1039/D0RA02394A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandurkar Y., Shinde A., Bhoye M. R., Jagadale S., Mhaske P. C.. Synthesis and biological screening of new 2-(5-aryl-1-phenyl-1 H-pyrazol-3-yl)-4-aryl thiazole derivatives as potential antimicrobial agents. ACS Omega. 2023;8(9):8743–8754. doi: 10.1021/acsomega.2c08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgania J., Gavadia R., Varma-Basil M., Chauhan V., Kumar S., Mor S., Singh D., Jakhar K.. Design and synthesis of isoniazid-based pyrazolines as potential inhibitors of Mycobacterium tuberculosis with promising radical scavenging action: in-vitro and in-silico evaluations. J. Mol. Struct. 2024;1295:136657. doi: 10.1016/j.molstruc.2023.136657. [DOI] [Google Scholar]
- Săcărescu L., Chibac-Scutaru A.-L., Roman G., Săcărescu G., Simionescu M.. Selective detection of metal ions, sulfites and glutathione with fluorescent pyrazolines: a review. Environ. Chem. Lett. 2023;21(1):561–596. doi: 10.1007/s10311-022-01508-8. [DOI] [Google Scholar]
- Zhou M.-Y., Tong J., Lu H.-L., Wang X.-Y., Yu S.-Y.. Hierarchical self-assembly and packing models of dipalladium (II, II)-based metallacapsules and metallacages based on amide-functionalized multi-pyrazoles. Inorg. Chem. Commun. 2022;136:109145. doi: 10.1016/j.inoche.2021.109145. [DOI] [Google Scholar]
- Wang P., Tong J., Meng C., Yuan Q., Deng W., Yu S.-Y., Ma H.-W.. Self-assembly of tripyrazolate-linked [M6L2] cages for the selective sensing of HSO3– and gaseous SO2 by turn-on fluorescence. Dalton Trans. 2023;52(18):6129–6137. doi: 10.1039/D3DT00083D. [DOI] [PubMed] [Google Scholar]
- Wen J., Tong J., Kou Y.-L., Wang J., Yu S.-Y.. Multi-responsive smart fluorophores from pyrazole-functionalized tetraphenylethene AIEgens in response to Hg (II) ion, temperature, and mechanical force. Dyes Pigm. 2024;223:111954. doi: 10.1016/j.dyepig.2024.111954. [DOI] [Google Scholar]
- Yadav A. K., Rajak R., Ghule V. D., Dharavath S.. Energetic coordination compounds: self-assembled from the nitrogen-rich energetic C–C bonded pyrazoles and triazoles. Dalton Trans. 2023;52(35):12271–12281. doi: 10.1039/D3DT02410E. [DOI] [PubMed] [Google Scholar]
- Iguarbe V., Romero P., Barberá J., Elduque A., Giménez R.. Dual liquid Crystalline/Gel behavior with AIE effect promoted by Self-assembly of pyrazole dendrons. J. Mol. Liq. 2022;365:120109. doi: 10.1016/j.molliq.2022.120109. [DOI] [Google Scholar]
- Ghorbanpour M., Shayanfar A., Soltani B.. Copper pyrazole complexes as potential anticancer agents: Evaluation of cytotoxic response against cancer cells and their mechanistic action at the molecular level. Coord. Chem. Rev. 2024;498:215459. doi: 10.1016/j.ccr.2023.215459. [DOI] [Google Scholar]
- Gómez de Segura D., Corral-Zorzano A., Alcolea E., Moreno M. T., Lalinde E.. Phenylbenzothiazole-Based Platinum (II) and Diplatinum (II) and (III) Complexes with Pyrazolate Groups: Optical Properties and Photocatalysis. Inorg. Chem. 2024;63(3):1589–1606. doi: 10.1021/acs.inorgchem.3c03532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S., Cordes D. B., Slawin A. M., Saha N. C.. Synthesis, characterization, X-ray Crystallography, Photoluminescence property and DNA binding activity of Co (II) and Ni (II) complexes of a pyrazole-containing Schiff-base ligand. J. Coord. Chem. 2024;77(1–2):170–187. doi: 10.1080/00958972.2024.2309629. [DOI] [Google Scholar]
- Hamodin A. G., Elgammal W. E., Eid A. M., Ibrahim A. G.. Synthesis, characterization, and biological evaluation of new chitosan derivative bearing diphenyl pyrazole moiety. Int. J. Biol. Macromol. 2023;243:125180. doi: 10.1016/j.ijbiomac.2023.125180. [DOI] [PubMed] [Google Scholar]
- Song X., Wang Y., Wang C., Wang D., Zhuang G., Kirlikovali K. O., Li P., Farha O. K.. Design rules of hydrogen-bonded organic frameworks with high chemical and thermal stabilities. J. Am. Chem. Soc. 2022;144(24):10663–10687. doi: 10.1021/jacs.2c02598. [DOI] [PubMed] [Google Scholar]
- Jo J.-H., Singh N., Kim D., Cho S. M., Mishra A., Kim H., Kang S. C., Chi K.-W.. Coordination-driven self-assembly using ditopic pyridyl–pyrazolyl donor and p-cymene Ru (II) Acceptors:[2] Catenane synthesis and anticancer activities. Inorg. Chem. 2017;56(14):8430–8438. doi: 10.1021/acs.inorgchem.7b01101. [DOI] [PubMed] [Google Scholar]
- Develay S., Blackburn O., Thompson A. L., Williams J. G.. Cyclometalated platinum (II) complexes of pyrazole-based, N∧ C∧ N-coordinating, terdentate ligands: The contrasting influence of pyrazolyl and pyridyl rings on luminescence. Inorg. Biochem. 2008;47(23):11129–11142. doi: 10.1021/ic8014157. [DOI] [PubMed] [Google Scholar]
- Lancheros A., Goswami S., Mian M. R., Zhang X., Zarate X., Schott E., Farha O. K., Hupp J. T.. Modulation of CO2 adsorption in novel pillar-layered MOFs based on carboxylate–pyrazole flexible linker. Dalton Trans. 2021;50(8):2880–2890. doi: 10.1039/D0DT03166F. [DOI] [PubMed] [Google Scholar]
