Abstract
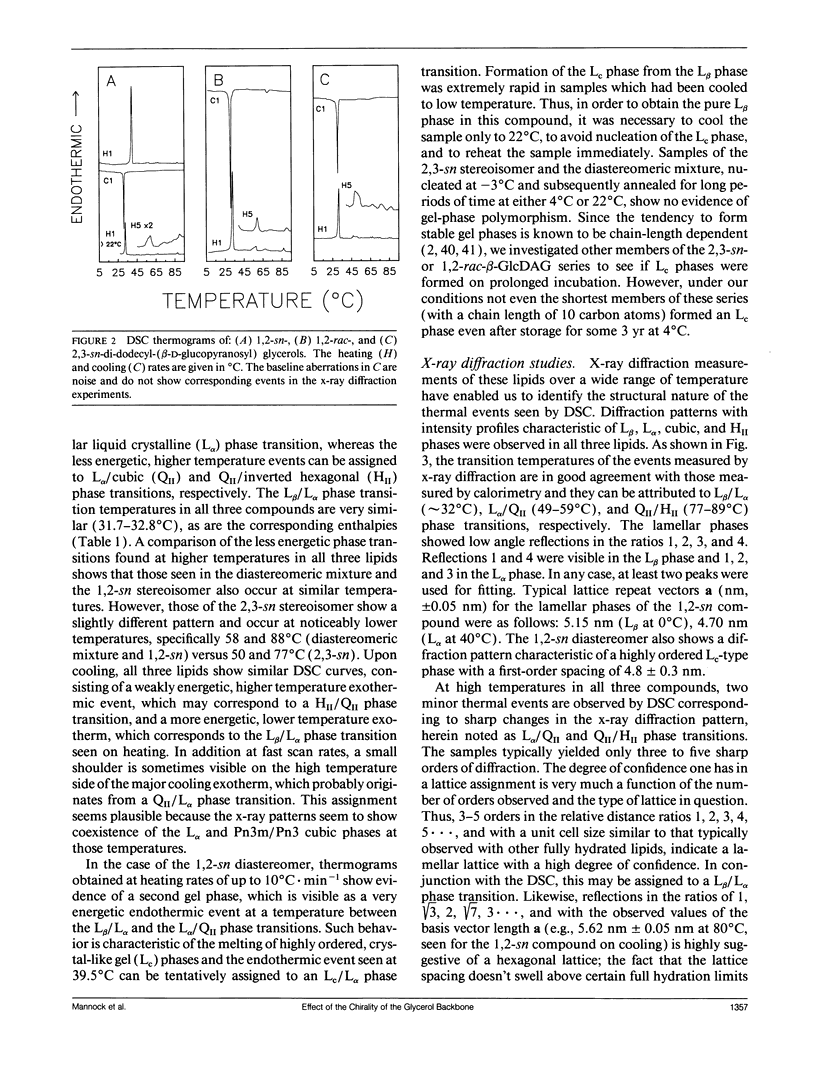
We have studied the physical properties of aqueous dispersions of 1,2-sn- and 2,3-sn-didodecyl-beta-D-glucopyranosyl glycerols, as well as their diastereomeric mixture, using differential scanning calorimetry and low angle x-ray diffraction. Upon heating, both the chiral lipids and the diastereomeric mixture exhibit characteristically energetic L beta/L alpha phase transitions at 31.7-32.8 degrees C and two or three weakly energetic thermal events between 49 degrees C and 89 degrees C. In the diastereomeric mixture and the 1,2-sn glycerol derivative, these higher temperature endotherms correspond to the formation of, and interconversions between, several nonlamellar structures and have been assigned to L alpha/QIIa, QIIa/QIIb, and QIIb/HII phase transitions, respectively. The cubic phases QIIa and QIIb, whose cell lattice parameters are strongly temperature dependent, can be identified as belonging to space groups Ia3d and Pn3m/Pn3, respectively. In the equivalent 2,3-sn glucolipid, the QIIa phase is not observed and only two transitions are seen at 49 degrees C and 77 degrees C, which are identified as L alpha/QIIb and QIIb/HII phase transitions, respectively. These phase transitions temperatures are some 10 degrees C lower than those of the corresponding phase transitions observed in the diastereomeric mixture and the 1,2-sn glycerol derivative. On cooling, all three lipids exhibit a minor higher temperature exothermic event, which can be assigned to a HII/QIIb phase transition. An exothermic L alpha/L beta phase transition is observed at 30-31 degrees C. A shoulder is sometimes discernible on the high temperature side of the L alpha/L beta event, which may originate from a QIIb/L alpha phase transition prior to the freezing of the hydrocarbon chains. None of the lipids show evidence of a QIIa phase on cooling. No additional exothermic transitions are observed on further cooling to -3 degrees C. However, after nucleation at 0 degrees C followed by a short period of annealing at 22 degrees C, the 1,2-sn glucolipid forms an Lc phase that converts to an L alpha phase at 39.5 degrees C on heating. Neither the diastereomeric mixture nor the 2,3-sn glycerol derivative shows such behavior even after extended periods of annealing. Our results suggest that the differences in the phase behavior of these glycolipid isomers may not be attributable to headgroup size per se, but rather to differences in the stereochemistry of the lipid polar/apolar interfacial region, which consequently effects hydrogen-bonding, hydration, and the hydrophilic/hydrophobic balance.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asgharian B., Cadenhead D. A., Mannock D. A., Lewis R. N., McElhaney R. N. A comparative monomolecular film study of 1,2-di-O-palmitoyl-3-O-(alpha- and beta-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1989 Aug 22;28(17):7102–7106. doi: 10.1021/bi00443a047. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim Biophys Acta. 1987 Oct 5;906(3):353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Boyanov A. I., Koynova R. D., Tenchov B. G. Effect of lipid admixtures on the L-dipalmitoylphosphatidylcholine subtransition. Chem Phys Lipids. 1986 Jan;39(1-2):155–163. doi: 10.1016/0009-3084(86)90109-x. [DOI] [PubMed] [Google Scholar]
