Abstract
ATP-sensitive K+ (KATP) channels in the heart are normally closed by high intracellular ATP, but are activated during ischemia to promote cellular survival. These channels are heteromultimers composed of Kir6.2 subunit, an inwardly rectifying K+ channel core, and SUR2A, a regulatory subunit implicated in ligand-dependent regulation of channel gating. Here, we have shown that the muscle form (M-LDH), but not heart form (H-LDH), of lactate dehydrogenase is directly physically associated with the sarcolemmal KATP channel by interacting with the Kir6.2 subunit via its N-terminus and with the SUR2A subunit via its C-terminus. The species of LDH bound to the channel regulated the channel activity despite millimolar concentration of intracellular ATP. The presence of M-LDH in the channel protein complex was required for opening of KATP channels during ischemia and ischemia-resistant cellular phenotype. We conclude that M-LDH is an integral part of the sarcolemmal KATP channel protein complex in vivo, where, by virtue of its catalytic activity, it couples the metabolic status of the cell with the KATP channels activity that is essential for cell protection against ischemia.
Keywords: heart/KATP channels/Kir6.2/lactate dehydrogenase/SUR2A
Introduction
Sarcolemmal ATP-sensitive K+ (KATP) channels belong to the group of intracellular ATP sensors, coupling the metabolic status of the cell with the membrane excitability (Noma, 1983). These channels are selectively permeable to K+ ions and are closed by high intracellular ATP levels (Terzic et al., 1995; Ashcroft and Gribble, 1998). It has been suggested that the opening of sarcolemmal KATP channels promotes survival of cardiomyocytes during metabolic stress (Jovanović and Jovanović, 2001a; Light et al., 2001). Ischemia opens sarcolemmal KATP channels before a significant fall in the level of intracellular ATP occurs, suggesting that the intracellular level of ATP is not the only factor that regulates the channel activity (Knopp et al., 1999). Indeed, it is believed that the activity of KATP channels is controlled by a complex interaction of many intracellular factors and signalling pathways. In addition to ATP, the activity of these channels may be regulated by other nucleotides, intracellular pH, lactate, cytoskeleton, protein kinase C (PKC), phosphatidylinositol-4,5-bisphosphate and by the operative condition of the channel itself (reviewed by Tanemoto et al., 2001).
Structurally, the cardiac subtype of KATP channels are heteromultimers composed of the Kir6.2 subunit, an inwardly rectifying K+ channel core primarily responsible for K+ permeance, and SUR2A, a regulatory subunit implicated in ligand-dependent regulation of channel gating (Inagaki et al., 1996). More recently, evidence has been provided that adenylate kinase and creatine kinase, two main regulators of intracellular ATP levels in the heart, are also parts of the cardiac KATP channel protein complex (Carrasco et al., 2001; Crawford et al., 2002). The whole channel protein complex seems to interact with the actin cytoskeleton (Korchev et al., 2000). However, the complexity of channel regulation would further suggest the sarcolemmal KATP channel to be composed of more proteins than those currently recognized.
In this regard, we have employed several strategies to determine whether the sarcolemmal KATP channel protein complex is composed of more proteins than Kir6.2, SUR2A, adenylate kinase and creatine kinase. We have found that the muscle form of lactate dehydrogenase (M-LDH) is also an integral part of the sarcolemmal KATP channel, where it regulates channel activity. Moreover, the presence of M-LDH in the channel complex seems to be required for early opening of KATP channels during ischemia and cellular resistance against metabolic stress.
Results
Co-immunoprecipitation of the cardiac membrane fraction
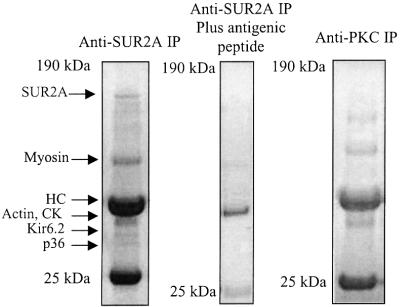
To immunoprecipitate proteins associated with the cardiac KATP channels, we used anti-SUR2A antibody (see Ranki et al., 2001; Crawford et al., 2002). Immunoprecipitation revealed putative accessory polypeptides migrated at ∼36 (p36), ∼38 (p38), ∼46 (p46), ∼48 (p48), ∼98 (p98) and ∼150 kDa (p150) (Figure 1). The profile of immunoprecipitated peptides was different when anti-PKC antibody [nPKCε(n-17); Santa Cruz Biotechnology, Santa Cruz, CA] was used instead of anti-SUR2A antibody (Figure 1), and anti-SUR2A immunoprecipitation was blocked with an excess of the corresponding antigenic peptide (Figure 1).
Fig. 1. Immunoprecipitation of cardiac membrane fraction. Coomassie Blue stain of immunoprecipitate pellets (IP) obtained from cardiac membrane fraction precipitated with either anti-SUR2A (without and with antigenic peptide) or anti-PKC antibody.
LDH is a part of the sarcolemmal KATP channel complex
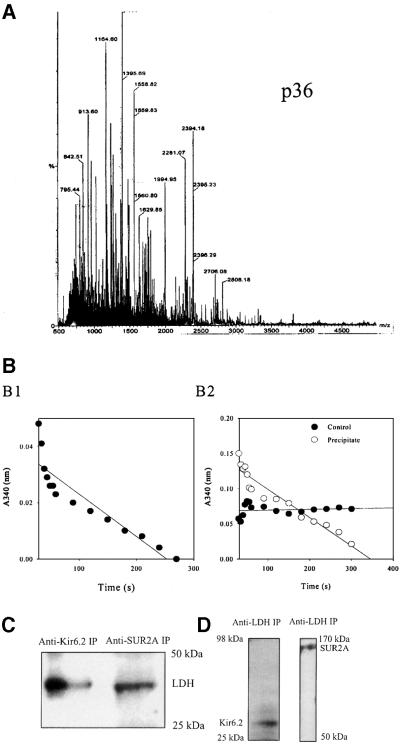
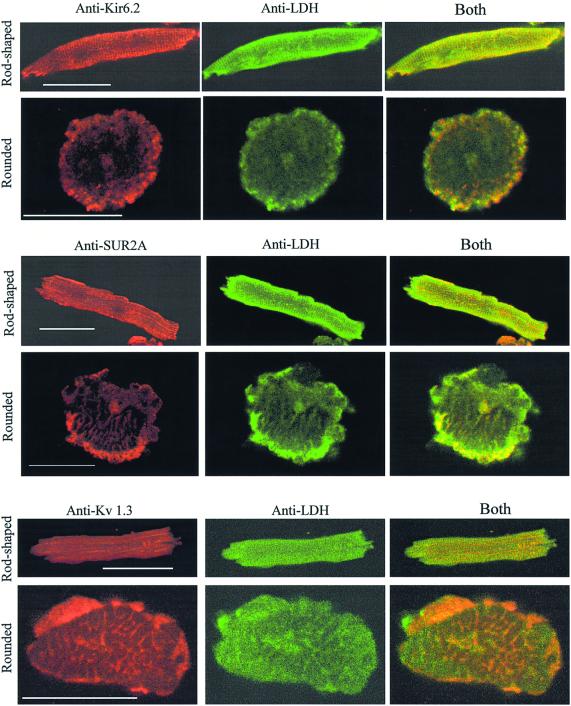
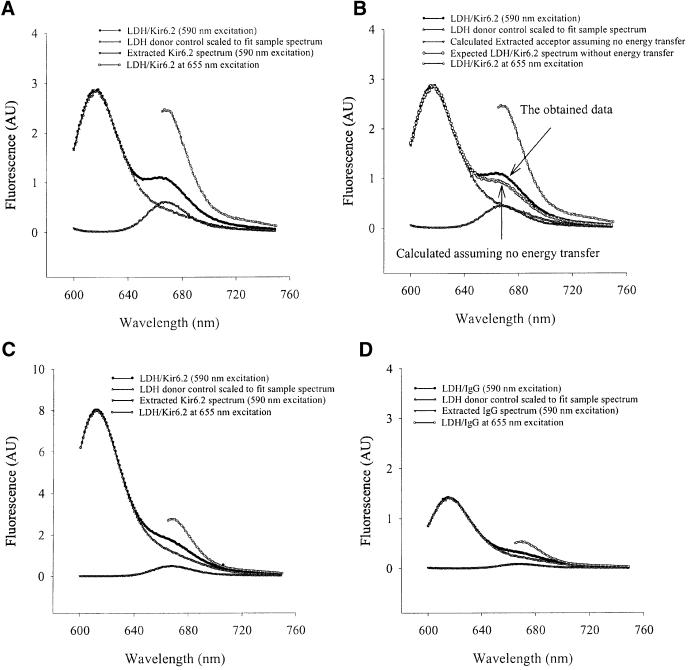