- Jin J.-K., Wu K., Liu X.-Y., Huang G.-Q., Huang Y.-L., Luo D., Xie M., Zhao Y., Lu W., Zhou X.-P.. et al. Building a pyrazole–benzothiadiazole–pyrazole photosensitizer into metal–organic frameworks for photocatalytic aerobic oxidation. J. Am. Chem. Soc. 2021;143(50):21340–21349. doi: 10.1021/jacs.1c10008. [DOI] [PubMed] [Google Scholar]
- Younas, M. ; Ul Azam, S. ; Farukh, S. ; Ullah, N. ; Ihsan, H. ; Mukhtar, H. ; Rezakazemi, M. . Metal– Organic Frameworks for Carbon Dioxide Capture. In Metal– Organic Frameworks for Carbon Capture and Energy; ACS Publications, 2021; pp 203–238. [Google Scholar]
- Anwar M. I., Asad M., Ma L., Zhang W., Abbas A., Khan M. Y., Zeeshan M., Khatoon A., Gao R., Manzoor S.. et al. Nitrogenous MOFs and their composites as high-performance electrode material for supercapacitors: Recent advances and perspectives. Coord. Chem. Rev. 2023;478:214967. doi: 10.1016/j.ccr.2022.214967. [DOI] [Google Scholar]
- Cuerva C., Cano M., Lodeiro C.. Advanced functional luminescent metallomesogens: the key role of the metal center. Chem. Rev. 2021;121(20):12966–13010. doi: 10.1021/acs.chemrev.1c00011. [DOI] [PubMed] [Google Scholar]
- Cook T. R., Stang P. J.. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015;115(15):7001–7045. doi: 10.1021/cr5005666. [DOI] [PubMed] [Google Scholar]
- Baruah J. B.. Inorganic Molecular Complexes: Potential for Growth of a New Subject Area in Self-Assembly. Top. Curr. Chem. 2020;378:30. doi: 10.1007/s41061-020-0294-8. [DOI] [PubMed] [Google Scholar]
- Chkirate K., Karrouchi K., Chakchak H., Mague J. T., Radi S., Adarsh N., Li W., Talbaoui A., Essassi E. M., Garcia Y.. Coordination complexes constructed from pyrazole–acetamide and pyrazole–quinoxaline: effect of hydrogen bonding on the self-assembly process and antibacterial activity. RSC Adv. 2022;12(9):5324–5339. doi: 10.1039/D1RA09027E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti F., Pettinari C., Di Nicola C., Tombesi A., Pettinari R.. Coordination chemistry of pyrazolone-based ligands and applications of their metal complexes. Coord. Chem. Rev. 2019;401:213069. doi: 10.1016/j.ccr.2019.213069. [DOI] [Google Scholar]
- Gusev A., Shulgin V., Kiskin M.. Self-Assembly of Polynuclear Complexes Based on Spacer-Armed Pyridylazoles. J. Struct. Chem. 2019;60:335–355. doi: 10.1134/S0022476619030016. [DOI] [Google Scholar]
- Carrión M. C., Dura G., Jalon F. A., Manzano B. R., Rodriguez A. M.. Polynuclear complexes containing ditopic bispyrazolylmethane ligands. Influence of metal geometry and supramolecular interactions. Cryst. Growth Des. 2012;12(4):1952–1969. doi: 10.1021/cg201677s. [DOI] [Google Scholar]
- Liu J., Chen L., Cui H., Zhang J., Zhang L., Su C.-Y.. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014;43(16):6011–6061. doi: 10.1039/C4CS00094C. [DOI] [PubMed] [Google Scholar]
- Kumar V., Vellingiri K., Kukkar D., Kumar S., Kim K.-H.. Recent advances and opportunities in the treatment of hydrocarbons and oils: Metal-organic frameworks-based approaches. Crit. Rev. Environ. Sci. Technol. 2019;49(7):587–654. doi: 10.1080/10643389.2018.1554402. [DOI] [Google Scholar]
- Yuan S., Feng L., Wang K., Pang J., Bosch M., Lollar C., Sun Y., Qin J., Yang X., Zhang P.. et al. Stable metal–organic frameworks: design, synthesis, and applications. Adv. Mater. 2018;30(37):1704303. doi: 10.1002/adma.201704303. [DOI] [PubMed] [Google Scholar]
- Firooz S. K., Armstrong D. W.. Metal-organic frameworks in separations: A review. Anal. Chim. Acta. 2022;1234:340208. doi: 10.1016/j.aca.2022.340208. [DOI] [PubMed] [Google Scholar]
- Hossack C., Cahill C., Besson C.. Utility of all-pyrazole heteroscorpionates in f-element chemistry. Dalton Trans. 2023;52(47):17656–17665. doi: 10.1039/D3DT02737F. [DOI] [PubMed] [Google Scholar]
- Zhao D., Timmons D. J., Yuan D., Zhou H.-C.. Tuning the topology and functionality of metal– organic frameworks by ligand design. Acc. Chem. Res. 2011;44(2):123–133. doi: 10.1021/ar100112y. [DOI] [PubMed] [Google Scholar]
- Lu W., Wei Z., Gu Z.-Y., Liu T.-F., Park J., Park J., Tian J., Zhang M., Zhang Q., Gentle III T.. et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014;43(16):5561–5593. doi: 10.1039/c4cs00003j. [DOI] [PubMed] [Google Scholar]
- Zhang J.-P., Zhang Y.-B., Lin J.-B., Chen X.-M.. Metal azolate frameworks: from crystal engineering to functional materials. Chem. Rev. 2012;112(2):1001–1033. doi: 10.1021/cr200139g. [DOI] [PubMed] [Google Scholar]
- Biradha K., Su C.-Y., Vittal J. J.. Recent developments in crystal engineering. Cryst. Growth Des. 2011;11(4):875–886. doi: 10.1021/cg101241x. [DOI] [Google Scholar]
- Wang Q., Yu Y., Li Y., Min X., Zhang J., Sun T.. Methane separation and capture from nitrogen rich gases by selective adsorption in microporous materials: A review. Sep. Purif. Technol. 2022;283:120206. doi: 10.1016/j.seppur.2021.120206. [DOI] [Google Scholar]
