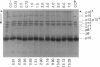
Abstract
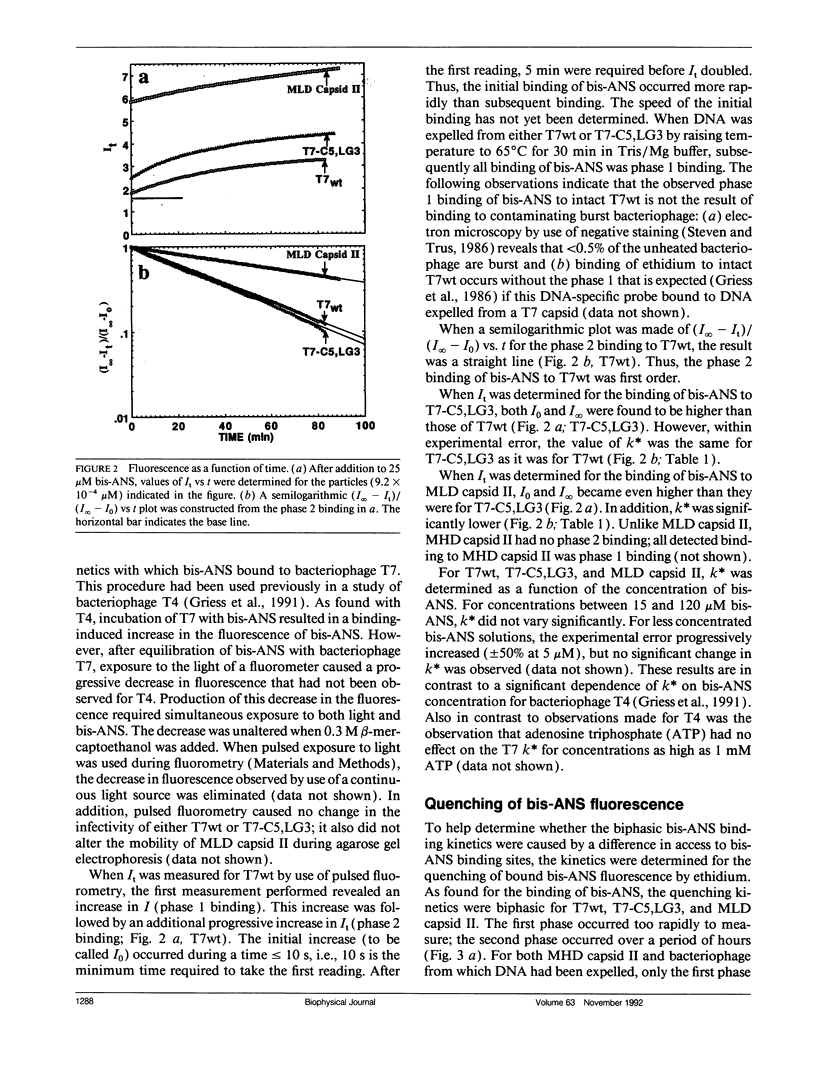
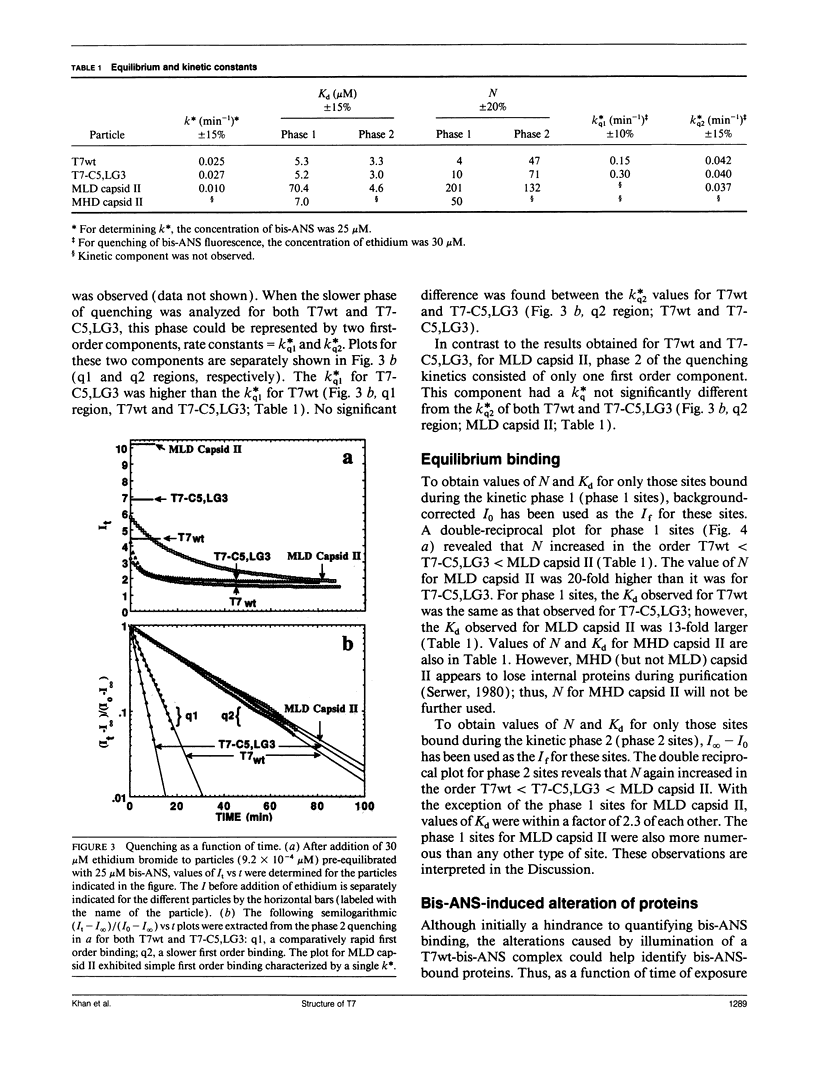
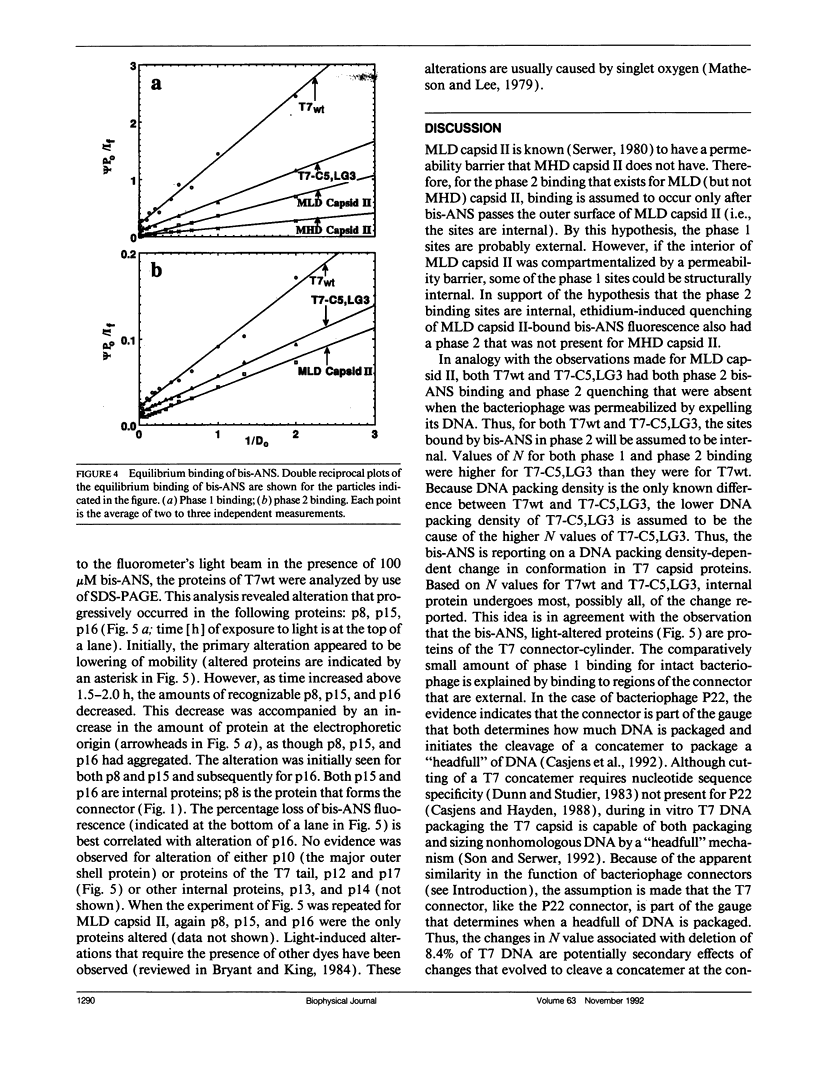
To detect changes in capsid structure that occur when a preassembled bacteriophage T7 capsid both packages and cleaves to mature-size longer (concatameric) DNA, the kinetics and thermodynamics are determined here for the binding of the protein-specific probe, 1,1'-bi(4-anilino)naphthalene-5,5'-di-sulfonic acid (bis-ANS), to bacteriophage T7, a T7 DNA deletion (8.4%) mutant, and a DNA-free T7 capsid (metrizamide low density capsid II) known to be a DNA packaging intermediate that has a permeability barrier not present in a related capsid (metrizamide high density capsid II). Initially, some binding to either bacteriophage or metrizamide low density capsid II occurs too rapidly to quantify (phase 1, duration < 10 s). Subsequent binding (phase 2) occurs with first-order kinetics. Only the phase 1 binding occurs for metrizamide high density capsid II. These observations, together with both the kinetics of the quenching by ethidium of bound bis-ANS fluorescence and the nature of bis-ANS-induced protein alterations, are explained by the hypothesis that the phase 2 binding occurs at internal sites. The number of these internal sites increases as the density of the packaged DNA decreases. The accompanying change in structure is potentially the signal for initiating cleavage of a concatemer. Evidence for the following was also obtained: (a) a previously undetected packaging-associated change in the conformation of the major protein of the outer capsid shell and (b) partitioning by a permeability barrier of the interior of the T7 capsid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft F. C., Freifelder D. Molecular weights of coliphages and coliphage DNA. I. Measurement of the molecular weight of bacteriophage T7 by high-speed equilibrium centrifugation. J Mol Biol. 1970 Dec 28;54(3):537–546. doi: 10.1016/0022-2836(70)90124-5. [DOI] [PubMed] [Google Scholar]