Previously, p38, p48 and p150 were identified as Kir6.2, creatine kinase and SUR2A respectively (Crawford et al., 2002). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) analysis identified p98, p46 and p36 as β-myosin, α-actin (data not shown) and LDH (Figure 2A), respectively. LDH activity was present in anti-SUR2A immunoprecipitation pellets, whereas no activity was observed when the experimental protocol was applied without the antibody (Figure 2B). Western blotting with anti-LDH antibody (Abcam, Cambridge, UK; this antibody targets all isoforms of LDH) of both anti-Kir6.2 and anti-SUR2A immunoprecipitates revealed signals for LDH (Figure 2C). Conversely, western blotting with anti-Kir6.2 and anti-SUR2A antibodies detected Kir6.2 and SUR2A subunits in anti-LDH immunoprecipitate of cardiac membrane fraction (Figure 2D). Furthermore, double labelling immunofluorescence showed that both Kir6.2 and SUR2A co-localize with a substantial portion of LDH in cardiomyocytes (Figure 3). This was observed in both rod-shaped and rounded cells. In contrast, when cardiomyocytes were labelled with antibody against Kv 1.3, much less spatial overlap was noted (Figure 3). To provide even more evidence for physical association between LDH and sarcolemmal KATP channels we performed fluorescence resonance energy transfer (FRET) analysis using antibodies labelled with Alexa 594 (Molecular Probes, Eugene, OR) (anti-LDH antibody, donor) and Alexa 647 (anti-Kir6.2 antibody, acceptor) applied on cardiac membrane fraction. The emission spectrum for this sample is shown in Figure 4A, as are the donor and acceptor components. The same data are presented in Figure 4B, except that the magnitude of the extracted acceptor spectrum is calculated from the (ratio)A observed for a sample with only the anti-Kir6.2 antibody. Comparison of the two panels shows that there is significant energy transfer between the fluorophores attached to the two antibodies. The (ratio)A value is 0.246, one-third greater than the value of 0.179 determined for the acceptor-only control. SDS (1%), a disrupter of protein–protein complexes, abolished the observed energy transfer [Figure 4C; (ratio)A decreased from 0.246 in the absence to 0.188 in the presence of SDS, which was not significantly different from the acceptor-only control (0.192)]. When the same type of experiment was carried out with anti-IgG antibody (Santa Cruz) in place of anti-Kir6.2 antibody, no energy transfer was observed [Figure 4D; (ratio)A was 0.177 with acceptor alone and 0.165 with acceptor–donor]. In contrast, the energy transfer between anti-Kir6.2 (donor) and anti-SUR2A (acceptor) antibodies was detected [(ratio)A was 0.197 with acceptor alone and 0.213 with acceptor–donor].
Fig. 2. LDH co-immunoprecipitates with sarcolemmal cardiac KATP channel protein complex and vice versa. (A) MALDI-TOF mass spectrum of tryptic mass fingerprint obtained from an ∼36 kDa migrating protein (identified as LDH). (B) LDH assay with purified LDH (B1) and anti-SUR2A immunoprecipitate of cardiac membrane fraction (B2). (C) Western blotting of anti-Kir6.2 and anti-SUR2A immunoprecipitate of cardiac membrane fraction with anti-LDH antibody. (D) Western blotting of anti-LDH immunoprecipitate (IP) with anti-Kir6.2 and anti-SUR2A antibodies.
Fig. 3. Sarcolemmal KATP channel subunits, Kir6.2 and SUR2A, co-localize with LDH in cardiomyocytes. Original images of rod-shaped and rounded cardiomyocytes stained with either anti-Kir6.2 or anti-SUR2A or anti-Kv 1.3 and anti-LDH antibodies labelled with rhodamine (red channel) or fluorescein (green channel) as indicated in the figure. Yellow colour is suggestive of co-localization. Scale bar, 45 µm.
Fig. 4. Energy transfer between LDH and Kir6.2. (A) The emission spectra of LDH/Kir6.2, LDH donor control and Kir6.2 acceptor in PBS. (B) The same as in (A) except that the magnitude of the extracted acceptor spectrum is calculated from the (ratio)A value from a sample with only the anti-Kir6.2 antibody. The difference between the sample data and calculated LDH/Kir6.2 spectrum (the sum of donor and acceptor spectra) shows energy transfer. (C) The sample from (A) after the treatment with SDS (1%). (D) The emission spectra of LDH/IgG, LDH donor control and IgG acceptor in PBS. AU, arbitrary units.
M-LDH, but not the heart form of LDH, is the isoform directly physically associated with the cardiac KATP channel subunits
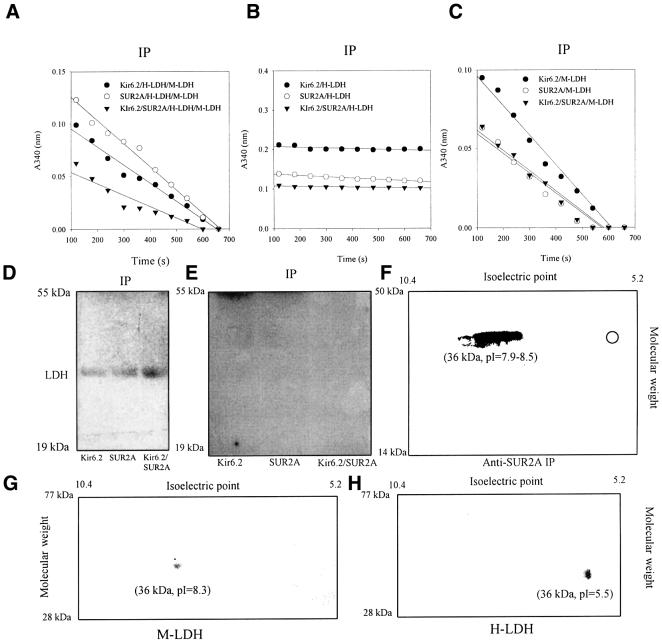
LDH is a tetramer composed of either M (muscle) and/or H (heart) subunits, which may be combined to form five LDH isozymes (Van Hall, 2000). We have co-expressed Kir6.2 and/or SUR2A with M- and H-LDH subunits, and examined which of the isozymes associate with the channel subunits. When both H- and M-LDH subunits were co-expressed with Kir6.2, SUR2A and Kir6.2/SUR2A in A549 cells, cells natively devoid of KATP channels, LDH activity was found in anti-Kir6.2 [antibody published in Crawford et al. (2002), and it was used for immunoprecipitation cells expressing Kir6.2 alone] and anti-SUR2A (used for immunoprecipitation cells expressing Kir6.2 alone and Kir6.2/SUR2A) immunoprecipitates of cells expressing subunits both alone and combined (Figure 5A). However, when either H- or M-LDH was co-expressed with channel subunits, LDH activity was detected in immunoprecipitate from cells transfected with M-LDH, but not in immunoprecipitate from cells transfected with H-LDH (Figure 5B and C). Western blotting with the anti-LDH antibody of anti-Kir6.2 and anti-SUR2A immunoprecipitates identified the presence of LDH in cells transfected with Kir6.2, SUR2A, Kir6.2/SUR2A and M-LDH, but not in those transfected with H-LDH (Figure 5D and E). To determine whether M-LDH is the only LDH subunit that associates with the KATP channel subunits in vivo, we subjected anti-SUR2A immunoprecipitate from cardiac membrane fraction to two-dimensional (2D) gel analysis using an anti-LDH antibody. 2D gel analysis revealed a signal corresponding to M-LDH without any visible tracings of H subunits in immunoprecipitate (Figure 5F).
