Abstract
Mammalian epidermis is renewed throughout life by proliferation of a multipotential stem cell population and terminal differentiation of stem cell progeny. In recent years, extracellular matrix receptors of the integrin family have been identified as important regulators of epidermal homeostasis, influencing the balance between stem cell renewal and differentiation. Integrin expression is altered when the epidermis is damaged or diseased, and there is good evidence that specific integrins can contribute positively or negatively to pathogenesis. In this review I summarize what is known about the expression and function of epidermal integrins, and highlight the challenges for future research.
Keywords: cancer/differentiation/epidermis/integrins/stem cells
Introduction
Integrins are heterodimeric transmembrane receptors consisting of one α and one β subunit. The two subunits collaborate to bind ligands, which are extracellular matrix proteins or counter-receptors of the Ig superfamily. Ligand specificity is determined by heterodimer composition and cellular context. The strength of ligand binding is modulated by divalent cations, by receptor clustering and by the association of integrins with accessory molecules (Bazzoni and Hemler, 1998; Liu et al., 2000; van der Flier and Sonnenberg, 2001). Integrins can transduce signals between cells and the extracellular milieu, and the structural basis of how changes in the extracellular domain can influence the conformation of the cytoplasmic domain is the subject of intense investigation.
The focus of this review is the expression and function of integrins in keratinocytes, the cells that make up the epidermis and other stratified squamous epithelia. Back in 1977, it was demonstrated that when keratinocytes are held in suspension they withdraw from the cell cycle and initiate terminal differentiation (Green, 1977). More than 20 years later, we know that the suspension effect reflects the fact that anchorage of keratinocytes to extracellular matrix negatively regulates their differentiation (Adams and Watt, 1989; Watt et al., 1993; Levy et al., 2000). The advent of tissue-specific knockout mice and the characterization of certain human heritable skin disorders have expanded our understanding of the importance and multiple roles of integrins in the epidermis.
Which integrins are expressed?
The integrins expressed by keratinocytes can be placed into different categories, according to whether they are abundant or weakly expressed, and whether they are expressed constitutively, or induced by wounding or pathological changes (Table I). The most abundant, constitutive integrins in the epidermis are α2β1 (collagen receptor), α3β1 (predominantly a laminin 5 receptor) and α6β4 (laminin receptor) (Watt and Hertle, 1994). αvβ5 (vitronectin receptor) is also a constitutive epidermal integrin, but is expressed at lower levels than the others (Adams and Watt, 1991; Watt and Hertle, 1994).
Table I. Keratinocyte integrins.
| Integrin |
Major ligand |
Expression |
|---|---|---|
| α2β1 | Collagen | Constitutive |
| α3β1 | Laminin | Constitutive |
| α6β4 | Laminin | Constitutive |
| αvβ5 | Vitronectin | Weak |
| α5β1 | Fibronectin | Induced in culture, on wounding, in pathological conditions |
| αvβ6 | Fibronectin; tenascin | As α5β1 |
| α9β1 | Tenascin | Upregulated during wound healing |
| αvβ8 | Vitronectin | Suprabasal |
α5β1 (fibronectin receptor) and αvβ6 (receptor for fibronectin and tenascin) are induced in culture and on wounding (Watt and Hertle, 1994; Breuss et al., 1995; Zambruno et al., 1995; Häkkinen et al., 2000). αvβ6 is often expressed in squamous cell carcinomas, tumours of keratinocytes (Jones et al., 1997). α9β1 (tenascin receptor; Palmer et al., 1993) is expressed in undamaged epidermis (Stepp et al., 1995) and is upregulated during wound healing, albeit with different kinetics from αvβ6 (Häkkinen et al., 2000).
Two other integrins that have been found in the epidermis are α8β1 (receptor for tenascin, fibronectin and vitronectin; Schnapp et al., 1995a) and αvβ8 (receptor for vitronectin and potentially also for laminin, collagen and fibronectin; Nishimura et al., 1994; Venstrom and Reichardt, 1995). Although originally reported in the epidermis of developing chick embryos (Bossy et al., 1991), α8β1 is confined to the arrector pili muscle in postnatal mammalian skin (Schnapp et al., 1995b). Unlike the other keratinocyte integrins, αvβ8 is absent from the basal layer of adult epidermis and is found exclusively in the suprabasal layers (Stepp, 1999).
Where are the integrins expressed?
In normal, undamaged epidermis, integrin expression is confined to the basal layer and outer root sheath of the hair follicles (an exception being αvβ8, as described above). α6β4 is primarily concentrated at the basement membrane zone and is a component of hemidesmosomes. The other integrins are distributed over the basal, lateral and apical surfaces of basal cells (Watt and Hertle, 1994). Whole-mount labelling of human epidermis reveals that on the basal surface of basal cells there are clusters of β1 integrins interspersed with hemidesmosomes, but the majority of β1 integrins appear to form an ‘O’ ring at the cell periphery (Jensen et al., 1999).
In vitro, α6β4 is found in rudimentary hemidesmosomes that are associated with intermediate filaments and also on the leading lamellae of migrating keratinocytes in association with filamentous actin (Adams and Watt, 1991; Mercurio et al., 2001). The β1 integrins are found in focal adhesions, but also, as in vivo, concentrate in an ‘O’ ring at the peripheral membrane in contact with the culture substrate (Braga et al., 1998).