- Browning J. L. Motions and interactions of phospholipid head groups at the membrane surface. 3. Dynamic properties of amine-containing head groups. Biochemistry. 1981 Dec 8;20(25):7144–7151. doi: 10.1021/bi00528a014. [DOI] [PubMed] [Google Scholar]
- Bruzik K. S. Conformation of the polar headgroup of sphingomyelin and its analogues. Biochim Biophys Acta. 1988 Apr 7;939(2):315–326. doi: 10.1016/0005-2736(88)90076-4. [DOI] [PubMed] [Google Scholar]
- Bruzik K. S., Tsai M. D. A calorimetric study of the thermotropic behavior of pure sphingomyelin diastereomers. Biochemistry. 1987 Aug 25;26(17):5364–5368. doi: 10.1021/bi00391a022. [DOI] [PubMed] [Google Scholar]
- Bruzik K., Jiang R. T., Tsai M. D. Phospholipids chiral at phosphorus. Preparation and spectral properties of chiral thiophospholipids. Biochemistry. 1983 May 10;22(10):2478–2486. doi: 10.1021/bi00279a026. [DOI] [PubMed] [Google Scholar]
- Caffrey M. Kinetics and mechanism of transitions involving the lamellar, cubic, inverted hexagonal, and fluid isotropic phases of hydrated monoacylglycerides monitored by time-resolved X-ray diffraction. Biochemistry. 1987 Oct 6;26(20):6349–6363. doi: 10.1021/bi00394a008. [DOI] [PubMed] [Google Scholar]
- Cevc G. How membrane chain melting properties are regulated by the polar surface of the lipid bilayer. Biochemistry. 1987 Oct 6;26(20):6305–6310. doi: 10.1021/bi00394a002. [DOI] [PubMed] [Google Scholar]
- Dorset D. L. Aliphatic chain packing in three crystalline polymorphs of a saturated racemic phosphatidylethanolamine. A quantitative electron diffraction study. Biochim Biophys Acta. 1976 Mar 26;424(3):396–403. doi: 10.1016/0005-2760(76)90029-1. [DOI] [PubMed] [Google Scholar]
- Endo T., Inoue K., Nojima S. Physical properties and barrier functions of synthetic glyceroglycolipids. J Biochem. 1982 Sep;92(3):953–960. doi: 10.1093/oxfordjournals.jbchem.a134011. [DOI] [PubMed] [Google Scholar]
- Endo T., Inoue K., Nojima S., Sekiya T., Ohki K., Nozawa Y. Electron microscopic study on the structures formed by mixtures containing synthetic glyceroglycolipids. J Biochem. 1983 Jan;93(1):1–6. doi: 10.1093/oxfordjournals.jbchem.a134143. [DOI] [PubMed] [Google Scholar]
- Hauser H., Paltauf F., Shipley G. G. Structure and thermotropic behavior of phosphatidylserine bilayer membranes. Biochemistry. 1982 Mar 2;21(5):1061–1067. doi: 10.1021/bi00534a037. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Sundell S. Preferred conformation and dynamics of the glycerol backbone in phospholipids. An NMR and X-ray single-crystal analysis. Biochemistry. 1988 Dec 27;27(26):9166–9174. doi: 10.1021/bi00426a014. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Kuttenreich H., Meyer R., Renner M., Fründ R., Koynova R., Boyanov A. I., Tenchov B. G. Stereochemistry and size of sugar head groups determine structure and phase behavior of glycolipid membranes: densitometric, calorimetric, and X-ray studies. Biochemistry. 1991 May 28;30(21):5125–5138. doi: 10.1021/bi00235a003. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Six L., Ruess K. P., Liefländer M. Head-group contributions to bilayer stability: monolayer and calorimetric studies on synthetic, stereochemically uniform glucolipids. Biochemistry. 1985 Jan 29;24(3):806–813. doi: 10.1021/bi00324a041. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Jarrell H. C., Wand A. J., Giziewicz J. B., Smith I. C. The dependence of glyceroglycolipid orientation and dynamics on head-group structure. Biochim Biophys Acta. 1987 Feb 12;897(1):69–82. doi: 10.1016/0005-2736(87)90316-6. [DOI] [PubMed] [Google Scholar]