Fig. 5. The muscle, but not heart, form of LDH directly associates with Kir6.2 and SUR2A subunits. (A–C) LDH assay with anti-SUR2A or anti-Kir6.2 immunoprecipitate of untransfected A549 cells or cells transfected with Kir6.2 and SUR2A (alone and in combination) plus M-LDH plus H-LDH (A), H-LDH (B) or M-LDH (C). Anti-Kir6.2 antibody was used for immunoprecipitation of Kir6.2 cells, while for all other groups anti-SUR2A antibody was applied. (D) Western blotting of anti-Kir6.2 and anti-SUR2A immunoprecipitate under conditions in (C). (E) Western blotting of anti-Kir6.2 and anti-SUR2A immunoprecipitate under conditions in (B). (F–H) Western blotting 2D gel analysis with anti-LDH antibody of anti-SUR2A immunoprecipitate (IP) of cardiac membrane fraction or purified M- and H-LDH [Sigma; (G) and (H)]. Hollow circle in 2D gel in (F) indicates the expected position for H-LDH signal.
M-LDH associates with Kir6.2 and SUR2A subunits via its N- and C-termini, respectively
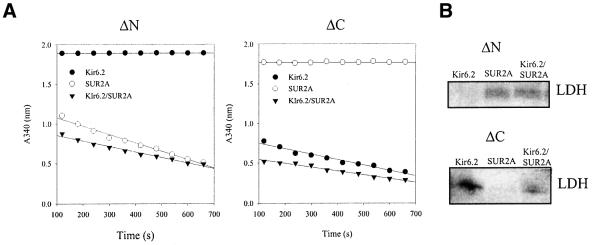
The results obtained with transfected A549 cells suggested that M-LDH is associated with the KATP channel protein complex interacting with both Kir6.2 and SUR2A subunits. Based on known LDH structure and primary sequence differences between M- and H-LDH subunits (see Discussion), we hypothesized that M-LDH interacts with Kir6.2 and SUR2A peptides via terminal ends. To test this hypothesis, we made N- and C-terminus-truncated forms of M-LDH (see Materials and methods). When an N-terminus-truncated form of M-LDH was co-expressed with the channel subunits in A549 cells, the activity was lost only in immunoprecipitate from cells express ing Kir6.2 subunit alone (Figure 6A). Conversely, a C-terminus truncated form of M-LDH was absent only in immunoprecipitate of cells expressing the SUR2A subunit alone (Figure 6A). The same pattern of LDH presence was observed when western blotting with anti-LDH antibody was applied instead of measuring LDH activity (Figure 6B).
Fig. 6. M-LDH associates with Kir6.2 and SUR2A subunits via N- and C-terminus, respectively. (A) LDH assay with anti-SUR2A or anti-Kir6.2 immunoprecipitate of A549 cells transfected with Kir6.2 and SUR2A (alone and in combination) plus N(ΔN)- or C(ΔC)-termini deletion mutants of M-LDH. (B) Western blotting with anti-LDH antibody in conditions under (A).
LDH substrates regulate sarcolemmal KATP channels activity
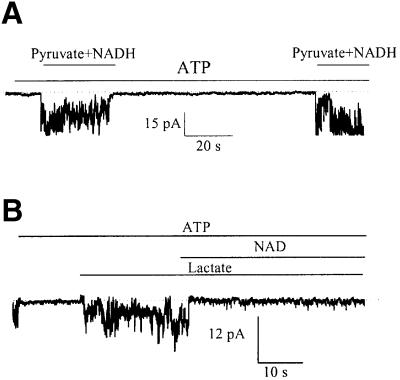
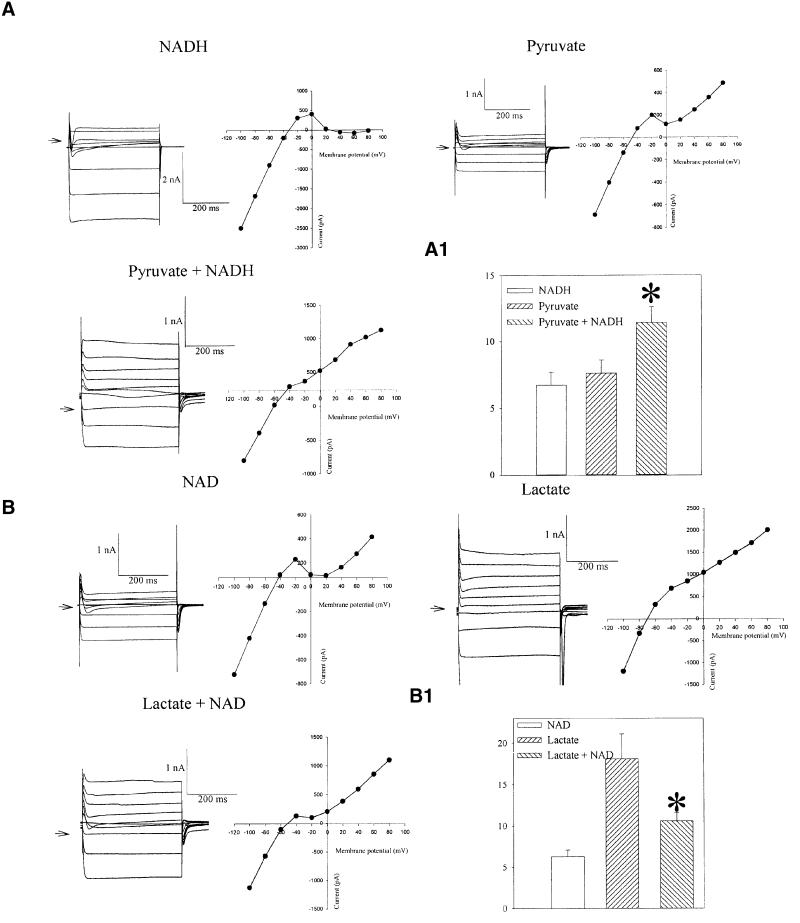
The defining attribute of KATP channels is their inhibition by intracellular ATP (Noma, 1983). Upon excision of a membrane patch from guinea pig ventricular cardiomyocyte, opening of KATP channels was inhibited by 1 mM of ATP. Addition of pyruvate plus NADH (20 mM each) on the intracellular face of the excised membrane patch opened KATP channels despite the presence of ATP (1 mM; Figure 7A). On the other hand, the opening of KATP channels with 20 mM of lactate, in the presence of ATP (1 mM), was inhibited by NAD (20 mM; Figure 7B). Furthermore, we measured membrane currents from a guinea pig ventricular myocyte in a whole-cell configuration. When NADH (20 mM) or pyruvate (20 mM) was present in the patch pipette solution alone, the steady-state current–voltage relationship (Figure 8A) was in an N shape due to the strong inward rectification of Ik1 channels and absence of active KATP channels (Terzic et al., 1995). However, when pyruvate (20 mM) was combined with NADH (20 mM), the outward K+ current became greater and inward rectification weaker (Figure 8A). The current density was 6.6 ± 0.8 and 7.5 ± 0.8 pA/pF in the presence of NADH and pyruvate alone, respectively, and 11.4 ± 1.2 pA/pF in the presence of NADH plus pyruvate (P = 0.01, n = 6–9; Figure 8A1). Lactate (20 mM)-induced outward K+ current significantly increased at potentials more positive than –70 mV, and inward rectification of the current–voltage relationship was much weaker (Figure 8B), which is a typical finding when measuring K+ current flows through KATP channels (Terzic et al., 1995). When NAD (20 mM) was also present in the pipette solution with lactate (20 mM), the outward current was decreased and inward rectification was stronger (Figure 8B). The current density was 6.8 ± 1.0 and 18.1 ± 3.2 pA/pF in the presence of NAD and lactate alone, respectively, and 10.2 ± 1.8 pA/pF in the presence of NAD plus lactate (P = 0.019, n = 6–9; Figure 8B1).
Fig. 7. LDH substrates regulate the opening of KATP channels in excised membrane patches. Recording of KATP channel activity in membrane patch treated with ATP (1 mM) alone, ATP (1 mM) plus pyruvate (20 mM) plus NADH (20 mM), again with ATP (1 mM) alone and again with ATP (1 mM) plus pyruvate (20 mM) plus NADH (20 mM) (A) or treated with ATP (1 mM), ATP (1 mM) plus lactate (20 mM) and ATP (1 mM) plus lactate (20 mM) plus NAD (20 mM) (B). Holding potential –60 mV. Dotted lines correspond to the zero current levels. Both types of experiment were repeated four times with essentially similar results.
Fig. 8. LDH substrates regulate KATP channel-mediated membrane current. (A) Membrane currents and corresponding current–voltage relationships in cells filled with pipette solution containing NADH (20 mM) or pyruvate (20 mM) or both. Arrow points to the zero current level. (A1) Current densities at 80 mV for conditions in (A). Vertical bars represent mean ± SEM (n = 6–9). *P <0.01. (B) Membrane currents and corresponding current–voltage relationships in cells filled with pipette solution containing NAD (20 mM) or lactate (20 mM) or both. Arrow points to the zero current level. In all experiments depicted cells were dialysed for ∼5 min. (B1) Current densities at 80 mV under conditions in (B). Vertical bars represent mean ± SEM (n = 6–9). *P <0.05.