- Jiao L., Wang J., Jiang H.-L.. Microenvironment modulation in metal–organic framework-based catalysis. Acc. Mater. Res. 2021;2(5):327–339. doi: 10.1021/accountsmr.1c00009. [DOI] [Google Scholar]
- Chen K., Ni L., Guo X., Xiao C., Yang Y., Zhou Y., Zhu Z., Qi J., Li J.. Introducing pyrazole-based MOF to polymer of intrinsic microporosity for mixed matrix membranes with enhanced CO2/CH4 separation performance. J. Membr. Sci. 2023;688:122110. doi: 10.1016/j.memsci.2023.122110. [DOI] [Google Scholar]
- Ashraf M., Ahmad M. S., Inomata Y., Ullah N., Tahir M. N., Kida T.. Transition metal nanoparticles as nanocatalysts for Suzuki, Heck and Sonogashira cross-coupling reactions. Coord. Chem. Rev. 2023;476:214928. doi: 10.1016/j.ccr.2022.214928. [DOI] [Google Scholar]
- Kashyap S., Singh R., Singh U. P.. Inorganic and organic anion sensing by azole family members. Coord. Chem. Rev. 2020;417:213369. doi: 10.1016/j.ccr.2020.213369. [DOI] [Google Scholar]
- Cui Y., Yue Y., Qian G., Chen B.. Luminescent functional metal–organic frameworks. Chem. Rev. 2012;112(2):1126–1162. doi: 10.1021/cr200101d. [DOI] [PubMed] [Google Scholar]
- Desai A. V., Sharma S., Let S., Ghosh S. K.. N-donor linker based metal-organic frameworks (MOFs): Advancement and prospects as functional materials. Coord. Chem. Rev. 2019;395:146–192. doi: 10.1016/j.ccr.2019.05.020. [DOI] [Google Scholar]
- Howarth A. J., Liu Y., Li P., Li Z., Wang T. C., Hupp J. T., Farha O. K.. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 2016;1(3):15018. doi: 10.1038/natrevmats.2015.18. [DOI] [Google Scholar]
- Van Vleet M. J., Weng T., Li X., Schmidt J.. In situ, time-resolved, and mechanistic studies of metal–organic framework nucleation and growth. Chem. Rev. 2018;118(7):3681–3721. doi: 10.1021/acs.chemrev.7b00582. [DOI] [PubMed] [Google Scholar]
- El Boutaybi M., Taleb A., Touzani R., Bahari Z.. Metal-organic frameworks based on pyrazole subunit for batteries applications: A systematic review. Mater. Today: Proc. 2020;31:S96–S102. doi: 10.1016/j.matpr.2020.06.249. [DOI] [Google Scholar]
- Dhakshinamoorthy A., Li Z., Garcia H.. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018;47(22):8134–8172. doi: 10.1039/C8CS00256H. [DOI] [PubMed] [Google Scholar]
- Li N., Xu J., Feng R., Hu T.-L., Bu X.-H.. Governing metal–organic frameworks towards high stability. Chem. Commun. 2016;52(55):8501–8513. doi: 10.1039/C6CC02931K. [DOI] [PubMed] [Google Scholar]
- Kumar S., Arora A., Kumar A., Tomar K.. An unsymmetrical tritopic pyrazole carboxylate ligand based porous Cd (II) MOF sensor for acetone molecule. Inorg. Chem. Commun. 2018;96:16–19. doi: 10.1016/j.inoche.2018.07.042. [DOI] [Google Scholar]
- Roy M., Adhikary A., Saha S., Mondal R.. Toxic metal–organic gels using a unique pyridine–pyrazole based ligand with Pb (II), Cd (II) and Hg (II) salts: Multi-stimuli responsiveness and toxic dye adsorption. Mater. Adv. 2022;3(19):7328–7338. doi: 10.1039/D2MA00483F. [DOI] [Google Scholar]
- Boles M. A., Engel M., Talapin D. V.. Self-assembly of colloidal nanocrystals: from intricate structures to functional materials. Chem. Rev. 2016;116(18):11220–11289. doi: 10.1021/acs.chemrev.6b00196. [DOI] [PubMed] [Google Scholar]
- Yong M. T., Linder-Patton O. M., Bloch W. M.. Assembly of a heterometallic Cu (II)-Pd (II) cage by post-assembly metal insertion. Inorg. Chem. 2022;61(32):12863–12869. doi: 10.1021/acs.inorgchem.2c02046. [DOI] [PubMed] [Google Scholar]
- Dutta D., Sharma P., Gomila R. M., Frontera A., Barcelo-Oliver M., Verma A. K., Baishya T., Bhattacharyya M. K.. Supramolecular assemblies involving unconventional non-covalent contacts in pyrazole-based coordination compounds of Co (II) and Cu (II) pyridinedicarboxylates: Antiproliferative evaluation and theoretical studies. Polyhedron. 2022;224:116025. doi: 10.1016/j.poly.2022.116025. [DOI] [Google Scholar]
- Yu S. Y., Lu H. L.. From Metal-Metal Bonding to Supra-Metal-Metal Bonding Directed Self-Assembly: Supramolecular Architectures of Group 10 and 11 Metals with Ligands from Mono-to Poly-Pyrazoles. Isr. J. Chem. 2019;59(3–4):166–183. doi: 10.1002/ijch.201800097. [DOI] [Google Scholar]
- Griffiths K., Greenfield J. L., Halcovitch N. R., Fuchter M. J., Griffin J. M.. Systematic Investigation into the Photoswitching and Thermal Properties of Arylazopyrazole-based MOF Host–Guest Complexes. Cryst. Growth Des. 2023;23(10):7044–7052. doi: 10.1021/acs.cgd.2c01384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Zhao Y.. Biomedical applications of supramolecular systems based on host–guest interactions. Chem. Rev. 2015;115(15):7794–7839. doi: 10.1021/cr500392w. [DOI] [PubMed] [Google Scholar]