- Bazinet C., King J. The DNA translocating vertex of dsDNA bacteriophage. Annu Rev Microbiol. 1985;39:109–129. doi: 10.1146/annurev.mi.39.100185.000545. [DOI] [PubMed] [Google Scholar]
- Black L. W. DNA packaging in dsDNA bacteriophages. Annu Rev Microbiol. 1989;43:267–292. doi: 10.1146/annurev.mi.43.100189.001411. [DOI] [PubMed] [Google Scholar]
- Bryant J. L., Jr, King J. DNA injection proteins are targets of acridine-sensitized photoinactivation of bacteriophage P22. J Mol Biol. 1984 Dec 25;180(4):837–863. doi: 10.1016/0022-2836(84)90260-2. [DOI] [PubMed] [Google Scholar]
- Carazo J. M., Donate L. E., Herranz L., Secilla J. P., Carrascosa J. L. Three-dimensional reconstruction of the connector of bacteriophage phi 29 at 1.8 nm resolution. J Mol Biol. 1986 Dec 20;192(4):853–867. doi: 10.1016/0022-2836(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Casjens S., Hayden M. Analysis in vivo of the bacteriophage P22 headful nuclease. J Mol Biol. 1988 Feb 5;199(3):467–474. doi: 10.1016/0022-2836(88)90618-3. [DOI] [PubMed] [Google Scholar]
- Casjens S., Wyckoff E., Hayden M., Sampson L., Eppler K., Randall S., Moreno E. T., Serwer P. Bacteriophage P22 portal protein is part of the gauge that regulates packing density of intravirion DNA. J Mol Biol. 1992 Apr 20;224(4):1055–1074. doi: 10.1016/0022-2836(92)90469-z. [DOI] [PubMed] [Google Scholar]
- Chung Y. B., Nardone C., Hinkle D. C. Bacteriophage T7 DNA packaging. III. A "hairpin" end formed on T7 concatemers may be an intermediate in the processing reaction. J Mol Biol. 1990 Dec 20;216(4):939–948. doi: 10.1016/S0022-2836(99)80012-6. [DOI] [PubMed] [Google Scholar]