Kir6.2/SUR2A/M-LDH confer resistance to ischemia in A549 cells
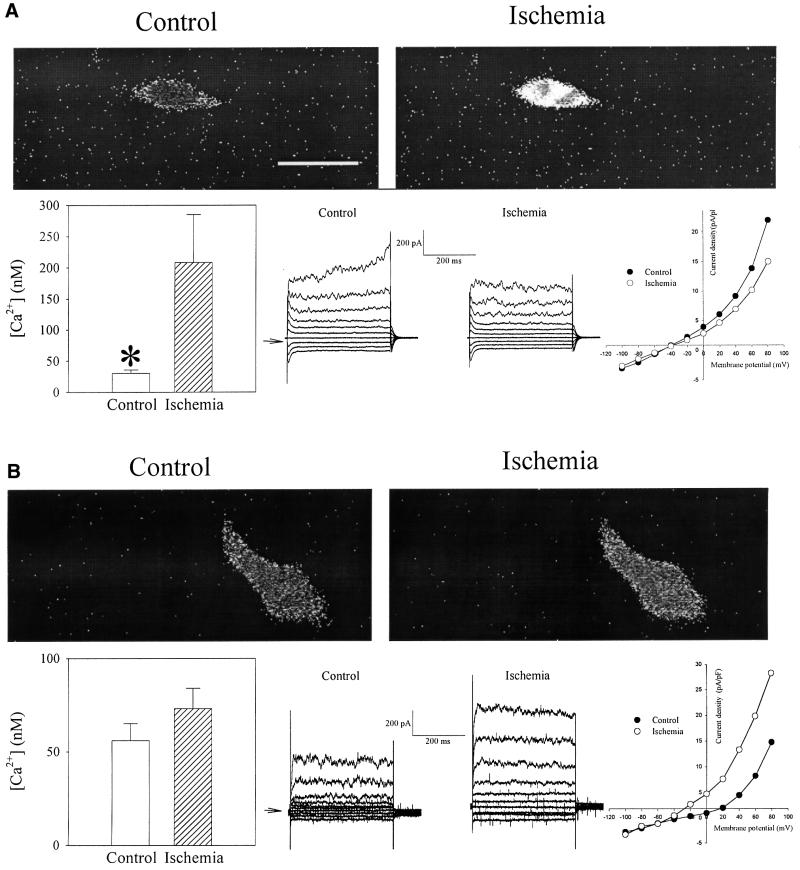
At rest, A549 cells, which are natively devoid of KATP channels, have a low cytosolic Ca2+ concentration (30.2 ± 5.7 nM, n = 16; Figure 9A). Exposure to ischemia produced rapid and significant Ca2+ loading (208.5 ± 71.1 nM, n = 16, P = 0.03; Figure 9), which was associated with statistically non-significant decrease in whole-cell K+ current as measured by perforated patch–clamp electrophysiology (current density at +80 mV was 21.9 ± 1.9 pA/pF in control and 15.0 ± 2.6 pA/pF in ischemia, n = 6, P = 0.13; Figure 9). Transfection of Kir6.2/SUR2A/M-LDH almost completely prevented ischemia-induced Ca2+ loading (resting Ca2+ was 55.6 ± 9.0 nM prior to and 73.3 ± 10.8 nM following ischemia, P = 0.22, n = 9; Figure 9). In transfected cells, ischemia also induced significant increase in whole-cell K+ membrane current as measured by perforated patch–clamp electrophysiology [in this mode intracellular milieu remains largely undisturbed (Jovanović et al., 2001); current density at +80 mV was 14.7 ± 2.0 pA/pF under control conditions and 28.2 ± 3.1 pA/pF following ischemia, P = 0.007, n = 5, Figure 9].
Fig. 9. Kir6.2/SUR2A/M-LDH confers resistance against ischemia in A549 cells. Original frames and whole-cell recordings under control and ischemic conditions with corresponding bar (intracellular Ca2+ levels, frames) and scatter-line graphs (whole-cell recordings) of (A) untransfected Fura-2 loaded A549 cells and (B) transfected with Kir6.2/SUR2A/M-LDH. Horizontal bar represents 20 µm. Vertical bars represent mean ± SEM, whereas points represent the mean (n = 6–16). *P <0.05.
The opening of recombinant KATP channels as well as intact LDH activity is required for cell protection afforded by Kir6.2/SUR2A/M-LDH
The resistance of Kir6.2/SUR2A/M-LDH A549 phenotype to ischemia was significantly reduced when glybenclamide (10 µM), a KATP channel blocker, was present during the experiments (intracellular Ca2+ was 31.2 ± 5.3 nM prior to and 96.4 ± 31.6 nM following ischemia, P = 0.008, n = 9; Figure 10). In addition, glybenclamide (10 µM) abolished the ischemia-induced increase in whole-cell membrane K+ current (current density at +80 mV was 23.1 ± 2.9 pA/pF in control and 24.2 ± 3.2 pA/pF in ischemia, n = 5, P = 0.63; Figure 10). When Kir6.2/SUR2A were co-transfected with the inactive, mutated form of M-LDH (His193 was mutated into glycine, which greatly reduced the catalytic activity; Clarke et al., 1986) instead of wild-type enzyme, the protection against ischemia was abolished (intracellular Ca2+ was 37.0 ± 6.5 nM prior to and 193.8 ± 62.7 nM following ischemia, P = 0.026, n = 6; Figure 10), as well as ischemia-induced increase in whole-cell membrane K+ current (current density at +80 mV was 20.1 ± 3.1 pA/pF in control and 19.3 ± 2.9 pA/pF in ischemia, n = 5, P = 0.32; Figure 10).
Fig. 10. The opening of KATP channels and intact activity of M-LDH is required for Kir6.2/SUR2A/M-LDH-mediated resistance. Original frames and whole-cell recordings under control and ischemic conditions with corresponding bar (intracellular Ca2+ levels, frames) and scatter-line graphs (whole-cell recordings) of A549 cells transfected with Kir6.2/SUR2A/M-LDH in the presence of 10 µM glybenclamide (A) and transfected with Kir6.2/SUR2A plus 193gly-M-LDH (B) (single-amino acid mutated form of M-LDH with greatly reduced LDH activity; glybenclamide was absent). Scale bar, 20 µm. Vertical bars represent mean ± SEM; points represent mean (n = 5–9). *P <0.05.
Discussion
It has recently been suggested that sarcolemmal KATP channel complexes are composed of Kir6.2 and SUR2A subunits as well as two enzymes, regulators of ATP levels, adenylate kinase and creatine kinase (Inagaki et al., 1996; Carrasco et al., 2001; Crawford et al., 2002). In the present study, Coomassie Blue staining of the anti-SUR2A immunoprecipitate revealed polypeptides that were previously identified as Kir6.2, SUR2A and creatine kinase (Crawford et al., 2002). The presence of adenylate kinase in immunoprecipitate was not visualized, which is plausible since the molecular weight of adenylate kinase would overlap with the primary antibody heavy chain under the conditions applied (Carrasco et al., 2001). MALDI-TOF analysis identified p46 and p98 as α-actin and β-myosin, which is in accord with the cardiac KATP channel protein complex being physically associated with the cytoskeleton (Korchev et al., 2000).