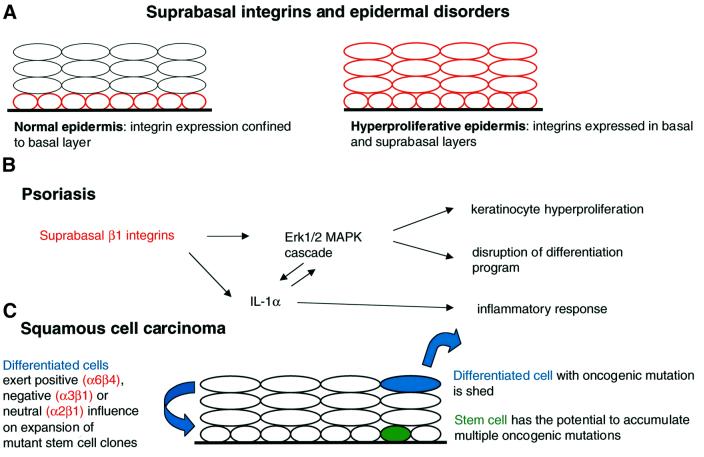
In addition to the upregulation of certain integrins that occurs in culture, during wound healing or in disease (see Table I), two changes in epidermal integrin expression are frequently observed. The first is expression of integrins in the suprabasal, differentiating cell layers, which is generally associated with benign hyperproliferation, for example during wound healing, in psoriatic lesions and in normal oral mucosa, and is also seen in some squamous cell carcinomas (Watt and Hertle, 1994; Figure 1A). The second is focal or generalized loss of integrins, and this is a feature of squamous cell carcinomas (Jones et al., 1993; Bagutti et al., 1998). Focal integrin loss in tumours tends to correlate with a loss of basement membrane proteins, and has been proposed to play a role in invasion (Downer et al., 1993; Jones et al., 1993).
Fig. 1. Schematic representation of how suprabasal integrin expression in the epidermis (A) contributes to the pathogenesis of psoriasis (B) and squamous cell carcinoma (C).
Extracellular matrix adhesion and migration
The earliest experiments to define the role of integrins in keratinocyte adhesion and migration relied on the use of integrin-specific antibodies to perturb adhesion of cultured human keratinocytes on different extracellular matrix substrates (reviewed by Watt and Hertle, 1994). Studies with adhesion-blocking antibodies also established a role for integrins in keratinocyte motility, different integrins mediating movement on different substrates (Watt and Hertle, 1994; Nguyen et al., 2000).
Complementary roles for α3β1 and α6β4 in mediating keratinocyte adhesion and motility on laminin have been proposed, with α3β1 being required at the leading edge of the cell and α6β4 stabilizing attachment distally (DiPersio et al., 1997; Goldfinger et al., 1999; Nguyen et al., 2000). However, it is now clear that α6β4 has distinct functions according to whether it is associated with hemidesmosomes or with membrane protrusions, and α6β4 and α3β1 at the leading edge may collaborate to promote motility and wound healing (Nguyen et al., 2000; Mercurio et al., 2001). One of the mechanisms by which α6β4 promotes migration is through activation of phosphatidylinositol 3-kinase (PI3-K) (Mercurio et al., 2001); however, signalling via Rac and Rho (O’Connor et al., 2000) and the MAPK pathway (Mainiero et al., 1997) are also important.
The generation of mice that lack specific integrins has confirmed their importance in keratinocyte adhesion to the underlying basement membrane (Table II). Mice with a targeted deletion of the α6 or β4 integrin subunit die shortly after birth; they have severe blistering of the skin and other stratified squamous epithelia, and lack hemidesmosomes (Dowling et al., 1996; Georges-Labouesse et al., 1996; van der Neut et al., 1996). Humans who have pyloric atresia with junctional epidermolysis bullosa, an autosomal recessive disorder with epidermal blistering, have mutations in the α6 or β4 integrin genes, the degree of severity of the phenotype reflecting the nature of the mutation (reviewed by Ashton et al., 2001).
Table II. Epidermal phenotype of integrin knockout mice.
| Integrin subunit | Phenotype | Reference |
|---|---|---|
| α3 | Disorganized basement membrane; occasional epidermal/dermal blistering, primarily on legs and footpads | DiPersio et al. (1997) |
| β6 | Juvenile hair loss due to macrophage infiltration into skin; wound healing normal | Huang et al. (1996) |
| α9 | No defects observed | Huang et al. (2000b) |
| β5 | Wound healing normal | Huang et al. (2000a) |
| β1 | Floxed β1 × K5Cre | Brakebusch et al. (2000) |
| Abnormal hair follicles; hair loss with removal of follicles by infiltrating macrophages; epidermal/dermal blisters; reduced proliferation and abnormal differentiation of IFE; disruption of basement membrane; reduced α6β4 expression; reduced hemidesmosomes; dermal fibrosis; impaired wound healing | Grose et al. (2002) | |
| Floxed β1 × K14Cre | Raghavan et al. (2000) | |
| Epidermal/dermal blistering; basement membrane disruption; reduced hemidesmosomes; thin epidermis; reduced number of hair follicles; reduced α6β4 expression | ||
| α6 | Severe epidermal blistering | Georges-Labouesse et al. (1996) |
| β4 | Severe epidermal blistering; absence of hemidesmosomes | Van der Neut et al. (1996) |
| Dowling et al. (1996) | ||
| α3 + α6 | Epidermal blistering; proliferation, stratification and hair follicle morphogenesis normal in adherent epidermis | DiPersio et al. (2000a) |
| α2 | No epidermal defects observed, wound healing not impaired | Chen et al. (2002) Holtkötter et al. (2002) |
In contrast to the α6 and β4 knockouts, the β1 knockout is early embryonic lethal. To study the consequences of epidermal-specific β1 deletion, mice with floxed β1 alleles have been crossed with mice expressing Cre under the control of a promoter (keratin 5 or 14) that is active in the basal layer of the epidermis (Brakebusch et al., 2000; Raghavan et al., 2000). These animals have epidermal blistering, although not as severe as in the α6 or β4 knockouts. Some mice with epidermal deletion of β1 survive long enough to allow wound-healing studies to be performed and these confirm that β1 is essential for keratinocyte migration in vivo (Grose et al., 2002).