- Sahandi Zangabad P., Karimi M., Mehdizadeh F., Malekzad H., Ghasemi A., Bahrami S., Zare H., Moghoofei M., Hekmatmanesh A., Hamblin M. R.. Nanocaged platforms: modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale. 2017;9(4):1356–1392. doi: 10.1039/c6nr07315h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M. A., Cooper A. I.. The chemistry of porous organic molecular materials. Adv. Funct. Mater. 2020;30(41):1909842. doi: 10.1002/adfm.201909842. [DOI] [Google Scholar]
- Ariga K., Shionoya M.. Nanoarchitectonics for coordination asymmetry and related chemistry. Bull. Chem. Soc. Jpn. 2021;94(3):839–859. doi: 10.1246/bcsj.20200362. [DOI] [Google Scholar]
- Benaissa H., Wolff M., Robeyns K., Knör G. n., Van Hecke K., Campagnol N., Fransaer J., Garcia Y.. Syntheses, crystal structures, luminescent properties, and electrochemical synthesis of group 12 element coordination polymers with 4-substituted 1, 2, 4-triazole ligands. Cryst. Growth Des. 2019;19(9):5292–5307. doi: 10.1021/acs.cgd.9b00734. [DOI] [Google Scholar]
- Montà-González G., Sancenón F., Martínez-Máñez R., Martí-Centelles V.. Purely covalent molecular cages and containers for guest encapsulation. Chem. Rev. 2022;122(16):13636–13708. doi: 10.1021/acs.chemrev.2c00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Starvaggi N., Pentzer E.. Capsules with responsive polymeric shells for applications beyond drug delivery. Polym. Chem. 2023;14(35):4033–4047. doi: 10.1039/D3PY00434A. [DOI] [Google Scholar]
- Sethi S., Jena S., Das P. K., Behera N.. Synthetic approach and structural diversities of pyridylpyrazole derived late transition metal complexes. J. Mol. Struct. 2019;1193:495–521. doi: 10.1016/j.molstruc.2019.04.112. [DOI] [Google Scholar]
- Li J., Li B., Pan M., Liu B., Cheng J., Li R., Gao X., Wang S., Hou H., Liu Z.. Five Multidimensional Co (II)-Complexes (Zero-Dimensional to Three-Dimensional) Derived from an Asymmetric 5-(Pyridin-3-yl)-1 H-pyrazole-3-carboxylic Acid: Syntheses, Structures, and Magnetic Properties. Cryst. Growth Des. 2017;17(6):2975–2986. doi: 10.1021/acs.cgd.6b01478. [DOI] [Google Scholar]
- Olguín J., Brooker S.. Spin crossover active iron (II) complexes of selected pyrazole-pyridine/pyrazine ligands. Coord. Chem. Rev. 2011;255(1–2):203–240. doi: 10.1016/j.ccr.2010.08.002. [DOI] [Google Scholar]
- Pettinari C., Tăbăcaru A., Galli S.. Coordination polymers and metal–organic frameworks based on poly (pyrazole)-containing ligands. Coord. Chem. Rev. 2016;307:1–31. doi: 10.1016/j.ccr.2015.08.005. [DOI] [Google Scholar]
- Ali Akbar Razavi S., Morsali A.. Linker functionalized metal-organic frameworks. Coord. Chem. Rev. 2019;399:213023. doi: 10.1016/j.ccr.2019.213023. [DOI] [Google Scholar]
- Gupta R. K., Kallem P., Luo G.-G., Cui P., Wang Z., Banat F., Tung C.-H., Sun D.. 1H-Pyrazole-4-carboxylic acid-based metal–organic frameworks: multifaceted materials. Coord. Chem. Rev. 2023;497:215436. doi: 10.1016/j.ccr.2023.215436. [DOI] [Google Scholar]
- Chen L.-J., Yang H.-B.. Construction of stimuli-responsive functional materials via hierarchical self-assembly involving coordination interactions. Acc. Chem. Res. 2018;51(11):2699–2710. doi: 10.1021/acs.accounts.8b00317. [DOI] [PubMed] [Google Scholar]
- Berdiell I. C., Kulak A. N., Warriner S. L., Halcrow M. A.. Heterometallic coordination polymer gels supported by 2, 4, 6-tris (pyrazol-1-yl)-1, 3, 5-triazine. ACS Omega. 2018;3(12):18466–18474. doi: 10.1021/acsomega.8b02508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. G., El-Mahdy A. F., Kotp M. G., Kuo S.-W.. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022;3(2):707–733. doi: 10.1039/D1MA00771H. [DOI] [Google Scholar]
- Ariga K.. Liquid interfacial nanoarchitectonics: Molecular machines, organic semiconductors, nanocarbons, stem cells, and others. Curr. Opin. Colloid Interface Sci. 2023;63:101656. doi: 10.1016/j.cocis.2022.101656. [DOI] [Google Scholar]
- Lunev A. M., Sidoruk A. V., Gontcharenko V. E., Kiskin M. A., Taydakov I. V., Belousov Y. A., Drozdov A. A.. Novel pyrazole-based carboxylate ligand as a building block for assembling lanthanides in luminescent 2D and 3D MOFs. Inorg. Chim. Acta. 2022;537:120956. doi: 10.1016/j.ica.2022.120956. [DOI] [Google Scholar]
- Lim J. Y., Goh S. S., Liow S. S., Xue K., Loh X. J.. Molecular gel sorbent materials for environmental remediation and wastewater treatment. J. Mater. Chem. A. 2019;7(32):18759–18791. doi: 10.1039/C9TA05782J. [DOI] [Google Scholar]
- Patel A. M., Bhardwaj V., Ballabh A.. New Ureas and Amides-An Account of Recent Trends and Developments in Low Molecular Weight Gelators. Curr. Org. Chem. 2024;28(13):1046–1058. doi: 10.2174/0113852728277924231124094902. [DOI] [Google Scholar]
- Bhat M., Poojary B., Kalal B. S., Gurubasavaraja Swamy P. M., Kabilan S., Kumar V., Shruthi N., Alias Anand S. A., Pai V. R.. Synthesis and evaluation of thiazolidinone–pyrazole conjugates as anticancer and antimicrobial agents. Future Med. Chem. 2018;10(9):1017–1036. doi: 10.4155/fmc-2017-0191. [DOI] [PubMed] [Google Scholar]