- Donate L. E., Murialdo H., Carrascosa J. L. Production of lambda-phi 29 phage chimeras. Virology. 1990 Dec;179(2):936–940. doi: 10.1016/0042-6822(90)90172-n. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Ensign L. D., Arnold G. E., Roberts L. M. Proposed molten globule intermediates in fd phage penetration and assembly. FEBS Lett. 1991 Nov 4;292(1-2):275–278. doi: 10.1016/0014-5793(91)80883-5. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Griess G. A., Khan S. A., Serwer P. Variation of the permeability of bacteriophage T4: analysis by use of a protein-specific probe for the T4 interior. Biopolymers. 1991 Jan;31(1):11–21. doi: 10.1002/bip.360310103. [DOI] [PubMed] [Google Scholar]
- Griess G. A., Serwer P., Kaushal V., Horowitz P. M. Kinetics of ethidium's intercalation in packaged bacteriophage T7 DNA: effects of DNA packing density. Biopolymers. 1986 Jul;25(7):1345–1357. doi: 10.1002/bip.360250713. [DOI] [PubMed] [Google Scholar]
- Horowitz P. M., Criscimagna N. L. Differential binding of the fluorescent probe 8-anilinonaphthalene-2-sulfonic acid to rhodanese catalytic intermediates. Biochemistry. 1985 May 21;24(11):2587–2593. doi: 10.1021/bi00332a001. [DOI] [PubMed] [Google Scholar]
- Javor G. T., Sood S. M., Chang P., Slattery C. W. Interactions of triply phosphorylated human beta-casein: fluorescence spectroscopy and light-scattering studies of conformation and self-association. Arch Biochem Biophys. 1991 Aug 15;289(1):39–46. doi: 10.1016/0003-9861(91)90439-p. [DOI] [PubMed] [Google Scholar]
- Kellenberger E. Form determination of the heads of bacteriophages. Eur J Biochem. 1990 Jun 20;190(2):233–248. doi: 10.1111/j.1432-1033.1990.tb15568.x. [DOI] [PubMed] [Google Scholar]
- Lawson R. C., Jr, York S. S. Stoichiometry of lac repressor binding to nonspecific DNA: three different complexes form. Biochemistry. 1987 Jul 28;26(15):4867–4875. doi: 10.1021/bi00389a039. [DOI] [PubMed] [Google Scholar]