Other integrin knockouts have also shed light on which integrins mediate epidermal adhesion and wound healing in vivo. When the α3 subunit is deleted, there is occasional epidermal blistering and more extensive disorganization of the basement membrane (DiPersio et al., 1997). Interestingly, the double knockout of α3β1 and α6β4 has an epidermal phenotype that is no more severe than either one alone (DiPersio et al., 2000a). The α2 (Chen et al., 2002; Holtkötter et al., 2002), α9 (Huang et al., 2000b) and β5 (Huang et al., 2000a) knockouts have no reported skin phenotype, although in vitro β5-null keratinocytes show severely impaired migration (Huang et al., 2000a).
Terminal differentiation and apoptosis
When cultured human or mouse keratinocytes are placed in suspension as single cells, they withdraw from the cell cycle and undergo terminal differentiation (Green, 1977; Adams and Watt, 1989; Drozdoff and Pledger, 1993; Romero et al., 1999). The initiation of terminal differentiation can be partially inhibited by fibronectin or adhesion-blocking antibodies to β1 integrins (Adams and Watt, 1989; Watt et al., 1993; Levy et al., 2000). Fab fragments of anti-β1 antibodies are as effective as whole IgG in blocking differentiation, and the antibodies do not act by promoting actin polymerization in suspended cells (Adams and Watt, 1989; Watt et al., 1993). It thus appears that ligation of β1 integrins is a negative regulator of terminal differentiation and that the mechanism by which β1 integrins exert their effect differs from β1-mediated cell spreading in that it does not require receptor clustering or polymerization of the actin cytoskeleton.
The role of β1 integrins in regulating keratinocyte differentiation has been further investigated by introducing wild-type and mutant chicken β1 subunits into primary human keratinocytes (Levy et al., 2000). These studies demonstrate that the absolute number, and not the proportion, of occupied receptors regulates differentiation. The signal transduced by β1 integrins is an instruction not to differentiate (transduced by occupied receptors) rather than a positive signal to differentiate (transduced by unoccupied receptors). β1 subunits with point mutations inactivating one or both of the cytoplasmic domain NPXY motifs can still regulate differentiation even though they cannot target focal adhesions (Levy et al., 2000). Deletions affecting the juxtamembrane region are, however, inactive, and investigation of the signalling pathways affected by removal of this region should provide information as to the mechanism by which β1 integrins regulate differentiation.
Based on the in vitro experiments with human keratinocytes, one would predict that ablation of epidermal β1 integrins in vivo would stimulate the initiation of differentiation. Some of the mice in which the β1 gene is removed by crossing with keratin 5-Cre animals survive beyond 6 weeks after birth (Brakebusch et al., 2000). They have severe hair loss due to reduced proliferation of hair matrix keratinocytes, and at 7 weeks lack hair follicles and sebaceous glands. Proliferation is also reduced in the interfollicular epidermis. The proportion of suprabasal, terminally differentiating keratinocytes is increased from 20 to 40%, although the differentiation process itself is executed largely normally. Interpreting the effects of β1 ablation is complicated by the fact that the mice display dermal fibrosis and inflammation, both of which can influence keratinocyte proliferation and differentiation. The β1 K14-Cre mice, which die shortly after birth, also show hair abnormalities, blistering and impaired epidermal proliferation; the differentiation programme itself is normal, but the proportion of differentiating cells has not been determined (Raghavan et al., 2000).
The primary effect of deletion of α6 or β4 is massive epidermal blistering, and those regions that remain attached to the basement membrane show normal differentiation (Georges-Labouesse et al., 1996; van der Neut et al., 1996). This is also the case in epidermis lacking both α6β4 and α3β1 (DiPersio et al., 2000a). Clusters of basal keratinocytes are found in the suprabasal layers of β4-null epidermis (Dowling et al., 1996). This might reflect clonal expansion of keratinocytes that have escaped detachment-induced cell death (Dowling et al., 1996); however, since similar clusters of displaced basal cells are found in the roof of newly formed epidermal suction blisters (Hertle et al., 1992), it may simply reflect catapulting of basal cells into the suprabasal layers as a result of disrupted basement membrane adhesion.
The conclusion from analysis of mice lacking β1 or α6β4 integrins is that β1 integrins are indeed important for normal epidermal proliferation, whereas the role of α6β4 is primarily one of anchorage. The finding that mice lacking the α3β1 and α6β4 integrins show no evidence of reduced epidermal proliferation or abnormal differentiation (DiPersio et al., 2000a) suggests that all the β1 integrins must be removed in order to decrease proliferation and increase the proportion of differentiating keratinocytes. The reasons why the β1-null keratinocytes do not all undergo spontaneous terminal differentiation, as occurs when cultured keratinocytes are held in suspension, could be that intact intercellular contacts are maintained in vivo and that a basement membrane, albeit an abnormal one, is present. In vitro, β1-null keratinocytes show greatly impaired adhesion to extracellular matrix proteins and a 5-fold higher proportion of terminally differentiating cells than β1-positive keratinocytes (Grose et al., 2002).
While there are clear parallels between epidermal terminal differentiation and apoptosis, the two processes are distinct and primary human keratinocytes do not undergo suspension-induced apoptosis, known as anoikis (Gandarillas et al., 1999). It has been suggested that in mouse epidermis, loss of β4 causes apoptosis (Dowling et al., 1996), but this has not been confirmed in α6-null epidermis or in epidermis lacking both α3 and α6 integrins (DiPersio et al., 2000a). Targeted deletion of all β1 integrins does not stimulate epidermal apoptosis either (Brakebusch et al., 2000). Although unligated integrins are reported to stimulate apoptosis in adherent cells by recruiting caspase-8 to the plasma membrane (Stupack et al., 2001), this is not seen in transgenic mouse epidermis in which a variety of integrins are expressed suprabasally in their unligated form (Carroll et al., 1995; Romero et al., 1999; Owens and Watt, 2001). Thus, integrins do not appear to play a significant role in regulating apoptosis within the epidermis, which is perhaps not surprising since cells that have left the basal layer in human skin remain viable and metabolically active for several days or weeks before being lost from the cell surface.