- Hirst A. R., Smith D. K.. Two-Component Gel-Phase MaterialsHighly Tunable Self-Assembling Systems. Chem.Eur. J. 2005;11(19):5496–5508. doi: 10.1002/chem.200500241. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Sengupta S., Bala S., Goswami A., Ganguly S., Mondal R.. Pyrazole-based metallogels showing an unprecedented colorimetric ammonia gas sensing through gel-to-gel transformation with a rare event of time-dependent morphology transformation. Cryst. Growth Des. 2014;14(5):2366–2374. doi: 10.1021/cg5000827. [DOI] [Google Scholar]
- Ugwu D. I., Conradie J.. Bidentate ligands in self-assembly: synthesis, structure and applications. J. Mol. Struct. 2023;1293:136275. doi: 10.1016/j.molstruc.2023.136275. [DOI] [Google Scholar]
- Mahmudov I., Ibrahimova B., Taslimi P., Sadeghian N., Karaoğlan Z., Taskin-Tok T., Abdullayev Y., Farzaliyev V., Sujayev A., Alwasel S. H.. et al. Synthesis, characterization, crystal structure, molecular docking, and biological studies of Cu, Ni and Co metal complexes of pyrazole. J. Mol. Struct. 2024;1309:138205. doi: 10.1016/j.molstruc.2024.138205. [DOI] [Google Scholar]
- Budagumpi S., Haque R. A., Salman A. W.. Stereochemical and structural characteristics of single-and double-site Pd (II)–N-heterocyclic carbene complexes: Promising catalysts in organic syntheses ranging from CC coupling to olefin polymerizations. Coord. Chem. Rev. 2012;256(17–18):1787–1830. doi: 10.1016/j.ccr.2012.04.003. [DOI] [Google Scholar]
- Otero A., Fernández-Baeza J., Lara-Sánchez A., Sánchez-Barba L. F.. Metal complexes with heteroscorpionate ligands based on the bis (pyrazol-1-yl) methane moiety: Catalytic chemistry. Coord. Chem. Rev. 2013;257(11–12):1806–1868. doi: 10.1016/j.ccr.2013.01.027. [DOI] [Google Scholar]
- Martins L. M., Pombeiro A. J.. Tris (pyrazol-1-yl) methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014;265:74–88. doi: 10.1016/j.ccr.2014.01.013. [DOI] [Google Scholar]
- Jeong S., Joo J. M.. Transition-Metal-Catalyzed Divergent C–H Functionalization of Five-Membered Heteroarenes. Acc. Chem. Res. 2021;54(24):4518–4529. doi: 10.1021/acs.accounts.1c00547. [DOI] [PubMed] [Google Scholar]
- Kadu B. S.. Suzuki–Miyaura cross coupling reaction: recent advancements in catalysis and organic synthesis. Catal. Sci. Technol. 2021;11(4):1186–1221. doi: 10.1039/D0CY02059A. [DOI] [Google Scholar]
- Al Isawi W. A., Ahmed B. M., Hartman C. K., Seybold A. N., Mezei G.. Are nanojars unique to copper? Solution and solid state characterization of high-symmetry octanuclear nickel (II)-pyrazolate complexes. Inorg. Chim. Acta. 2018;475:65–72. doi: 10.1016/j.ica.2017.08.013. [DOI] [Google Scholar]
- Kumar V., El-Massaoudi M., Radi S., Van Hecke K., Rotaru A., Garcia Y.. Iron (ii) coordination pyrazole complexes with aromatic sulfonate ligands: the role of ether. New J. Chem. 2020;44(32):13902–13912. doi: 10.1039/D0NJ02823A. [DOI] [Google Scholar]
- Tang X., Wu W., Zeng W., Jiang H.. Copper-catalyzed oxidative carbon–carbon and/or carbon–heteroatom bond formation with O2 or internal oxidants. Acc. Chem. Res. 2018;51(5):1092–1105. doi: 10.1021/acs.accounts.7b00611. [DOI] [PubMed] [Google Scholar]
- Pascual-Escudero A., Ortiz-Rojano L., Simon-Fuente S., Adrio J., Ribagorda M.. Aldehydes as photoremovable directing groups: synthesis of pyrazoles by a photocatalyzed [3+ 2] cycloaddition/norrish type fragmentation sequence. Org. Lett. 2021;23(12):4903–4908. doi: 10.1021/acs.orglett.1c01665. [DOI] [PubMed] [Google Scholar]
- Brown, A. T. ; Cotterell, S. J. ; Denny, M. R. ; Downer-Riley, N. K. . Copper-Based Nanomaterials in the Synthesis of Heterocycles. In Copper-Based Nanomaterials in Organic Transformations; ACS Publications, 2024; pp 193–230. [Google Scholar]
- Sun C.-L., Li B.-J., Shi Z.-J.. Direct C– H transformation via iron catalysis. Chem. Rev. 2011;111(3):1293–1314. doi: 10.1021/cr100198w. [DOI] [PubMed] [Google Scholar]
- Bouroumane N., El Kodadi M., Touzani R., El Boutaybi M., Oussaid A., Hammouti B., Nandiyanto A. B. D.. New pyrazole-based ligands: Synthesis, characterization, and catalytic activity of their copper complexes. Arabian J. Sci. Eng. 2022;47(1):269–279. doi: 10.1007/s13369-021-05343-x. [DOI] [Google Scholar]
- Sadjadi S., M. Heravi M.. Recent advances in applications of POMs and their hybrids in catalysis. Curr. Org. Chem. 2016;20(13):1404–1444. doi: 10.2174/1385272820666160216225330. [DOI] [Google Scholar]
- Phukan P., Sarma D.. Synthesis of medicinally relevant scaffolds-triazoles and pyrazoles in green solvent ionic liquids. Curr. Org. Chem. 2021;25(13):1523–1538. doi: 10.2174/1385272825666210412160142. [DOI] [Google Scholar]
- Zhang D., Zou X.-N., Wang X.-G., Su J., Luan T.-X., Fan W., Li P.-Z., Zhao Y.. Highly effective photocatalytic radical reactions triggered by a photoactive metal–organic framework. ACS Appl. Mater. Interfaces. 2022;14(20):23518–23526. doi: 10.1021/acsami.2c04331. [DOI] [PubMed] [Google Scholar]