Several lines of evidence, including MALDI-TOF, LDH assay and western blotting, suggested that a previously unidentified 36 kDa protein found in the immunoprecipitate was LDH. Since immunoprecipitations could have non-specific contaminants (Harlow and Lane, 1999), we used extreme care to classify LDH as a protein associated with the sarcolemmal KATP channel complex. The following results suggest that the LDH presence in immunoprecipitate is due to the physical association with KATP channel subunits: (i) the appearance of p36 was specific for the anti-SUR2A antibody and was blocked by the antigenic peptide, suggesting that interaction between the anti-SUR2A antibody and membrane fraction is required for the presence of LDH in the immunoprecipitate; (ii) there was no cross-reactivity between the anti-SUR2A antibody and purified LDH itself, which excludes the possibility that LDH was directly precipitated with the anti-SUR2A antibody; (iii) LDH was detected in immunoprecipitates obtained with structurally unrelated antibodies against two members of the protein complex, Kir6.2 and SUR2A subunits; and (iv) not only was LDH found in both anti-SUR2A and anti-Kir6.2 immunoprecipitates but, vice versa, both SUR2A and Kir6.2 subunits were identified in anti-LDH immunoprecipitate of cardiac membrane fraction. Immunofluorescence and FRET analysis were also employed to test the conclusions drawn with immunoprecipitation data. Immunofluorescence demonstrated co-localization of Kir6.2 or SUR2A subunits with LDH in both rod-shaped and rounded cardiomyocytes, i.e. in both healthy and dead cells, respectively (see S.Jovanović et al., 1998). In healthy cardiomyocytes, it could be argued that LDH might appear to be co-localized with channel subunits due to ubiquitous intracellular presence of the enzyme. However, since dead cardiac cells release a major fraction of intracellular LDH (Altschuld et al., 1994), this argument does not stand, suggesting that the observed co-localization is specific for LDH and the channel subunits. This conclusion is supported by finding that much less spatial overlap was observed between Kv 1.3, another K+ channel subunit expressed in the heart (England et al., 1995), and LDH. In addition, FRET analysis detected energy transfer between Kir 6.2 and LDH in cardiac membrane fraction suggesting that these two proteins are in close proximity (see Clegg, 1992). As the Ro for this donor–acceptor pair is 8.4 nm (see the manufacturer’s web site: www.probes.com), the average distance between the fluorophores must be <17 nm, as neglible FRET is observed at distances greater than twice the Ro. Bearing in mind that this distance includes a sum of anti-Kir6.2 antibody, Kir6.2 protein, LDH protein and anti-LDH antibody lengths, it is very unlikely that in pure membrane fraction vigorously cleaned of cytosolic elements Kir6.2 and LDH protein could be so close without being physically associated. This conclusion is supported by the finding that treatment which disrupts protein complexes abolished the energy transfer between Kir6.2 and LDH, while no energy transfer was observed between LDH and IgG antibodies. Taken all together, it reasonable to conclude that LDH is an integral part of the sarcolemmal KATP channel protein complex in vivo.
LDH is a tetramer composed of M (muscle) and/or H (heart) subunits, which may be combined to form five LDH isozymes. In the heart, all LDH isozymes are present; LDH1 (H4) is the predominant form, whereas LDH5 (M4) is present in trace amounts (Van Hall, 2000). When H- and M-LDH subunits in combination and alone were co-expressed with Kir6.2, SUR2A and Kir6.2/SUR2A in A549 cells, the pattern of LDH presence in anti-Kir6.2 and anti-SUR2A immunoprecipitate suggested that M-LDH, but not H-LDH, is the isoform that associates with the channel subunits. Since M- and H-LDH subunits are indistinguishable by size but have different isoelectric points (Li, 1990; Read et al., 2001; this study), we applied isoelectric focusing and 2D gel analysis to determine whether M-LDH is the only LDH subunit that associates with the KATP channel subunits in vivo. This technique revealed a signal corresponding to M-LDH without any visible tracings of H subunits in immunoprecipitate, confirming the data obtained with the heterologues expression system and suggesting that M-LDH is indeed a part of the cardiac KATP channel complex in vivo. LDH is composed of 331 amino acids, with the active site localized approximately in the middle of the protein (Li, 1990; Van Hall, 2000). In order to be associated with both Kir6.2 and SUR2A subunits and still retain its activity, interacting parts of M-LDH with channel subunits probably have to be localized on its terminal sides. In addition, differences in amino acid sequence between M-LDH (associates with the channel) and H-LDH (does not associate with the channel) are found primarily in the first 22 and the last 38 residues (Li, 1990), again suggesting that termini are involved in interaction between M-LDH and Kir6.2/SUR2A. To test this hypothesis, we made N- and C-terminus-truncated forms of M-LDH. When an N-terminus-truncated form of M-LDH was co-expressed with the channel subunits in A549 cells, LDH was lost only in immunoprecipitate from cells expressing Kir6.2 subunit alone, while a C-terminus-truncated form of M-LDH was absent only in immunoprecipitate of cells expressing SUR2A subunit alone. These results suggest that M-LDH associates with the Kir6.2 subunit via its N-terminus and with the SUR2A via its C-terminus. Although LDH is normally a tetramer (Li, 1990; Takatani et al., 2001), the catalytic function of LDH does not depend upon interaction between LDH subunits, and single subunits retain catalytic activity (King and Weber, 1986). This means that the complex of Kir6.2, SUR2A and M-LDH subunits may be functional even if LDH subunits do not interact with each other. Also, since a single KATP channel is composed of four Kir6.2 and four SUR2A subunits (Shyng and Nichols, 1997), it is possible that four M-LDH subunits associated with the channel subunits form a three-dimensional structure that has a spatial relationship similar to those in the traditional enzyme tetramer without necessarily being physically associated.
It is generally accepted that M-LDH primarily controls the formation of lactate (Van Hall, 2000). Lactate is reported to open sarcolemmal KATP channels in cells despite the presence of millimolar concentrations of ATP (Han et al., 1993). Since LDH catalyses NADH + pyruvate ↔ lactate + NAD reaction, this enzyme has the potential both to open (by lactate production) and to close (by lactate removal) KATP channels. The results obtained, at both single channel and whole-cell levels, demonstrate that LDH substrates activate or inhibit KATP channels opening in a manner consistent with the hypothesis that LDH is a part of the sarcolemmal KATP channels. The regulation of KATP channel activity was observed despite the continuous intracellular presence of millimolar concentrations of ATP, suggesting that M-LDH, by virtue of its activity, could open KATP channels at physiological concentrations of ATP. In the heart, KATP channels are opened during ischemia and act in a cardioprotective manner (Knopp et al., 1999; Light et al., 2001). Although Kir6.2/SUR2A reconstitutes basic properties of sarcolemmal KATP channels in cell lines natively devoid of these channels (Inagaki et al., 1996), recombinant KATP channels remain mostly closed throughout the metabolic challenge (Jovanović and Jovanović, 2001c). Yet, when recombinant channels are pharmacologically open host cells are protected against metabolic stress (A.Jovanović et al., 1998; Jovanović et al., 1999). Inability of Kir6.2/SUR2A proteins to create cellular phenotype resistant to metabolic stress may be due to incomplete reconstitution of the channel and impaired metabolic sensing. We tested the hypothesis that M-LDH, as a part of the sarcolemmal KATP channel complex, may be important for co-ordinated channel opening and cellular protection. A549 cells responded to ischemia with intracellular Ca2+ loading, which is a major indicator of the cell injury (A.Jovanović et al., 1998), showing that these cells are vulnerable to such an insult. When Kir6.2/SUR2A were co-expressed with M-LDH in A549 cells, ischemia-induced Ca2+ increase was almost abolished and this was associated with significant activation of the outward K+ current. Both increase in K+ current and resistance towards ischemia was inhibited by glybenclamide, an antagonist of KATP channels, suggesting that the opening of recombinant KATP channels was responsible for the observed cellular resistance to ischemia. When Kir6.2/SUR2A was co-transfected with the inactive, mutated form of M-LDH, protection against ischemia was abolished, as well as ischemia-induced increase in whole-cell membrane K+ current, implying that the presence of M-LDH with unimpaired activity is required in the cardiac KATP channel protein complex for early opening of KATP channels, clamping the membrane potential and maintaining the intracellular Ca2+ homeostasis.
M-LDH as a part of the cardiac KATP channel could explain how the channel open during ischemia to protect the cells despite the presence of millimolar levels of ATP. Under anaerobic conditions, pyruvate, NAD and H+ cannot be metabolized further and accumulate (see Kantor et al., 2001), leading to the M-LDH-catalysed lactate production within microenvironment surrounding the channel. Lactate opens the channel despite high levels of ATP (Han et al., 1993; this study) and protects the cell against Ca2+ overload and death.
In conclusion, we have demonstrated that M-LDH is an integral part of the sarcolemmal KATP channel protein complex in vivo, where it couples the metabolic status of the cell with the KATP channels activity which, in turn, is essential for cell protection against ischemia.