- Koynova R. D., Kuttenreich H. L., Tenchov B. G., Hinz H. J. Influence of head-group interactions on the miscibility of synthetic, stereochemically pure glycolipids and phospholipids. Biochemistry. 1988 Jun 28;27(13):4612–4619. doi: 10.1021/bi00413a005. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Mak N., McElhaney R. N. A differential scanning calorimetric study of the thermotropic phase behavior of model membranes composed of phosphatidylcholines containing linear saturated fatty acyl chains. Biochemistry. 1987 Sep 22;26(19):6118–6126. doi: 10.1021/bi00393a026. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Mannock D. A., McElhaney R. N., Turner D. C., Gruner S. M. Effect of fatty acyl chain length and structure on the lamellar gel to liquid-crystalline and lamellar to reversed hexagonal phase transitions of aqueous phosphatidylethanolamine dispersions. Biochemistry. 1989 Jan 24;28(2):541–548. doi: 10.1021/bi00428a020. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Mantsch H. H., McElhaney R. N. Thermotropic phase behavior of phosphatidylcholines with omega-tertiary-butyl fatty acyl chains. Biophys J. 1989 Jul;56(1):183–193. doi: 10.1016/S0006-3495(89)82663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., Sykes B. D., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing cis-monounsaturated acyl chain homologues of oleic acid: differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry. 1988 Feb 9;27(3):880–887. doi: 10.1021/bi00403a007. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Sykes B. D., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing dl-methyl anteisobranched fatty acids. 1. Differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry. 1987 Jun 30;26(13):4036–4044. doi: 10.1021/bi00387a044. [DOI] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Interactions between neutral phospholipid bilayer membranes. Biophys J. 1982 Mar;37(3):657–665. [PMC free article] [PubMed] [Google Scholar]
- Mannock D. A., Lewis R. N., McElhaney R. N. Physical properties of glycosyl diacylglycerols. 1. Calorimetric studies of a homologous series of 1,2-di-O-acyl-3-O-(alpha-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1990 Aug 28;29(34):7790–7799. doi: 10.1021/bi00486a003. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., Lewis R. N., Sen A., McElhaney R. N. The physical properties of glycosyldiacylglycerols. Calorimetric studies of a homologous series of 1,2-di-O-acyl-3-O-(beta-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1988 Sep 6;27(18):6852–6859. doi: 10.1021/bi00418a030. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., McElhaney R. N. Differential scanning calorimetry and X-ray diffraction studies of a series of synthetic beta-D-galactosyl diacylglycerols. Biochem Cell Biol. 1991 Dec;69(12):863–867. doi: 10.1139/o91-128. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Hsi S. C., Butler K. W., Cameron D. G. Studies on the thermotropic behavior of aqueous phosphatidylethanolamines. Biochim Biophys Acta. 1983 Mar 9;728(3):325–330. doi: 10.1016/0005-2736(83)90502-3. [DOI] [PubMed] [Google Scholar]