- Zhao X., Li J., Li X., Huo P., Shi W.. Design of metal-organic frameworks (MOFs)-based photocatalyst for solar fuel production and photo-degradation of pollutants. Chin. J. Catal. 2021;42(6):872–903. doi: 10.1016/S1872-2067(20)63715-9. [DOI] [Google Scholar]
- Li J., Zeng W., Wang L., Shi G., Wang D.. Pyrazolyl-pyrimidine porous-organic-polymer supported single-site nickel composites as efficient catalysts for the synthesis of substituted pyrimidines. Chem. Eng. J. 2023;474:145642. doi: 10.1016/j.cej.2023.145642. [DOI] [Google Scholar]
- Pedrini A., Maspero A., Bracco S., Comotti A., Galli S., Marchiò L., Nardo L., Penoni A., Scapinello L., Sozzani P.. et al. Synthesis, crystal structure, and optical properties of fluorinated poly (pyrazole) ligands and in silico assessment of their affinity for volatile organic compounds. New J. Chem. 2020;44(16):6443–6455. doi: 10.1039/d0nj00259c. [DOI] [Google Scholar]
- Ansari A., Ali A., Asif M., Shamsuzzaman S.. Review: biologically active pyrazole derivatives. New J. Chem. 2017;41(1):16–41. doi: 10.1039/c6nj03181a. [DOI] [Google Scholar]
- Nurmamat M., Yan H., Wang R., Zhao H., Li Y., Wang X., Nurmaimaiti K., Kurmanjiang T., Luo D., Baodi J.. et al. Novel copper (II) complex with a 4-acylpyrazolone derivative and coligand induce apoptosis in liver cancer cells. ACS Med. Chem. Lett. 2021;12(3):467–476. doi: 10.1021/acsmedchemlett.0c00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancheros A., Goswami S., Mian M. R., Zhang X., Zarate X., Schott E., Farha O. K., Hupp J. T.. Modulation of CO 2 adsorption in novel pillar-layered MOFs based on carboxylate–pyrazole flexible linker. Dalton Trans. 2021;50(8):2880–2890. doi: 10.1039/D0DT03166F. [DOI] [PubMed] [Google Scholar]
- De A., Bala S., Saha S., Das K. S., Akhtar S., Adhikary A., Ghosh A., Huang G.-Z., Chowdhuri S. P., Das B. B.. et al. Lanthanide clusters of phenanthroline containing a pyridine–pyrazole based ligand: magnetism and cell imaging. Dalton Trans. 2021;50(10):3593–3609. doi: 10.1039/d0dt04122j. [DOI] [PubMed] [Google Scholar]
- Dhara A., Guchhait N., Mukherjee I., Mukherjee A., Chandra Bhattacharya S.. A novel pyrazole based single molecular probe for multi-analyte (Zn 2+ and Mg 2+) detection in human gastric adenocarcinoma cells. RSC Adv. 2016;6(107):105930–105939. doi: 10.1039/c6ra22869k. [DOI] [Google Scholar]
- Saha S., De A., Ghosh A., Ghosh A., Bera K., Das K. S., Akhtar S., Maiti N. C., Das A. K., Das B. B.. et al. Pyridine-pyrazole based Al (III)‘turn on’sensor for MCF7 cancer cell imaging and detection of picric acid. RSC Adv. 2021;11(17):10094–10109. doi: 10.1039/d1ra00082a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragay G., Pons J., Ros J., Merkoci A.. Aminopyrazole-based ligand induces gold nanoparticle formation and remains available for heavy metal ions sensing. A simple “mix and detect” approach. Langmuir. 2010;26(12):10165–10170. doi: 10.1021/la100288s. [DOI] [PubMed] [Google Scholar]
- Garcıa M., Romero I., Portilla J.. Synthesis of Fluorescent 1, 7-Dipyridyl-bis-pyrazolo [3, 4-b: 4 0, 3 0-e] pyridines: Design of Reversible Chemosensors for Nanomolar Detection of Cu2+ ACS Omega. 2019;4:6757–6768. doi: 10.1021/acsomega.9b00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel S., Sathish V., Mathivathanan L., Morozov A. N., Mebel A. M., Raptis R. G.. Aggregation induced emission enhancement (AIEE) of tripodal pyrazole derivatives for sensing of nitroaromatics and vapor phase detection of picric acid. New J. Chem. 2019;43(19):7251–7258. doi: 10.1039/C9NJ00166B. [DOI] [Google Scholar]
- Nelson M., Muniyasamy H., Ongi P., Balakrishnan S., Sepperumal M., Ayyanar S., Jegathalaprathaban R.. Incredible colorimetric sensing behavior of pyrazole-based imine chemosensor towards copper (II) ion detection: synthesis, characterization and theoretical investigations. Results Chem. 2022;4:100501. doi: 10.1016/j.rechem.2022.100501. [DOI] [Google Scholar]
- Wales D. J., Grand J., Ting V. P., Burke R. D., Edler K. J., Bowen C. R., Mintova S., Burrows A. D.. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015;44(13):4290–4321. doi: 10.1039/C5CS00040H. [DOI] [PubMed] [Google Scholar]
- Wei Y.-S., Zhang M., Zou R., Xu Q.. Metal–organic framework-based catalysts with single metal sites. Chem. Rev. 2020;120(21):12089–12174. doi: 10.1021/acs.chemrev.9b00757. [DOI] [PubMed] [Google Scholar]
- Yang S., Karve V. V., Justin A., Kochetygov I., Espín J., Asgari M., Trukhina O., Sun D. T., Peng L., Queen W. L.. Enhancing MOF performance through the introduction of polymer guests. Coord. Chem. Rev. 2021;427:213525. doi: 10.1016/j.ccr.2020.213525. [DOI] [Google Scholar]