Stem cells
Stem cells are a subpopulation of keratinocytes that are responsible for renewing the epidermis throughout adult life, giving rise to the differentiating cells of the interfollicular epidermis, hair follicles and sebaceous glands (Watt, 2001). Stem cell daughters that have left the stem cell compartment can divide a small number of times prior to terminal differentiation and are known as transit-amplifying cells or committed progenitors.
Both in cultures of human keratinocytes and in human interfollicular epidermis, it is possible to enrich for stem cells by selecting the cells that have highest levels of β1 integrins (Jones and Watt, 1993; Jones et al., 1995; Jensen et al., 1999). In human hair follicles, there is also high β1 integrin expression in the region, known as the bulge, where stem cells are concentrated (Jones et al., 1995; Lyle et al., 1998; Akiyama et al., 2000). High β1 expression within the stem cell compartment is of functional significance for two reasons. First, if β1 expression and function are downregulated via a dominant-negative integrin mutation, the cells behave like transit-amplifying cells, differentiating within a few rounds of division (Zhu et al., 1999). β1 integrins and MAP kinase cooperate to maintain the epidermal stem cell compartment in vitro (Zhu et al., 1999). Secondly, high β1 integrin expression helps to maintain the patterned distribution of stem cells; stem cells are less motile than transit-amplifying cells and thus tend to remain clustered within the epidermal basal layer (Jensen et al., 1999). Human keratinocytes in culture that express high levels of β1 integrins also express high levels of α6β4 (Jones and Watt, 1993) and β1 integrin ablation in mouse keratinocytes reduces α6β4 expression (see for example Grose et al., 2002). However, opinions differ as to whether α6β4 can be used to enrich for human epidermal stem cells as effectively as β1 integrins (Jones and Watt, 1993; Li et al., 1998; Jensen et al., 1999).
In cultures of mouse keratinocytes, as in human (Jones and Watt, 1993), stem cells are more adhesive to extracellular matrix than transit-amplifying cells (Bickenbach and Chism, 1998). In mouse epidermis, β1 integrins are reported to be upregulated in the bulge region of the hair follicles (Huelsken et al., 2001). A decrease in β1 integrin expression is associated with c-Myc-mediated depletion of stem cells in mouse epidermis (Waikel et al., 2001) and with the inhibition of proliferation and stimulation of differentiation that results from inhibition of Ras (Dajee et al., 2002). High expression of α6β4 is also thought to be a marker of stem cells in mouse epidermis (Tani et al., 2000), although the functional significance of this is questionable, given that α6β4 ablation does not affect epidermal proliferation (van der Neut et al., 1996; DiPersio et al., 2000a). Conversely, the reduced proliferation of epidermis lacking β1 integrins, the loss of hair follicles and impaired wound healing may all be indicative of depletion of the stem cell compartment, although detailed analysis remains to be carried out (Brakebusch et al., 2000; Raghavan et al., 2000; Grose et al, 2002).
Epidermal hyperproliferation and inflammation
Suprabasal integrin expression, which is a feature of hyperproliferative epidermis (Figure 1A), can contribute to the onset of psoriasis. This has been demonstrated by creating transgenic mice in which various integrin subunits are expressed under the control of the involucrin promoter (Carroll et al., 1995; Romero et al., 1999). The mice have sporadic epidermal hyperproliferation with accompanying histological features of psoriasis, including a lymphocytic infiltrate.
Recent experiments suggest a role for β1 integrin-mediated activation of the classical MAPK cascade in the pathogenesis of psoriasis (Haase et al., 2001; Figure 1B). In hyperproliferative wounded or psoriatic epidermis of human and mouse, but not in normal epidermis, MAPK is activated in basal and suprabasal keratinocytes. Constitutive activation of MAPK in cultured human keratinocytes results in increased proliferation and some of the abnormalities of terminal differentiation that are features of psoriasis. Ligation of suprabasal integrins activates MAPK, thus establishing that the receptors are capable of signal transduction. However, since most potential extracellular matrix ligands are not expressed suprabasally (Hertle et al., 1992), a second mechanism by which suprabasal integrins can activate MAPK, through stimulating keratinocytes to release interleukin (IL)-1α, may be more important (Haase et al., 2001; Figure 1B). This could explain the inflammation seen in affected transgenic mouse epidermis: IL-1α production by keratinocytes induces a dermal mononuclear infiltrate, leading to release of further cytokines and growth factors.
Even though suprabasal integrins are capable of activating MAPK directly or via stimulating release of IL-1α, examination of phenotypically normal transgenic epidermis establishes that suprabasal integrins do not activate MAPK constitutively (Haase et al., 2001). One explanation is that keratinocyte responsiveness to IL-1α does not correlate directly with the amount of IL-1α released (discussed by Haase et al., 2001). In addition, suprabasal integrins may sensitize the epidermis to other, as yet uncharacterized, environmental stimuli (Owens and Watt, 2001).
While suprabasal β1 expression may stimulate inflammation through IL-1α release (Haase et al., 2001), αvβ6 can contribute to skin inflammation via its interaction with transforming growth factor (TGF) β. β6-null mice have juvenile baldness and the degenerating hair follicles are surrounded by foci of monocytes and macrophages (Huang et al., 1996). αvβ6 binds and activates latent TGFβ1, thereby spatially restricting TGFβ1 activation (Munger et al., 1999). This, in turn, could have a profound effect on epidermal proliferation and skin inflammation.