- Mariani P., Luzzati V., Delacroix H. Cubic phases of lipid-containing systems. Structure analysis and biological implications. J Mol Biol. 1988 Nov 5;204(1):165–189. doi: 10.1016/0022-2836(88)90607-9. [DOI] [PubMed] [Google Scholar]
- Nyholm P. G., Pascher I., Sundell S. The effect of hydrogen bonds on the conformation of glycosphingolipids. Methylated and unmethylated cerebroside studied by X-ray single crystal analysis and model calculations. Chem Phys Lipids. 1990 Jan;52(1):1–10. doi: 10.1016/0009-3084(90)90002-9. [DOI] [PubMed] [Google Scholar]
- Ranck J. L., Keira T., Luzzati V. A novel packing of the hydrocarbon chains in lipids. The low temperature phases of dipalmitoyl phosphatidyl-glycerol. Biochim Biophys Acta. 1977 Sep 28;488(3):432–441. doi: 10.1016/0005-2760(77)90201-6. [DOI] [PubMed] [Google Scholar]
- Ranck J. L., Tocanne J. F. Choline and acetylcholine induce interdigitation of hydrocarbon chains in dipalmitoylphosphatidylglycerol lamellar phase with stiff chains. FEBS Lett. 1982 Jul 5;143(2):171–174. doi: 10.1016/0014-5793(82)80092-6. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Gruner S. M., Parsegian V. A. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 1990 Jan 9;29(1):76–87. doi: 10.1021/bi00453a010. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Marsh D. Calorimetric studies of the gel-fluid (L beta-L alpha) and lamellar-inverted hexagonal (L alpha-HII) phase transitions in dialkyl- and diacylphosphatidylethanolamines. Biochemistry. 1983 Mar 1;22(5):1280–1289. doi: 10.1021/bi00274a045. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Harlos K., Marsh D. Metastability and polymorphism in the gel and fluid bilayer phases of dilauroylphosphatidylethanolamine. Two crystalline forms in excess water. J Biol Chem. 1983 Mar 25;258(6):3850–3854. [PubMed] [Google Scholar]
- Sen A., Hui S. W. Direct measurement of headgroup hydration of polar lipids in inverted micelles. Chem Phys Lipids. 1988 Dec;49(3):179–184. doi: 10.1016/0009-3084(88)90005-9. [DOI] [PubMed] [Google Scholar]
- Sen A., Hui S. W., Mannock D. A., Lewis R. N., McElhaney R. N. Physical properties of glycosyl diacylglycerols. 2. X-ray diffraction studies of a homologous series of 1,2-Di-O-acyl-3-O-(alpha-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1990 Aug 28;29(34):7799–7804. doi: 10.1021/bi00486a004. [DOI] [PubMed] [Google Scholar]
- Shyamsunder E., Gruner S. M., Tate M. W., Turner D. C., So P. T., Tilcock C. P. Observation of inverted cubic phase in hydrated dioleoylphosphatidylethanolamine membranes. Biochemistry. 1988 Apr 5;27(7):2332–2336. doi: 10.1021/bi00407a014. [DOI] [PubMed] [Google Scholar]
- Tate M. W., Gruner S. M. Temperature dependence of the structural dimensions of the inverted hexagonal (HII) phase of phosphatidylethanolamine-containing membranes. Biochemistry. 1989 May 16;28(10):4245–4253. doi: 10.1021/bi00436a019. [DOI] [PubMed] [Google Scholar]
- Tenchov B. G., Lis L. J., Quinn P. J. Structural rearrangements during crystal-liquid-crystal and gel-liquid-crystal phase transitions in aqueous dispersions of dipalmitoylphosphatidylethanolamine. A time-resolved X-ray diffraction study. Biochim Biophys Acta. 1988 Jul 21;942(2):305–314. doi: 10.1016/0005-2736(88)90032-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. A., Nagle J. F. Dilatometry and calorimetry of saturated phosphatidylethanolamine dispersions. Biochemistry. 1981 Jan 6;20(1):187–192. doi: 10.1021/bi00504a031. [DOI] [PubMed] [Google Scholar]