- Chen X.-W., Yuan H.-L., He L.-H., Chen J.-L., Liu S.-J., Wen H.-R., Zhou G., Wang J.-Y., Wong W.-Y.. A sublimable dinuclear cuprous complex showing selective luminescence vapochromism in the crystalline state. Inorg. Biochem. 2019;58(21):14478–14489. doi: 10.1021/acs.inorgchem.9b01972. [DOI] [PubMed] [Google Scholar]
- Siu B., Chowdhury A. R., Yan Z., Humphrey S. M., Hutter T.. Selective adsorption of volatile organic compounds in metal-organic frameworks (MOFs) Coord. Chem. Rev. 2023;485:215119. doi: 10.1016/j.ccr.2023.215119. [DOI] [Google Scholar]
- Anand A., Khanapurmath N., Kulkarni M. V., Guru Row T. N.. Biheterocyclic Coumarins: A Simple Yet Versatile Resource for Futuristic Design and Applications in Bio-molecular and Material Chemistry. Curr. Org. Chem. 2022;26(5):444–506. doi: 10.2174/1385272826666220301124149. [DOI] [Google Scholar]
- Shaaban M. R., Mayhoub A. S., Farag A. M.. Recent advances in the therapeutic applications of pyrazolines. Expert Opin. Ther. Pat. 2012;22(3):253–291. doi: 10.1517/13543776.2012.667403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Wang K.-Y., Lv X.-L., Yan T.-H., Zhou H.-C.. Hierarchically porous metal–organic frameworks: synthetic strategies and applications. Natl. Sci. Rev. 2020;7(11):1743–1758. doi: 10.1093/nsr/nwz170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei L., Asadi Z., Samolova E., Dusek M., Amirghofran Z.. Pyrazolate as bridging ligand in stabilization of self-assemble Cu (II) Schiff base complexes: Synthesis, structural investigations, DNA/protein (BSA) binding and growth inhibitory effects on the MCF7, CT-26, MDA-MB-231 cell lines. Inorg. Chim. Acta. 2020;509:119674. doi: 10.1016/j.ica.2020.119674. [DOI] [Google Scholar]
- Havrylyuk D., Roman O., Lesyk R.. Synthetic approaches, structure activity relationship and biological applications for pharmacologically attractive pyrazole/pyrazoline–thiazolidine-based hybrids. Eur. J. Med. Chem. 2016;113:145–166. doi: 10.1016/j.ejmech.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.-L., Xu Q.. Porous metal–organic frameworks as platforms for functional applications. Chem. Commun. 2011;47(12):3351–3370. doi: 10.1039/c0cc05419d. [DOI] [PubMed] [Google Scholar]
- Pettinari C., Marchetti F., Mosca N., Tosi G., Drozdov A.. Application of metal– organic frameworks. Polym. Int. 2017;66(6):731–744. doi: 10.1002/pi.5315. [DOI] [Google Scholar]
- Moharramnejad M., Ehsani A., Shahi M., Gharanli S., Saremi H., Malekshah R. E., Basmenj Z. S., Salmani S., Mohammadi M.. MOF as nanoscale drug delivery devices: Synthesis and recent progress in biomedical applications. J. Drug Delivery Sci. Technol. 2023;81:104285. doi: 10.1016/j.jddst.2023.104285. [DOI] [Google Scholar]
- Tsai J. L.-L., Zou T., Liu J., Chen T., Chan A. O.-Y., Yang C., Lok C.-N., Che C.-M.. Luminescent platinum (II) complexes with self-assembly and anti-cancer properties: hydrogel, pH dependent emission color and sustained-release properties under physiological conditions. Chem. Sci. 2015;6(7):3823–3830. doi: 10.1039/C4SC03635B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M. B., Caviglia M., Santini C., Del Gobbo J., Zeppa L., Del Bello F., Giorgioni G., Piergentili A., Quaglia W., Battocchio C.. et al. Copper-Based Complexes with Adamantane Ring-Conjugated bis (3, 5-Dimethyl-pyrazol-1-yl) acetate Ligand as Promising Agents for the Treatment of Glioblastoma. J. Med. Chem. 2024;67(11):9662–9685. doi: 10.1021/acs.jmedchem.4c00821. [DOI] [PubMed] [Google Scholar]
- Mansha M., Abbas N., Altaf F., Khan S. A., Khan I., Ali S.. Nanomaterial-based probes for iodide sensing: synthesis strategies, applications, challenges, and solutions. J. Mater. Chem. C. 2024;12:4919–4947. doi: 10.1039/d3tc04611g. [DOI] [Google Scholar]
- Gao L., Zhang A.. Copper-instigated modulatory cell mortality mechanisms and progress in oncological treatment investigations. Front. Immunol. 2023;14:1236063. doi: 10.3389/fimmu.2023.1236063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baren M. H., Ibrahim S. A., Al-Rooqi M. M., Ahmed S. A., El-Gamil M. M., Hekal H. A.. A new class of anticancer activity with computational studies for a novel bioactive aminophosphonates based on pyrazole moiety. Sci. Rep. 2023;13(1):14680. doi: 10.1038/s41598-023-40265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A. R., França R. N., Silva-Jardim I., Silva R. J. S., Lima-Santos J., Salay L. C., Santos R. L. S. R.. Self-assembling micellar system based on Pluronic and pyrazole-dithiocarbazate-conjugate stimulates production of nitric oxide from macrophages. Colloid Interface Sci. Commun. 2021;41:100378. doi: 10.1016/j.colcom.2021.100378. [DOI] [Google Scholar]
- Paitandi R. P., Mukhopadhyay S., Singh R. S., Sharma V., Mobin S. M., Pandey D. S.. Anticancer activity of iridium (III) complexes based on a pyrazole-appended quinoline-based BODIPY. Inorg. Biochem. 2017;56(20):12232–12247. doi: 10.1021/acs.inorgchem.7b01693. [DOI] [PubMed] [Google Scholar]