- O'Brien T. A., Gennis R. B. Studies on the interaction between Escherichia coli pyruvate oxidase and a detergent activator by utilization of the fluorescence probe bis(8-p-toluidino-1-naphthalenesulfonate). Biochemistry. 1979 Mar 6;18(5):804–809. doi: 10.1021/bi00572a010. [DOI] [PubMed] [Google Scholar]
- Rontó G., Agamalyan M. M., Drabkin G. M., Feigin L. A., Lvov Y. M. Structure of bacteriophage T7. Small-angle X-ray and neutron scattering study. Biophys J. 1983 Sep;43(3):309–314. doi: 10.1016/S0006-3495(83)84354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secnik J., Wang Q., Chang C. M., Jentoft J. E. Interactions at the nucleic acid binding site of the avian retroviral nucleocapsid protein: studies utilizing the fluorescent probe 4,4'-bis(phenylamino)(1,1'-binaphthalene)-5,5'-disulfonic acid. Biochemistry. 1990 Aug 28;29(34):7991–7997. doi: 10.1021/bi00486a030. [DOI] [PubMed] [Google Scholar]
- Serwer P. A metrizamide-impermeable capsid in the DNA packaging pathway of bacteriophage T7. J Mol Biol. 1980 Mar 25;138(1):65–91. doi: 10.1016/s0022-2836(80)80005-2. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., Serwer P., Ross M. J. Assembly of bacteriophage T7. Dimensions of the bacteriophage and its capsids. Biophys J. 1981 Dec;36(3):743–757. doi: 10.1016/S0006-3495(81)84763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic analysis of non-essential bacteriophage T7 genes. J Mol Biol. 1973 Sep 15;79(2):227–236. doi: 10.1016/0022-2836(73)90002-8. [DOI] [PubMed] [Google Scholar]
- Valpuesta J. M., Fujisawa H., Marco S., Carazo J. M., Carrascosa J. L. Three-dimensional structure of T3 connector purified from overexpressing bacteria. J Mol Biol. 1992 Mar 5;224(1):103–112. doi: 10.1016/0022-2836(92)90579-9. [DOI] [PubMed] [Google Scholar]
- Wang J. L., Edelman G. M. Fluorescent probes for conformational states of proteins. IV. The pepsinogen-pepsin conversion. J Biol Chem. 1971 Mar 10;246(5):1185–1191. [PubMed] [Google Scholar]