Integrins and squamous cell carcinomas
Within the epidermis, it is the stem cells that have the greatest potential to found tumours. Stem cells are long-term residents and can thus accumulate multiple oncogenic mutations (Figure 1C), whereas transit- amplifying cells and their progeny are continually lost from the epidermis through terminal differentiation (see for example Brown et al., 1998; Jensen et al., 1999). Nevertheless, the differentiated keratinocytes of the epidermis do have a role to play in carcinogenesis as they can influence whether or not a potentially oncogenic clone of stem cell progeny expands to form a tumour or is held in check (Figure 1C).
Examination of human and mouse squamous cell carcinomas reveals considerable variation in integrin expression, both between tumours and in different regions of the same tumour. Normal expression, overexpression and focal or extensive loss of expression of the major keratinocyte integrins have all been observed, together with de novo expression of αvβ6 (see for example Jones et al., 1997; Bagutti et al., 1998). These changes could potentially influence growth and differentiation of the primary tumour and the ability of that tumour to invade and metastasize.
The integrin that has been most heavily implicated in epithelial carcinogenesis is α6β4 (Mercurio and Rabinovitz, 2001). Overexpression of α6β4, i.e. expression in suprabasal keratinocytes or keratinocytes that are not in the layer closest to the tumour stroma, is observed in human and mouse squamous cell carcinomas, correlating with poor prognosis in human oral cancer (van Waes et al., 1991) and with a high risk of malignant conversion in mouse carcinogenesis (Tennenbaum et al., 1993). Within a given tumour, both overexpression of α6β4 in the suprabasal layers and focal loss of α6β4 at the tumour margin can be observed, the latter pattern correlating with loss of the underlying basement membrane (Downer et al., 1993).
Expression of α6β4 is maintained in many invasive carcinomas in the absence of hemidesmosomes; in such cells it is associated with the actin cytoskeleton in areas of protrusive membrane activity (Mercurio and Rabinovitz, 2001). α6β4 is mobilized from the hemidesmosomes in response to chemotactic factors such as epidermal growth factor (EGF), and this is associated with increased phosphorylation of the cytoplasmic domain of β4 (Mainiero et al., 1996). α6β4 cooperates with growth factor receptors in activating PI3-K, and PI3-K activation is essential for invasion (Mercurio and Rabinovitz, 2001); other kinases are also be involved (Giancotti, 2000). In evaluating the role of α6β4 in tumours, attention must be paid not only to overall expression levels, but also to whether or not the integrin is in hemidesmosomes and to its ability to signal in both the ligated and non-ligated state (Mercurio and Rabinovitz, 2001).
The α6β4 studies have tended to emphasize the role of this integrin in promoting invasion by stimulating cell motility. However, another aspect of squamous cell carcinoma invasion is breakdown of the surrounding basement membrane, and there is some evidence that specific integrins influence matrix metalloproteinase expression by keratinocytes (DiPersio et al., 2000b; Thomas et al., 2001). In addition to their effects on tumour invasion, integrins can influence the genesis of tumours and their differentiation status. Introduction of αv into a poorly differentiated αv-negative oral squamous cell carcinoma line results in cell surface expression of αvβ5 and inhibition of anchorage-independent growth (Jones et al., 1996b). In contrast, repair of a β4-negative tumour line did not stimulate differentiation (Jones et al., 1996a), although this is perhaps not surprising in view of the phenotype of α6β4-negative mice.
Since tumorigenesis is a multi-step process, the timing of altered integrin expression may be critical, and early changes may have a greater effect on the course of the disease than the pattern of integrin expression that characterizes a mature tumour. Evidence to support this comes from studies of the transgenic mice in which integrins are expressed suprabasally via the involucrin promoter (Figure 1C). The mice do not develop spontaneous tumours; however, integrin-specific effects become evident when tumours are induced by applying DMBA to initiate Ras mutations, followed by repeated treatments with phorbol ester to promote expansion of mutant clones. Benign tumours (papillomas) appear first; some of these regress, but others convert to malignant squamous cell carcinomas. Mice expressing transgenic α2β1 respond in the same way as wild-type animals (Owens and Watt, 2001). Mice expressing α3β1 develop papillomas at the same frequency as wild-type animals; however, the papillomas are more highly differentiated and show a decreased rate of conversion to malignant tumours. The mechanism of action of α3β1 is unknown, but there is correlative evidence that it involves suppression of the TM4SF protein CD81 (Owens and Watt, 2001). In contrast, overexpression of α6β4 increases papilloma and squamous cell carcinoma formation (D.M.Owens and F.M.Watt, in preparation). Thus, whereas stem cells are responsible for the genesis of most tumours, altered integrin expression in the differentiated cell layers can profoundly affect the course of the disease (Figure 1C).
Conclusions
From this review, it is clear that integrins do far more than simply anchor the epidermis to the underlying basement membrane. They are required for wound repair and contribute to skin inflammation. They regulate the balance between proliferation and differentiation, and perturbed integrin expression contributes to the pathogenesis of benign and neoplastic conditions. Ten years ago, scientists working in this area were largely preoccupied with identifying new integrins and cataloguing their expression; now the focus is on understanding how integrins exert their diverse functions in keratinocytes.
Acknowledgments
Acknowledgement
F.M.W. is a recipient of the C.E.R.I.E.S. Research Award.