Materials and methods
Cardiac cells isolation
Ventricular cardiomyocytes were dissociated from guinea pig hearts as described previously (Ranki et al., 2001). In brief, hearts were retrogradely perfused (at 37°C) with medium 199, followed by Ca2+-EGTA-buffered low-Ca2+ medium (pCa = 7), and finally low-Ca2+ medium containing pronase E (8 mg/100 ml), proteinase K (1.7 mg/100 ml), bovine serum albumin (BSA; 0.1 g/100 ml, fraction V) and 200 µM CaCl2. Ventricles were cut into fragments in the low-Ca2+ medium enriched with 200 µM CaCl2. Single cells were isolated by stirring the tissue (at 37°C) in a solution containing pronase E and proteinase K supplemented with collagenase (5 mg/10 ml).
A549 cells, genes, truncations, site-directed mutagenesis and transfection
A549 cells (ATCC), which are natively devoid of KATP channels, were cultured in a tissue flask (at 5% CO2) containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum and 2 mM glutamine. Cells were transfected using 8–24 µl lipofectAMINE (Gibco), with 2–6 µg of total plasmid DNA [full-length Kir6.2, SUR2A, M-LDH, H-LDH, ΔN-LDH, ΔC-LDH, gly193-M-LDH cDNA and green fluorescent protein (GFP), subcloned into the mammalian expression vector pcDNA3.1+]. Kir6.2 was a gift from Dr S.Seino (Chiba University, Chiba, Japan) and SUR2A was kindly provided by Dr Y.Kurachi (Osaka University, Osaka, Japan). H- and M-LDH were cloned from mouse heart RNA. First strand cDNA was synthesized from 1 µg total RNA using 200 U of MML-V reverse transcriptase (Promega) and an anchor-oligo(dT) [5′-GTCATGGCATGGGATCCTG(T)15; total volume, 20 µl], according to the manufacturer’s instructions. The total PCR volume was 25 µl (50 µl for H-LDH), including 3 µl of the cDNA, 25 pmol (20 pmol for H-LDH) of each primer and 2.5 U PromegaTag (Promega). PCR was performed in a thermal cycler Model Phenix (Helena Biosciences) or a thermal cycler Mastercycler Gradient (Eppendorf) under the following conditions: the PCR was run with a hot start for 5 min at 95°C (initial melting), followed by 40 cycles of 0.5 min at 94°C, 0.5 min at 55°C and 1.5 min at 72°C (final extension). The primers had the following sequences. H-LDH-specific primers: sense, 5′-GACAAGATGGCAACCCTTAAGGA-3′; antisense, 5′-AGTGCAAACATCAAACAAGCCTGG-3′. M-LDH-specific primers: sense, 5′-ATGGCAACCCTCAAGGACCAG-3′; antisense, 5′-GCACAGCTGTGGGGAGTG-3′. The product obtained (full-length cDNA), which contain translatable parts of H-LDH or M-LDH sequence, was isolated from a 1% agarose gel using Qiagen gel extraction kit, sequenced, ligated into the pcDNA3.1+ mammalian expression vector and used for transfection. Truncation of the first 19 N-terminal amino acids of M-LDH was achieved using nested PCR of M-LDH cDNA and the following primers: sense: 5′-GAGCAGGCTCCCCAGAACAAGATTACAGTTATG-3′; antisense, 5′-GCAGAGCTGTGGGGAGTG-3′. The total PCR volume was 50 µl, including 3 µl of the cDNA, 20 pmol of each primer and 2.5 U PromegaTag (Promega). PCR was performed in a thermal cycler Mastercycler Gradient (Eppendorf) under the following conditions: the PCR was run with a hot start for 5 min at 94°C (initial melt), followed by for 35 cycles of 10 s at 94°C, 1.5 min at 56°C and 1.5 min at 71°C (final extension). Truncation of 48 C-terminal amino acids was achieved by PCR using site-directed mutagenesis to introduce a 3′ stop codon (TAG). The QuikChange site-directed mutagenesis kit (Stratagene) was used according to the manufacturer’s instructions. Mutagenic primers were sense: 5′-TGATTAAGGGTCTCTATGGAATCAATGTAGATGTCTTCCTCAGTGTCCCATGTATCCTG-3′; antisense, 5′-CAGGATACATGGGACACT GAGGAAGACATCTACATTGATTCCATAGAGACCCTTAATCA-3′. PCR conditions were the same as for N-terminus truncation.
For site-directed mutagenesis of His193, the same Stratagene kit as for C-terminus truncation was used. Mutagenic primers were sense: 5′-GCTGGGTCCTGGGAGAAGGTGGCGACTCC-3′; antisense, 5′-GGA GTCGCCACCTTCTCCCAGGACCCAGC-3′. The total PCR volume was 50 µl, including 25 ng of non-mutated M-LDH inserted into the pcDNA3.1+, 125 ng of each primer, 1 µl of dNTP and 2.5 U DNA polymerase. PCR was performed in a thermal cycler Model Phenix (Helena Biosciences) under the following conditions: the PCR was run with a hot start for 0.5 min at 95°C (initial melting), followed by 16 cycles of 0.5 min at 95°C, 1 min at 55°C and 14 min at 68°C. The parental DNA was destroyed according to the manufacturer’s instructions, and the mutated plasmids were further transformed, sequenced and used for experiments. All PCR products in experiments described above were verified by DNA sequencing.
Immunoprecipitation, Coomassie Blue staining and western blot analysis
Sheep anti-peptide antibodies were raised against synthetic peptides conjugated to a carrier protein, keyhole limpet hemocyanin, and used for immunoprecipitation and western blotting (Crawford et al., 2002). Cardiac membrane fraction was obtained as described previously (Crawford et al., 2002). In brief, guinea pig ventricular tissue was homogenized in buffer I [Tris 10 mM, NaH2PO4 20 mM, EDTA 1 mM, phenylmethylsulfonyl fluoride (PMSF) 0.1 mM, pepstatin 10 µg/ml, leupeptin 10 µg/ml pH 7.8] and incubated for 20 min (at 4°C). The osmolarity was restored with KCl, NaCl and sucrose and the obtained mixture was centrifugated at 500 g. The supernatant was diluted in buffer II (imidazole 30 mM, KCl 120 mM, NaCl 30 mM, NaH2PO4 20 mM, sucrose 250 mM, pepstatin 10 µg/ml, leupeptin 10 µg/ml pH 6.8) and centrifugated at 7000 g, the pellet removed and the supernatant was centrifugated at 30 000 g. The obtained pellet contains membrane fraction. A549 cells or membrane fraction were snap-frozen and ground to a powder under liquid nitrogen. The powder was resuspended in 10 ml of buffer (20 mM HEPES, 150 mM NaCl, 1% Triton X-100 pH 7.5) and homogenized. Protein concentration was determined using the method of Bradford. Forty micrograms of the epitope-specific anti-SUR2A or anti-Kir6.2 antibody was pre-bound to protein G–Sepharose beads and used to immunoprecipitate from 60 µg of protein extract. The pellets of this precipitation were run on two SDS polyacrylamide gels, one for Coomassie Blue stain and one for western blot analysis as described in Crawford et al. (2002). In a separate series of experiments, immunoprecipitation was performed with the anti-LDH antibody (Abcam) using the same protocol as described above. For the 2D gel analysis, immunoprecipitation was performed using 20 µg of SUR2A specific anti-peptide antibody to precipitate from 20 µg guinea pig cardiac membrane fraction. The resultant precipitate was run on a Novex (Invitrogen) isoelectric focusing gel encompassing a pH range of 3–10, followed by excision of the lane and further protein separation on the second dimension according to molecular weight. Control samples were purified H-LDH and H-LDH from Sigma run using the same protocol (Crawford et al., 2002).
Immunofluorescence and laser confocal microscopy
Cardiomyocytes were fixed with 4% paraformaldehyde for 20 min. After washing with phosphate-buffered saline (PBS), cells were permeabilized with 0.1% Triton X-100 in PBS for 15 min, washed and blocked with 5% BSA in PBS for 30 min. Cells were incubated overnight at 4°C with primary antibodies (rabbit anti-Kir6.2, rabbit anti-SUR2A, goat anti-LDH and rabbit anti-Kv1.3) in PBS and incubated in the dark with the secondary antibodies [anti-sheep fluorescein (Diagnostic Scotland, Edinburgh, UK) and anti-rabbit rhodamine (Santa Cruz)] at 1:500 dilution for 1 h. Samples were washed with PBS, mounted on slides using 50% glycerol containing 1% n-propylgallate and sealed with nail polisher (Marks & Spencer, Dundee, UK). Slides were examined by laser confocal microscopy (LSM-510; Zeiss, Gottingem, Germany). Images were acquired and analysed using Zeiss Image Examiner Software.