- Kumar S., Gupta S., Rani V., Sharma P.. Pyrazole containing anti-HIV agents: An update. Med. Chem. 2022;18(8):831–846. doi: 10.2174/1573406418666220106163846. [DOI] [PubMed] [Google Scholar]
- Yin J., Huang L., Wu L., Li J., James T. D., Lin W.. Small molecule based fluorescent chemosensors for imaging the microenvironment within specific cellular regions. Chem. Soc. Rev. 2021;50(21):12098–12150. doi: 10.1039/D1CS00645B. [DOI] [PubMed] [Google Scholar]
- Jia-nan L., Wenbo B., Jianlin S.. Chemical Design and Synthesis of Functionalized Probes for Imaging and Treating Tumor Hypoxia. Chem. Rev. 2017;117:6160–6224. doi: 10.1021/acs.chemrev.6b00525. [DOI] [PubMed] [Google Scholar]
- Britel A., Tomagra G., Aprà P., Varzi V., Sturari S., Amine N.-H., Olivero P., Picollo F.. 3D printing in microfluidics: experimental optimization of droplet size and generation time through flow focusing, phase, and geometry variation. RSC Adv. 2024;14(11):7770–7778. doi: 10.1039/D4RA00752B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni M. B., Velmurugan K., Nirmal J., Goel S.. Development of dexamethasone loaded nanomicelles using a 3D printed microfluidic device for ocular drug delivery applications. Sens. Actuators, A. 2023;357:114385. doi: 10.1016/j.sna.2023.114385. [DOI] [Google Scholar]
- Chandrasekharan S. P., Dhami A., Kumar S., Mohanan K.. Recent advances in pyrazole synthesis employing diazo compounds and synthetic analogues. Org. Biomol. Chem. 2022;20(45):8787–8817. doi: 10.1039/D2OB01918C. [DOI] [PubMed] [Google Scholar]
- Zhan S.-Z., Feng T., Lu W., Razali M. R., Li D.. Substituent Influence on Structural and Luminescent Diversities of Cu3 (pyrazolate) 3-Cu n I n Coordination Supramolecular Isomers. Cryst. Growth Des. 2018;18(12):7663–7673. doi: 10.1021/acs.cgd.8b01476. [DOI] [Google Scholar]
- Fan H., Tang J., Yang H., Cheng G.. Azo Pyrazole Carboxylic Derivatives for Potential Energetic Materials. Cryst. Growth Des. 2024;24(15):6292–6299. doi: 10.1021/acs.cgd.4c00568. [DOI] [Google Scholar]
- Lobo-Checa J., Rodríguez S. J., Hernández-López L., Herrer L., Passeggi M. C., Cea P., Serrano J. L.. Discarding metal incorporation in pyrazole macrocycles and the role of the substrate on single-layer assemblies. Nanoscale. 2024;16(14):7093–7101. doi: 10.1039/D3NR03773H. [DOI] [PubMed] [Google Scholar]
- Ahmad M. G., Chanda K.. Ionic liquid coordinated metal-catalyzed organic transformations: A comprehensive review. Coord. Chem. Rev. 2022;472:214769. doi: 10.1016/j.ccr.2022.214769. [DOI] [Google Scholar]
- Mithra U., Sarveswari S.. A review on pyrazole moieties as organic chemosensors in the detection of cations and anions. Inorg. Chim. Acta. 2024;569:122118. doi: 10.1016/j.ica.2024.122118. [DOI] [Google Scholar]
- Zhang S.-H., Zhang S.-Y., Li J.-R., Huang Z.-Q., Yang J., Yue K.-F., Wang Y.-Y.. Rational synthesis of an ultra-stable Zn (ii) coordination polymer based on a new tripodal pyrazole ligand for the highly sensitive and selective detection of Fe 3+ and Cr 2 O 7 2– in aqueous media. Dalton Trans. 2020;49(32):11201–11208. doi: 10.1039/D0DT01996H. [DOI] [PubMed] [Google Scholar]
- Shi Y., Zou Y., Khan M. S., Zhang M., Yan J., Zheng X., Wang W., Xie Z.. Metal–organic framework-derived photoelectrochemical sensors: Structural design and biosensing technology. J. Mater. Chem. C. 2023;11(11):3692–3709. doi: 10.1039/D2TC05338A. [DOI] [Google Scholar]
- Chen C., Zhou L., Xie B., Wang Y., Ren L., Chen X., Cen B., Lv H., Wang H.. Novel fast-acting pyrazole/pyridine-functionalized N-heterocyclic carbene silver complexes assembled with nanoparticles show enhanced safety and efficacy as anticancer therapeutics. Dalton Trans. 2020;49(8):2505–2516. doi: 10.1039/C9DT04751D. [DOI] [PubMed] [Google Scholar]
- Xu M., Wang T., Zhang C., Kuang B.-l., Xie Z.-m., Yi Z.-x., Lu Z.-j., Li Y., Zhu S., Zhang J.-G.. Preparation of highly energetic coordination compounds with rich oxidants and lower sensitivity based on methyl groups. Inorg. Chem. 2023;62(51):21371–21378. doi: 10.1021/acs.inorgchem.3c03463. [DOI] [PubMed] [Google Scholar]
- Gruhne M. S., Lommel M., Wurzenberger M. H., Klapötke T. M., Stierstorfer J. r.. Investigation of ethylenedinitramine as a versatile building block in energetic salts, cocrystals, and coordination compounds. Inorg. Chem. 2021;60(7):4816–4828. doi: 10.1021/acs.inorgchem.0c03752. [DOI] [PubMed] [Google Scholar]
- Bu R., Xiong Y., Wei X., Li H., Zhang C.. Hydrogen bonding in CHON-containing energetic crystals: a review. Cryst. Growth Des. 2019;19(10):5981–5997. doi: 10.1021/acs.cgd.9b00853. [DOI] [Google Scholar]
- Ren J., Zhao D.. Recent advances in reticular chemistry for clean energy, global warming, and water shortage solutions. Adv. Funct. Mater. 2024;34(43):2307778. doi: 10.1002/adfm.202307778. [DOI] [Google Scholar]