References
- Adams J.C. and Watt,F.M. (1989) Fibronectin inhibits the terminal differentiation of human keratinocytes. Nature, 340, 307–309. [DOI] [PubMed] [Google Scholar]
- Adams J.C. and Watt,F.M. (1991) Expression of β1, β3, β4 and β5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J. Cell Biol., 115, 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama M., Smith,L.T. and Shimizu,H. (2000) Changing patterns of localization of putative stem cells in developing human hair follicles. J. Invest. Dermatol., 114, 321–327. [DOI] [PubMed] [Google Scholar]
- Ashton G.H., Sorelli,P., Mellerio,J.E., Keane,F.M., Eady,R.A. and McGrath,J.A. (2001) α6β4 integrin abnormalities in junctional epidermolysis bullosa with pyloric atresia. Br. J. Dermatol., 144, 408–414. [DOI] [PubMed] [Google Scholar]
- Bagutti C., Speight,P.M. and Watt,F.M. (1998) Comparison of integrin, cadherin, and catenin expression in squamous cell carcinomas of the oral cavity. J. Pathol., 186, 8–16. [DOI] [PubMed] [Google Scholar]
- Bazzoni G. and Hemler,M.E. (1998) Are changes in integrin affinity and conformation overemphasized? Trends Biochem. Sci., 23, 30–34. [DOI] [PubMed] [Google Scholar]
- Bickenbach J.R. and Chism,E. (1998) Selection and extended growth of murine epidermal stem cells in culture. Exp. Cell Res., 244, 184–195. [DOI] [PubMed] [Google Scholar]
- Bossy B., Bossy-Wetzel,E. and Reichardt,L.F. (1991) Characterization of the integrin α8 subunit: a new integrin β1-associated subunit, which is prominently expressed on axons and on cells in contact with basal laminae in chick embryos. EMBO J., 10, 2375–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga V.M., Hajibagheri,N. and Watt,F.M. (1998) Calcium-induced intercellular adhesion of keratinocytes does not involve accumulation of β1 integrins at cell–cell contacts and does not involve changes in the levels or phosphorylation of catenins. Cell Adhes. Commun., 5, 137–149. [DOI] [PubMed] [Google Scholar]
- Brakebusch C. et al. (2000) Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J., 19, 3990–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuss J.M. et al. (1995) Expression of the β6 integrin in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci., 108, 2241–2251. [DOI] [PubMed] [Google Scholar]
- Brown K., Strathdee,D., Bryson,S., Lambie,W. and Balmain A. (1998) The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr. Biol., 8, 516–524. [DOI] [PubMed] [Google Scholar]
- Carroll J.M., Romero,M.R. and Watt,F.M. (1995) Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell, 83, 957–968. [DOI] [PubMed] [Google Scholar]
- Chen J., Diacov,T.G., Grenache,D.G., Santoro,S.A. and Zutter,M.M. (2002) The α2 integrin subunit deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and homostasis. Am. J. Pathol., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajee M., Tarutani,M., Deng,H., Cai,T. and Khavari,P.A. (2002) Epidermal Ras blockade demonstrates spatially localized Ras promotion of proliferation and inhibition of differentiation. Oncogene, 21, 1527–1538. [DOI] [PubMed] [Google Scholar]
- DiPersio C.M., Hodivala-Dilke,K.M., Jaenisch,R., Kreidberg,J.A. and Hynes,R.O. (1997) α3β1 integrin is required for normal development of the epidermal basement membrane. J. Cell Biol., 137, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio C.M., van der Neut,R., Georges-Labouesse,E., Kreidberg,J.A., Sonnenberg,A. and Hynes,R.O. (2000a) α3β1 and α6β4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J. Cell Sci., 113, 3051–3062. [DOI] [PubMed] [Google Scholar]
- DiPersio C.M., Shao,M., Di Costanzo,L., Kreidberg,J.A. and Hynes,R.O. (2000b) Mouse keratinocytes immortalized with large T antigen acquire α3β1 integrin-dependent secretion of MMP-9/gelatinase B. J. Cell Sci., 113, 2909–2921. [DOI] [PubMed] [Google Scholar]
- Dowling J., Yu,Q.-C. and Fuchs,E. (1996) β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol., 134, 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer C.S., Watt,F.M. and Speight,P.M. (1993) Loss α6 and β4 integrin subunits coincides with loss of basement membrane components in oral squamous cell carcinomas. J. Pathol., 171, 183–190. [DOI] [PubMed] [Google Scholar]
- Drozdoff V. and Pledger,W.J. (1993) Commitment to differentiation and expression of early differentiation markers in murine keratinocytes in vitro are regulated independently of extracellular calcium concentrations. J. Cell Biol., 123, 909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandarillas A., Goldsmith,L.A., Gschmeissner,S., Leigh,I.M. and Watt,F.M. (1999) Evidence that apoptosis and terminal differentiation of epidermal keratinocytes are distinct processes. Exp. Dermatol., 8, 71–79. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E., Messaddeq,N., Yehia,G., Cadalbert,L., Dierich,A. and LeMeur,M. (1996) Absence of the α6 integrin leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet., 13, 370–373. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G. (2000) Complexity and specificity of integrin signalling. Nat. Cell Biol., 2, E13–E14. [DOI] [PubMed] [Google Scholar]
- Goldfinger L.E., Hopkinson,S.B., deHart,G.W., Collawn,S., Couchman,J.R. and Jones,J.C. (1999) The α3 laminin subunit, α6β4 and α3β1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J. Cell Sci., 112, 2615–2629. [DOI] [PubMed] [Google Scholar]