FRET
Cardiac membrane fraction was obtained as described in the immunoprecipitation section. Anti-Kir6.2, anti-LDH, anti-SUR2A or anti-IgG antibodies were labelled with Alexa 594 (donor) or Alexa 647 (acceptor) where appropriate, according to the manufacturers instruction (Molecular Probes). Cardiac membrane fraction was blocked with 1% BSA, incubated with labelled antibodies for 12 h at 4°C, pelleted, and the pellets washed and resuspended with PBS. Fluorescence spectra were recorded at 20°C using an SLM-Aminco 8100 fluorimeter, in PBS buffer except where otherwise specified. The excitation and emission band passes were 4 and 8 nm, respectively. Scattering of incident light was minimized by setting excitation and emission polarizers crossed at 90°. FRET was measured using the acceptor normalization method (Clegg, 1992), in which an extracted acceptor spectrum FA(ν1,ν′) (excitation at ν′ = 590 nm for Alexa 594, emission at ν1) is normalized to a second spectrum [F(ν1,ν”)] from the same sample excited at a wavelength (ν” = 655 nm for Alexa 647) at which only the acceptor is excited, with emission at ν2. This gives the acceptor ratio (ratio)A = FA(ν1,ν′)/F(ν,ν”). FRET is considered to be observed if a sample has a (ratio)A value in excess of that recorded for a control sample having only the acceptor fluorophore. Fluorescence intensities were quantified by integrating emission spectra over the interval from 665 to 700 nm. The extracted acceptor spectrum was obtained by using regression to fit the spectrum of a donor-only control sample to that part of the sample spectrum which has only donor fluorescence (600–620 nm). Subtracting the scaled donor spectrum from the sample spectrum was used to calculate the extracted acceptor spectrum.
MALDI-TOF analysis
The band of interest was excised from the gel, and the protein trapped within the gel was reduced and alkylated and then digested with trypsin (12.5 ng/µl) overnight. The peptides were extracted, first, by the addition of 25 mM ammonium bicarbonate, followed after a 10 min incubation by an equal volume of acetonitrile, and then by two extractions with 5% formic acid and acetonitrile. The pooled extracts were concentrated to near dryness in vacuo and redissolved in 10% formic acid. Half of the sample was desalted and concentrated using a micro C18 column (0.2 µl ZapTip; Millipore) according to the manufacturer’s instructions. The peptides were eluted directly from the tip onto the target in 1.5 µl α-cyano-4-hydroxycinnamic acid (10 mg/ml) in 75% acetonitrile/25% formic acid (10%). Spectra were obtained on a Micromass TofSpec 2E instrument (Micromass, Manchester, UK) equiped with a 337 nm laser and operated in reflectron mode. Three hundred shots were combined and data were calibrated against a mixture of known peptides. Peptide masses were searched against the Swiss-Prot/TREMBL databases using the ExPASy PeptIdent program and against the NCBI database using the MS-Fit search program (USCF Mass Spectrometry Facility). The searches were limited to rodent proteins and included an appropriate mass range limit (Shevchenko et al., 1996).
LDH assay
LDH activity was determined in a reagent solution containg 0.2 M Tris–HCl (100 ml), 6.6 M NADH (1 ml), 30 mM sodium pyruvate (10 ml); pH was adjusted to 7.3 at 25°C. LDH activity was measured using a spectrophotometer (Unicam UV2 Spec) set at wavelength 340 nm on pellets (20 µl) dissolved in PBS (total volume was 100 µl) and put in reagent solution (Tris–HCl 1.4 ml, NADH 50 µl, sodium pyruvate 50 µl). Following 5 min incubation of the reagent solution in the spectrophotometer (to achieve temperature equilibration) pellets were added and the absorbance at 340 nm was measured every min until steady-state is reached (steady-state was defined as steady reading of absorbance in five consecutive measurements). The reaction velocity was determined by a decrease in absorbance at 340 nm, resulting from the oxidation of NADH indicative of LDH activity.
Patch–clamp electrophysiology
To monitor on-line behaviour of single channel molecules, the gigaohm seal patch–clamp technique was applied in the inside-out configuration (Jovanović and Jovanović, 2001b). Cells were superfused with (in mM): KCl 140, MgCl2 1, EGTA 5, HEPES–KOH 5 (pH 7.4). Fire-polished pipettes, coated with Sylgard (resistance 5–7 MΩ), were filled with (in mM): KCl 140, CaCl2 1, MgCl2 1, HEPES–KOH 5 (pH 7.3). Recordings were made at room temperature (22°C), using a patch–clamp amplifier (Axopatch-200B). Single-channel activity was monitored on-line and stored on a PC. Data were reproduced, low-pass filtered at 1 KHz (–3 dB) and sampled at 100 µs rate for further analysis with the pClamp8 software. For whole-cell electrophysiology applied on cardiomyocytes cells were superfused with Tyrode solution (in mM: NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, glucose 5.5, HEPES–NaOH 5.5; pH 7.4). Pipettes (resistance 3–5 MΩ), were filled with (in mM): KCl 140, MgCl2 1, ATP 3, HEPES–KOH 5 (pH 7.3). The whole-cell K+ current in untransfected or transfected A549 cells (selection of the transfected cells was based on the GFP fluorescence) was measured using whole-cell perforated patch– clamp technique with essentially the same pipette solution as above, except ATP was omitted and amphotericin B (240 µg/ml; Sigma) was added (see Jovanović et al., 2001). Also, during ischemia, Tyrode solution was modified as described below (see section Ischemia). For all cells monitored, the membrane potential was normally held at –40 mV and the currents evoked by a series of 400 ms depolarizing and hyperpolarizing current steps (–100 to +80 mV in 20 mV steps) recorded directly to hard disk using an Axopatch-200B amplifier, Digidata-1321 interface and pClamp8 software (Axon Instruments, Inc., Forster City, CA). The capacitance compensation was adjusted to null the additional whole-cell capacitative current. The slow capacitance component measured by this procedure was used as an approximation of the cell surface area and allowed normalization of current amplitude (i.e. current density). Currents were low pass filtered at 2 kHz and sampled at 100-µs intervals. When the effect of LDH substrates on whole-cell K+ current was assessed either NADH (20 mM), pyruvate (20 mM), NADH plus pyruvate (20 mM each), NAD (20 mM), lactate (20 mM) or NAD plus pyruvate (20 mM each) was added into the pipette solution (Crawford et al., 2002).
Digital epifluorescent microscopy
A549 cells (transfected cells were selected based on GFP fluorescence) were superfused with Tyrode solution, and loaded with the esterified form of the Ca2+-sensitive fluorescent probe Fura-2 (Fura-2AM, dissolved in dimethyl sulfoxide plus pluronic acid; Molecular Probes). Cells were imaged using a digital epifluorescence imaging system coupled to an inverted microscope (Image Solutions, Standish, UK). A mercury lamp served as a source of light to excite Fura-2AM at 340 and 380 nm. Fluorescence emitted at 520 nm was captured, after crossing dichroic mirrors, by an intensified charge coupled device camera, and digitized using imaging software. An estimate of the cytosolic Ca2+ concentration, as a function of Fura-2 fluorescence, was calculated according to the equation: [Ca2+] = (R – Rmin/Rmax – R)Kdβ, where R is the fluorescence ratio recorded from the cell, Rmin and Rmax the minimal and maximal fluorescence ratio, Kd the dissociation constant of the dye (236 nM), and β the ratio of minimum to maximum fluorescence at 380 nm (Ranki et al., 2001).
Ischemia
Untransfected and transfected A549 cells were exposed to ischemia combining hypoxia with modified Tyrode solution (ischemic solution). The ischemic solution (solution otherwise similar to Tyrode solution, contained slightly increased K+ concentration, 16 mM, pH was reduced to 6.5, and glucose was omitted) was continuously bubbled with 100% argon and the exchange of O2 between solution in the chamber and air was prevented by a nitrogen jet. The PO2 under these conditions was ∼20 mmHg. The duration of ischemic perfusion was 10 min (Ranki et al., 2001).
Statistical analysis
Data are presented as mean ± SEM, with n representing the number of patched/imaged cells or examined hearts. Mean values between two groups were compared by the paired or unpaired Student’s t-test or rank tests where appropriate. Mean values between more then two groups were compared by the one-way or one-way rank ANOVA. All statistical tests were carried out using using the SigmaStat program (Jandel Scientific). P <0.05 was considered statistically significant.
Acknowledgments
Acknowledgements
We thank S.Seino (Chiba University) for the Kir6.2 gene, Y.Kurachi (Osaka University) for the SUR2A gene. We are grateful to C.H.Booting (University of St Andrews) and D.M.J.Lilley (University of Dundee) for MALDI-TOF and FRET analysis, respectively. This research was supported by grants from the Anonymous Trust, BBSRC, British Heart Foundation, MRC, National Heart Research Fund, TENOVUS and the Wellcome Trust.
References
- Altschuld R.A., Jung,D.W., Phillips,R.M., Narayan,P., Castillo,L.C., Whitaker,T.E., Hensley,J., Hohl,C.M. and Brierley,G.P. (1994) Evidence against norepinephrine-stimulated efflux of mitochondrial Mg2+ from intact cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol., 266, H1103–H1111. [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M. and Gribble,F.M. (1998) Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci., 21, 288–294. [DOI] [PubMed] [Google Scholar]
- Carrasco A.J. et al. (2001) Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc. Natl Acad. Sci. USA, 98, 7623–7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A.R., Wigley,D.B., Chia,W.N., Barstow,D., Atkinson,T. and Holbrook,J.J. (1986) Site-directed mutagenesis reveals role of mobile arginine residue in lactate dehydrogenase catalysis. Nature, 324, 699–702. [DOI] [PubMed] [Google Scholar]
- Clegg R.M. (1992) Fluorescence resonance energy transfer and nucleic acids. Methods Enzymol., 211, 353–388. [DOI] [PubMed] [Google Scholar]
- Crawford R.M., Ranki,H.J., Botting,C.H., Budas,G.R. and Jovanović,A. (2002) Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J., 16, 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S.K., Uebele,V.N., Kodali,J., Bennett,P.B. and Tamkun,M.M. (1995) A novel K+ channel β-subunit (hKv β 1.3) is produced via alternative mRNA splicing. J. Biol. Chem., 270, 28531–28534. [DOI] [PubMed] [Google Scholar]
- Han J., Kim,E.Y. and Earm,Y.E. (1993) ATP-sensitive potassium channels are modulated by intracellular lactate in rabbit ventricular myocytes. Pflügers Arch., 425, 546–548. [DOI] [PubMed] [Google Scholar]
- Harlow E. and Lane,D. (1999) Immunoprecipitation. In Harlow,E. and Lane,D. (eds), Using Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Woodbury, NY, pp. 221–266.
- Inagaki N., Gonoi,T., Clement,J.P., Wang,C.Z., Aguilar-Bryan,L., Bryan,J. and Seino,S. (1996) A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron, 16, 1011–1017. [DOI] [PubMed] [Google Scholar]
- Jovanović S. and Jovanović,A. (2001a) Pinacidil prevents membrane depolarisation and intracellular Ca2+ loading in single cardiomyocytes exposed to severe metabolic stress. Int. J. Mol. Med., 7, 639–643. [DOI] [PubMed] [Google Scholar]
- Jovanović S. and Jovanović,A. (2001b) Diadenosine tetraphosphate-gating of cardiac KATP channels requires intact actin cytoskeleton. Naunyn Schmiedebergs Arch. Pharmacol., 364, 276–280. [DOI] [PubMed] [Google Scholar]
- Jovanović S. and Jovanović,A. (2001c) Delivery of genes encoding KATP channel subunits in conjuction with pinacidil prevents membrane depolarisation in cells exposed to chemical hypoxia-reoxygenation. Biochem. Biophys. Res. Commun., 282, 1098–1102. [DOI] [PubMed] [Google Scholar]
- Jovanović S., Jovanović,A., Shen,W.K. and Terzic,A. (1998) Protective action of 17β-estradiol in cardiac cells: implications for hyperkalemic cardioplegia. Ann. Thorac. Surg., 66, 1658–1661. [DOI] [PubMed] [Google Scholar]
- Jovanović A., Jovanović,S., Lorenz,E. and Terzic,A. (1998) Recombinant cardiac ATP-sensitive K+ channel subunits confer resistance towards chemical hypoxia-reoxygenation injury. Circulation, 98, 1548–1555. [DOI] [PubMed] [Google Scholar]
- Jovanović N., Jovanović,S., Jovanović,A. and Terzic,A. (1999) Gene delivery of Kir6.2/SUR2A in conjunction with pinacidil handles intracellular Ca2+ homeostasis under metabolic stress. FASEB J., 13, 923–929. [DOI] [PubMed] [Google Scholar]
- Jovanović S., Land,S.C., Olver,R.E. and Wilson S.M. (2001) Hypoxic activation of an amiloride-sensitive cation conductance in alveolar epithelial cells. Biochem. Biophys. Res. Commun., 286, 622–627. [DOI] [PubMed] [Google Scholar]
- Kantor P.F., Lopaschuk,G.D. and Opie,L.H. (2001) Myocardial energy metabolism. In Sperelakis,N., Kurachi,Y., Terzic,A. and Cohen,M.V. (eds), Heart Physiology and Pathophysiology. Academic Press, San Diego, CA, pp. 543–569.
- King L. and Weber,G. (1986) Conformational drift of dissociated lactate dehydrogenases. Biochemistry, 25, 3632–3637. [DOI] [PubMed] [Google Scholar]
- Knopp A., Thierfelder,S., Koopmann,R., Biskup,C., Bohle,T. and Benndorf,K. (1999) Anoxia generates rapid and massive opening of KATP channels in ventricular cardiac myocytes. Cardiovasc. Res., 41, 629–640. [DOI] [PubMed] [Google Scholar]
- Korchev Y.E., Negulyaev,Y.A., Edwards,C.R., Vodyanoy,I. and Lab,M.J. (2000) Functional localization of single active ion channels on the surface of a living cell. Nat. Cell Biol., 2, 616–619. [DOI] [PubMed] [Google Scholar]
- Li S.S. (1990) Human and mouse lactate dehydrogenase genes A (muscle), B (heart) and C (testis): protein structure, genomic organization, regulation of expression and molecular evolution. Prog. Clin. Biol. Res., 344, 75–99. [PubMed] [Google Scholar]
- Light P.E., Kanji,H.D., Manning Fox,J.E. and French,R.J. (2001) Distinct myoprotective roles of cardiac sarcolemmal and mitochondrial KATP channels during metabolic inhibition and recovery. FASEB J., 15, 2586–2594. [DOI] [PubMed] [Google Scholar]
- Noma A. (1983) ATP-regulated K+ channels in cardiac muscle. Nature, 305, 147–148. [DOI] [PubMed] [Google Scholar]
- Ranki H.J., Budas,G.R., Crawford,R.M. and Jovanović,A. (2001) Gender-specific difference in cardiac ATP-sensitive K+ channels. J. Am. Coll. Cardiol., 38, 906–915. [DOI] [PubMed] [Google Scholar]
- Read J.A., Winter,V.J., Eszes,C.M., Sessions,R.B. and Brady,R.L. (2001) Structural basis for altered activity of M- and H-isozyme forms of human lactate dehydrogenase. Proteins, 43, 175–185. [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Wilm,M., Vorm,O. and Mann,M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem., 68, 850–858. [DOI] [PubMed] [Google Scholar]
- Shyng S. and Nichols,C.G. (1997) Octameric stoichiometry of the KATP channel complex. J. Gen. Physiol., 110, 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatani T., Takaoka,N., Tatsumi,M., Kawamoto,H., Okuno,Y., Morita,K., Masutani,K., Murakawa,K. and Okamoto,Y. (2001) A novel missense mutation in human lactate dehydrogenase B-subunit gene. Mol. Gen. Metab., 73, 344–348. [DOI] [PubMed] [Google Scholar]
- Tanemoto M., Fujita,A. and Kurachi,Y. (2001) Inwardly-rectifying K+ channels in the heart. In Sperelakis,N., Kurachi,Y., Terzic,A. and Cohen,M.V. (eds), Heart Physiology and Pathophysiology. Academic Press, San Diego, CA, pp. 281–308.
- Terzic A., Jahangir,A. and Kurachi,Y. (1995) Cardiac ATP-sensitive K+ channels regulation by intracellular nucleotides and K+ channel opening drugs. Am. J. Physiol. Heart Circ. Physiol., 269, C525–C545. [DOI] [PubMed] [Google Scholar]
- Van Hall G. (2000) Lactate as a fuel for mitochondrial respiration. Acta Physiol. Scand., 168, 643–656. [DOI] [PubMed] [Google Scholar]