- Green H. (1977) Terminal differentiation of cultured human epidermal cells. Cell, 11, 405–416. [DOI] [PubMed] [Google Scholar]
- Grose R., Hutter,C., Bloch,W., Thorey,I., Watt,F.M., Fässler,R., Brakebusch,C. and Werner,S. (2002) A crucial role of β1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development, 129, 2303–2315. [DOI] [PubMed] [Google Scholar]
- Haase I., Hobbs,R.M., Romero,M.R., Broad,S. and Watt,F.M. (2001) A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J. Clin. Invest., 108, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häkkinen L., Hildebrand,H.C., Berndt,A., Kosmehl,H. and Larjava,H. (2000) Immunolocalization of tenascin-C, α9 integrin subunit and αvβ6 integrin during wound healing in human oral mucosa. J. Histochem. Cytochem., 48, 985–998 [DOI] [PubMed] [Google Scholar]
- Hertle M.D., Kubler,M.-D., Leigh,I.M. and Watt,F.M. (1992) Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J. Clin. Invest., 89, 1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkötter O., Nieswandt,B., Smyth,N., Müller,W., Hafner,M., Schulte,V., Krieg ,T. and Eckes,B. (2002) Integrin α2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J. Biol. Chem., 277, 10789–10794. [DOI] [PubMed] [Google Scholar]
- Huang X.-Z., Wu,J.F., Cass,D., Erle,D.J., Corry,D., Young,S.G., Farese,R.V.,Jr and Sheppard,D. (1996) Inactivation of the integrin β6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lungs and skin. J. Cell Biol., 133, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Griffiths,M., Wu,J., Farese,R.V.,Jr and Sheppard,D. (2000a) Normal development, wound healing and adenovirus susceptibility in β5-deficient mice. Mol. Cell. Biol., 20, 755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.Z., Wu,J.F., Ferrando,R., Lee,J.H., Wang,Y.L., Farese,R.V.,Jr and Sheppard,D. (2000b) Fatal bilateral chylothorax in mice lacking the integrin α9β1. Mol. Cell. Biol., 20, 5208–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J., Vogel,R., Erdmann,B., Cotsarelis,G. and Birchmeier,W. (2001) β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell, 105, 533–545. [DOI] [PubMed] [Google Scholar]
- Jensen U.B., Lowell,S. and Watt,F.M. (1999) The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole mount labelling and lineage analysis. Development, 126, 2409–2418. [DOI] [PubMed] [Google Scholar]
- Jones J., Sugiyama,M., Watt,F.M. and Speight,P.M. (1993) Integrin expression in normal, hyperplastic, dysplastic and malignant oral epithelium. J. Pathol., 169, 235–243. [DOI] [PubMed] [Google Scholar]
- Jones J., Sugiyama,M., Giancotti,F., Speight,P.M. and Watt,F.M. (1996a) Transfection of β4 integrin subunit into a neoplastic keratinocyte line fails to restore terminal differentiation capacity or influence proliferation. Cell Adhes. Commun., 4, 307–316. [DOI] [PubMed] [Google Scholar]
- Jones J., Sugiyama,M., Speight,P.M. and Watt,F.M. (1996b) Restoration of αvβ5 integrin expression in neoplastic keratinocytes results in increased capacity for terminal differentiation and suppression of anchorage-independent growth. Oncogene, 12, 119–126. [PubMed] [Google Scholar]
- Jones J., Watt,F.M. and Speight,P.M. (1997) Changes in the expression of αv integrins in oral squamous cell carcinomas. J. Oral Pathol. Med., 26, 63–68. [DOI] [PubMed] [Google Scholar]
- Jones P.H. and Watt,F.M. (1993) Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell, 73, 713–724. [DOI] [PubMed] [Google Scholar]
- Jones P.H., Harper,S. and Watt,F.M. (1995) Stem cell patterning and fate in human epidermis. Cell, 80, 83–93. [DOI] [PubMed] [Google Scholar]
- Levy L., Broad,S., Diekmann,D., Evans,R.D. and Watt,F.M. (2000) β1 integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol. Biol. Cell, 11, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A., Simmons,P.J. and Kaur,P. (1998) Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl Acad. Sci. USA, 95, 3902–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Calderwood,D.A. and Ginsberg,M.H. (2000) Integrin cytoplasmic domain-binding proteins. J. Cell Sci., 113, 3563–3571. [DOI] [PubMed] [Google Scholar]
- Lyle S., Christofidou-Solomidou,M., Liu,Y., Elder,D.E., Albelda,S. and Cotsarelis,G. (1998) The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell Sci., 111, 3179–3188. [DOI] [PubMed] [Google Scholar]
- Mainiero F., Pepe,A., Yeon,M., Ren,Y. and Giancotti,F.G. (1996) The intracellular functions of α6β4 integrin are regulated by EGF. J. Cell Biol., 134, 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F., Murgia,C., Wary,K.K., Curatola,A.M., Pepe,A., Blumemberg,M., Westwick,J.K., Der,C.J. and Giancotti,F.G. (1997) The coupling of α6β4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J., 16, 2365–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio A.M. and Rabinovitz,I. (2001) Towards a mechanistic understanding of tumor invasion—lessons from the α6β4 integrin. Semin. Cancer Biol., 11, 129–141. [DOI] [PubMed] [Google Scholar]
- Mercurio A.M., Rabinovitz,I. and Shaw,L.M. (2001) The α6β4 integrin and epithelial cell migration. Curr. Opin. Cell Biol., 13, 541–545. [DOI] [PubMed] [Google Scholar]
- Munger J.S. et al. (1999) The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell, 96, 319–328. [DOI] [PubMed] [Google Scholar]
- Nguyen B.P., Ryan,M.C., Gil,S.G. and Carter,W.G. (2000) Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr. Opin. Cell Biol., 12, 554–562. [DOI] [PubMed] [Google Scholar]
- Nishimura S.L., Sheppard,D. and Pytela,R. (1994) Integrin αvβ8. Interaction with vitronectin and functional divergence of the β8 cytoplasmic domain. J. Biol. Chem., 269, 28708–28715. [PubMed] [Google Scholar]
- O’Connor K.L., Nguyen,B.K. and Mercurio,A.M. (2000) RhoA function in lamellae formation and migration is regulated by the α6β4 integrin and cAMP metabolism. J. Cell Biol., 148, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens D.M. and Watt,F.M. (2001) Influence of β1 integrins on epidermal squamous cell carcinoma formation in a transgenic mouse model: α3β1, but not α2β1, suppresses malignant conversion. Cancer Res., 61, 5248–5254. [PubMed] [Google Scholar]
- Palmer E.L., Rüegg,C., Ferrando,R., Pytela,R. and Sheppard,D. (1993) Sequence and tissue distribution of the integrin α9 subunit, a novel partner of β1 that is widely distributed in epithelia and muscle. J. Cell Biol., 123, 1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S., Bauer,C., Mundschau,G., Li,Q. and Fuchs,E. (2000) Conditional ablation of β1 integrin in skin: severe defects in epidermal proliferation, basement membrane formation and hair follicle invagination. J. Cell Biol., 150, 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero M.R, Carroll,J.M. and Watt,F.M. (1999) Analysis of cultured keratinocytes from a transgenic mouse model of psoriasis: effects of suprabasal integrin expression on keratinocyte adhesion, proliferation and terminal differentiation. Exp. Dermatol., 8, 53–67. [DOI] [PubMed] [Google Scholar]
- Schnapp L.M., Hatch,N., Ramos,D.M., Klimanskaya,I.V., Sheppard,D. and Pytela,R. (1995a) The human integrin α8β1 functions as a receptor for tenascin, fibronectin and vitronectin. J. Biol. Chem., 270, 23196–23202. [DOI] [PubMed] [Google Scholar]
- Schnapp L.M., Breuss,J.M., Ramos,D.M., Sheppard,D. and Pytela,R. (1995b) Sequence and tissue distribution of the human integrin α8 subunit: a β1-assoicated α subunit expressed in smooth muscle cells. J. Cell Sci., 108, 537–544. [DOI] [PubMed] [Google Scholar]
- Stepp M.A. (1999) α9 and β8 integrin expression correlates with the merger of the developing mouse eyelids. Dev. Dyn., 214, 216–228. [DOI] [PubMed] [Google Scholar]
- Stepp M.A., Zhu,L., Sheppard,D. and Cranfill,R.L. (1995) Localized distribution of α9 integrin in the cornea and changes in expression during corneal epithelial cell differentiation. J. Histochem. Cytochem., 43, 353–362. [DOI] [PubMed] [Google Scholar]
- Stupack D.G., Puente,X.S., Boutsaboualoy,S., Storgard,C.M. and Cheresh,D.A. (2001) Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol., 155, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H., Morris,R.J. and Kaur,P. (2000) Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc. Natl Acad. Sci. USA, 97, 10960–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennenbaum T., Weiner,A.K., Belanger,A.J., Glick,A.B., Hennings,H. and Yuspa,S.H. (1993) The suprabasal expression of α6β4 integrin is associated with a high risk for malignant progression in mouse skin carcinogenesis. Cancer Res., 53, 4803–4810. [PubMed] [Google Scholar]
- Thomas G.J., Lewis,M.P., Hart,I.R., Marshall,J.F. and Speight,P.M. (2001) αvβ6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int. J. Cancer, 92, 641–650. [DOI] [PubMed] [Google Scholar]
- van der Flier A. and Sonnenberg,A. (2001) Function and interactions of integrins. Cell Tissue Res., 305, 285–298. [DOI] [PubMed] [Google Scholar]
- van der Neut R., Krimpenfort,P., Calafat,J., Niessen,C.M. and Sonnenberg,A. (1996) Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat. Genet., 13, 366–369. [DOI] [PubMed] [Google Scholar]
- van Waes C., Kozarsky,K.F., Warren,A.B., Kidd,L., Paugh,D., Liebert,M. and Carey,T.E. (1991) The A9 antigen associated with aggressive human squamous carcinoma is structurally and functionally similar to the newly defined integrin α6β4. Cancer Res., 51, 2395–2402. [PubMed] [Google Scholar]
- Venstrom K. and Reichardt,L. (1995) β8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV and fibronectin. Mol. Biol. Cell, 6, 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waikel R.L., Kawachi,Y., Waikel,P.A., Wang,X.J. and Roop,D.R. (2001) Deregulated expression of c-Myc depletes epidermal stem cells. Nat. Genet., 28, 165–168. [DOI] [PubMed] [Google Scholar]
- Watt F.M. (2001) Stem cell fate and patterning in mammalian epidermis. Curr. Opin. Genet. Dev., 11, 410–417. [DOI] [PubMed] [Google Scholar]
- Watt F.M. and Hertle,M.D. (1994) Keratinocyte integrins. In Leigh,I.M., Lane,E.B. and Watt,F.M. (eds), The Keratinocyte Handbook. Cambridge University Press, Cambridge, UK, pp. 153–164.
- Watt F.M., Kubler,M.-D., Hotchin,N.A., Nicholson,L.J. and Adams,J.C. (1993) Regulation of keratinocyte terminal differentiation by integrin-extracellular matrix interactions. J. Cell Sci., 106, 175–182. [DOI] [PubMed] [Google Scholar]
- Zambruno G., Marchisio,P.C., Marconi,A., Vaschieri,C., Melchiori,A., Giannetti,A. and De Luca,M. (1995) Transforming growth factor-β1 modulates β1 and β5 integrin receptors and induces the de novo expression of the αvβ6 heterodimer in normal human keratinocytes: implications for wound healing. J. Cell Biol., 129, 853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A.J., Haase,I. and Watt,F.M. (1999) Signalling via β1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc. Natl Acad. Sci. USA, 96, 6728–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]